Abstract
As oxygen and selenium are in the same group (Family VI) in the Periodic Table, the site-specific mutagenesis at the atomic level by replacing RNA oxygen with selenium can provide insights on structure and function of catalytic RNAs. We report here the first Se-derivatized ribozymes transcribed with all nucleoside 5’-(α-P-seleno)triphosphates (NTPαSe, including A, C, G, and U). We found that T7 RNA polymerase recognizes NTP SeαSp diastereomers as well as the natural NTPs, while NTPαSe Rp diastereomers are neither substrates nor inhibitors. We also demonstrated the catalytic activity of these Se-derivatized hammerhead ribozymes by cleaving the RNA substrate, and we found that these phosphoroselenoate ribozymes can be as active as the native. These hammerhead ribozymes mutagenized site-specifically by selenium reveal the close relationship between the catalytic activities and the replaced oxygen atoms, which provides the insight of the oxygen participation in catalysis or intramolecular interaction. This demonstrates a convenient strategy for mechanistic study of functional RNAs. In addition, the active ribozymes derivatized site-specifically by selenium will allow convenient MAD phasing in X-ray crystal structure study.
Keywords: selenium derivatization, phosphoroselenoate, ribozyme, X-ray crystallography
Functional RNAs play important roles in biological systems, including rRNA processing, mRNA editing, gene regulation, and RNA cleavage catalysis (1-4). X-ray crystallography is a powerful method for 3D structural and functional studies of large RNA molecules (5-7). In addition to crystallization (8, 9), however, heavy atom derivatization for phase determination is still the major problem in novel structure determination of nucleic acid molecules, especially RNAs, by X-ray crystallography (6-8). On the other hand, the selenomethionine strategy was developed in order to derivatize proteins and solve the phasing problem via multi-wavelength anomalous dispersion (MAD). Recently over two thirds of novel protein structures have been determined via the selenomethionine strategy (10, 11), which has clearly revolutionized protein X-ray crystallography. As selenium, sulfur, and oxygen are in the same group (Family VI) in the Periodic Table, we are attempting to develop the selenium derivatization of nucleic acids by replacing oxygen with selenium for nucleic acid X-ray crystallography, similarly to the selenium derivatization of proteins by replacing sulfur with selenium (10, 11).
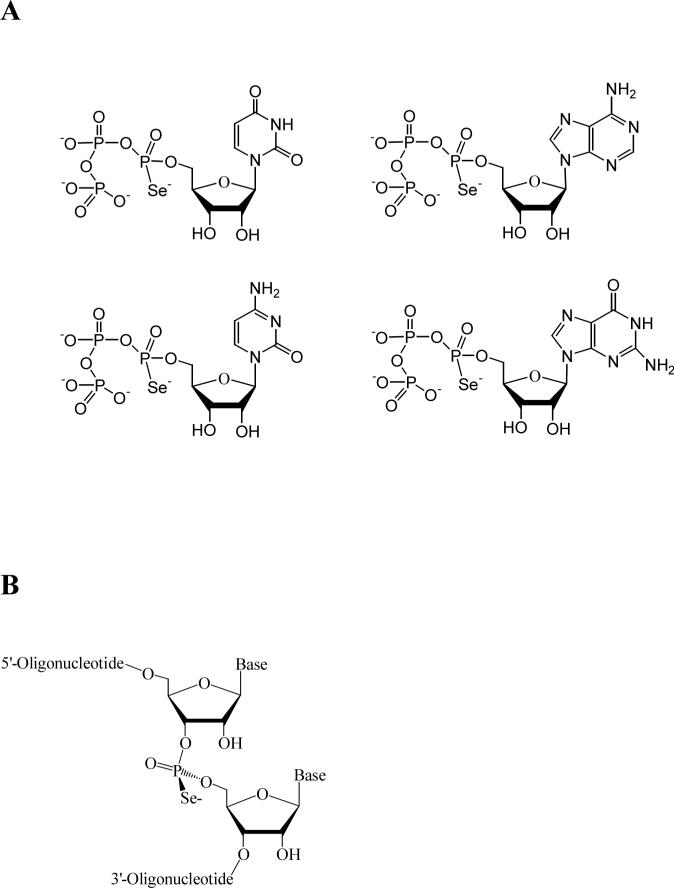
For this goal, our laboratory is in the process of developing selenium derivatization of nucleic acids for MAD phasing (12-18), and this novel derivatization methodology has been demonstrated in X-ray crystal structure studies of RNA and DNA molecules by several laboratories (14, 19, 20). To develop a general Se-derivatization strategy for the structural studies of functional RNAs, especially large ones, and to study the activity and structure after the selenium derivatization, we report here for the first time the successful enzymatic synthesis of Se-derivatized ribozymes using all four nucleoside 5’-(α-P-seleno)triphosphates (A, C, G, and U; Figure 1), and catalytic activities of these hammerhead ribozymes derivatized site-specifically with selenium. Our experimental results provide insights for structure and catalytic mechanism and demonstrate a useful strategy for the studies of ribozyme catalysis and structure.
FIGURE 1.
(A) NTPαSe structure. (B) Structure of Rp phosphoroselenoate RNA.
MATERIALS AND METHODS
Oligonucleotides and Triphosphate Derivatives
The NTPαSe analogs were prepared as described (17, 18), and all NTPαSe analogs were purified and then analyzed by HR-MS and RP-HPLC (Figure 2). A DNA template (55 nt, 5’-TGTACGTTTCGGCCTTTCGGCCTCATCAGGTTGCCTATAGTGAGTCGTATTACGC-3’,) was designed and synthesized on solid phase for in vitro transcription of the Se-modified hammerhead ribozymes using the NTPαSe analogs. T7 RNA polymerase promoter (top strand DNA, 5’-GCGTAATACGACTCACTATAG-3’) and an RNA substrate (5'-GGUCAUCUUUCCUAC-CUGUACGUCGUUGCCUAA-3') were also synthesized chemically.
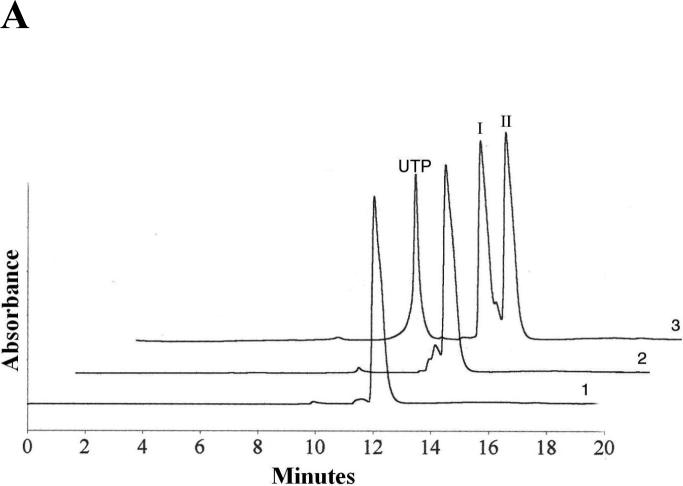
FIGURE 2.
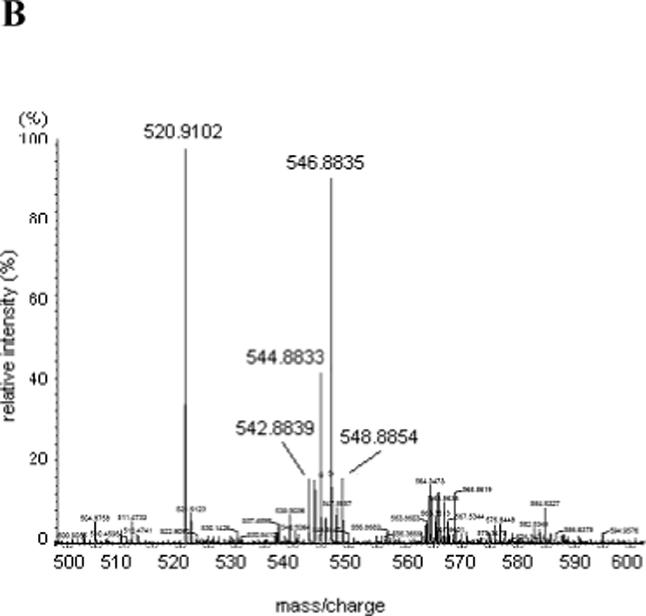
Analysis of UTPαSe by HPLC and HR-MS. (A) Typical HPLC profile: analysis of UTPαSe I and II, and normal UTP. 1. UTPαSe I; 2. UTPαSe II; 3. Co-injection of UTP, UTPαSe I and II. Their retention times were 9.7, 12.0, and 12.9 min, respectively. For the purpose of the HPLC presentation, the traces 2 and 3 are shifted to the right. (B) HR-MS analysis of UTPαSe I (UTPαSe II is almost the same). Molecular formula: C9H15N2O14P3Se; calculated isotopic mass (M-H+)−: 546.8823; measured mass: 546.8835.
Transcription of the Hammerhead Ribozyme using NTPs and NTPαSe Analogs
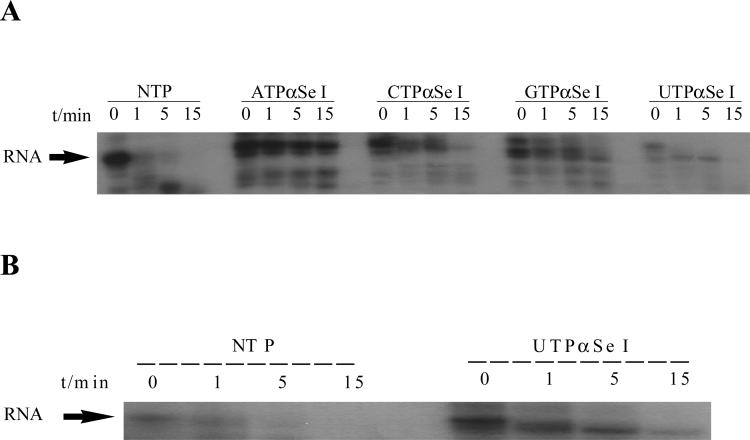
T7 RNA polymerase promoter and DNA template (Figure 3) were prepared by oligonucleotide solid-phase synthesis. Ampliscribe™ T7 Transcription Kit (Epicentre) was used for in vitro transcription, where DNA template and the top strand were added in equal molar amounts to a cocktail containing DTT and the buffer. The cocktail was split into two equal portions. α-32P-CTP was added to one portion, and α-32P-ATP was added to the other portion. Each portion was split equally into three Eppendorf tubes. The α-32P-CTP-containing mixture was split equally into three tubes labeled ATPαSe I, UTPαSe I, and NTP, respectively. The α-32P-ATP-containing mixture was split equally into three tubes labeled CTPαSe I, GTPαSe I, and NTP, respectively. Equal molar amounts of NTPαSe I and NTP were added to each tube as labeled (e.g., to the ATPαSe I tube, ATPαSe I, CTP, GTP, and UTP were added). Typical reactions were performed under the conditions of 0.1 μM top strand and template, NTP (1 mM), NTPαSe (1 mM), and 0.06 μL enzyme per 1 μL of reaction mixture. The reactions were initiated by the addition of T7 RNA polymerase, and were incubated at 37 °C. Aliquots (3.5 μL each) were removed from the reaction mixture at the corresponding time points, and the transcription reactions were quenched by the addition of the same volume of the loading dye containing EDTA (100 mM), followed by placing on dry ice. The comparison experiments of NTPαSe I and II diastereomers were done in the similar way, where the incubation time at 37 °C was 1 hour.
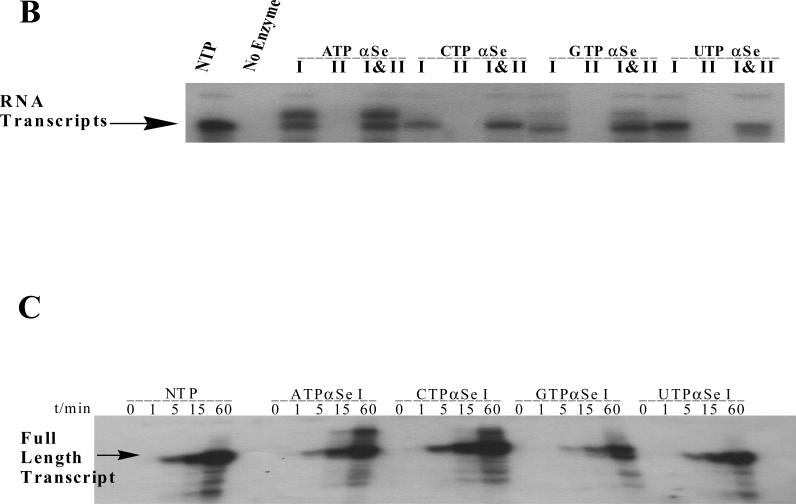
FIGURE 3.
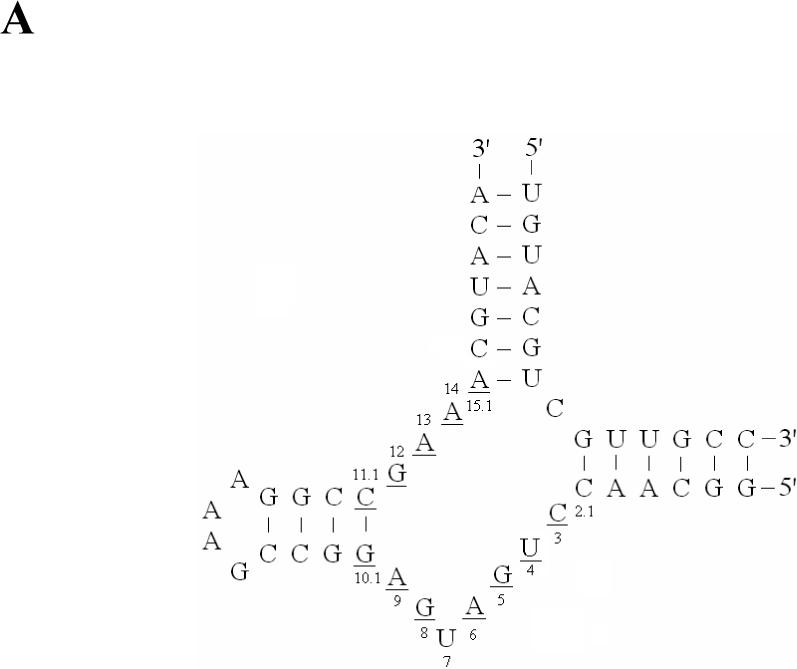
(A) The secondary structure of the hammerhead ribozymes; the underline sequences are highly conserved. (B) The transcription with native NTP, ATPαSe I & II, CTPαSe I & II, GTPαSe I & II UTPαSe I & II. (C) The time-course experiment of the transcription with native NTP, ATPαSe I, CTPαSe I, GTPαSe I, and UTPαSe I.
Kinase Reaction of the RNA Substrate
The RNA substrate (33 nt.) was kinased by T4 polynucleotide kinase and γ-32P-ATP for 1 hr at 37 °C. After the kinase reaction, extra γ-32P-ATP and salts were removed by NaCl/EtOH precipitation.
Activity Study of the Se-Hammerhead Ribozymes
The ribozymes were transcribed for 1 hour as previously discussed without the addition of any radioactive NTPs, and were desalted by centrifugation using a membrane (3000 Dalton cut-off) three times. The ribozymes were concentrated and adjusted to the same concentration on the basis of the time-course transcription experiment (Figure 3C). A cocktail containing the Tris-Cl buffer (pH 7.6, 10 mM MgCl2) and the 32P-labeled RNA substrate was made, and it was split into 5 equal portions. The ribozymes transcribed with ATPαSe I, CTPαSe I, GTPαSe I, UTPαSe I, and NTPs were individually added to each portion to initiate the substrate digestion reaction incubated at 27 °C. Aliquots (3.5 μL each) were removed from the reaction mixture at the corresponding time points and quenched individually with the loading dye (3.5 μL) containing EDTA (100 mM), followed by placing on dry ice. The digestion reactions were analyzed by PAGE (Figure 4A). The gel image quantification was done by using phosphorimager.
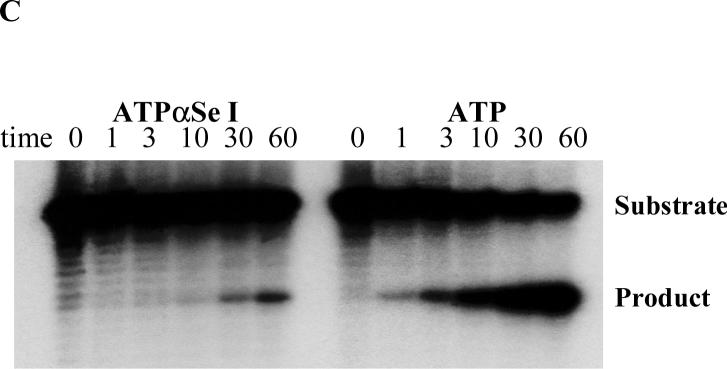
FIGURE 4.
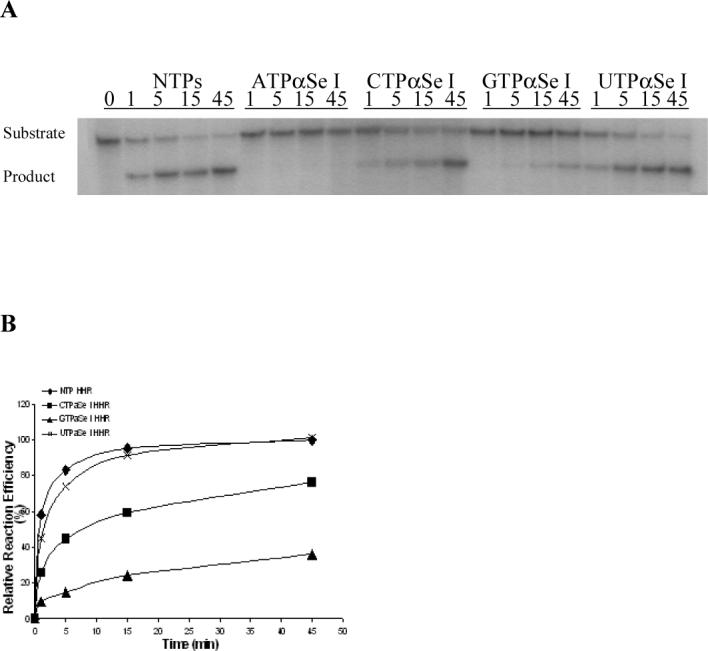
Catalysis and analysis of the modified and native hammerhead ribozymes: 5’-GGCAACCUGAUGAGGCCGAAAGGCCGAAACGUACA-3’. The highly conserved sequences are underlined. (A) The time-course ribozyme digestion of the RNA substrate: 5'-GGUCAUCUUUCCUACC-UGUACGUCGUUGCCUAA-3'. (B) The plot of the catalytic experimental results, relative to the activity of the native ribozyme. (C) The time-course Mn2+ rescue experiment of the ribozymes transcribed with ATPαSe I and ATP.
Se-Ribozyme Resistance to the Digestion of Snake Venom Phosphodiesterase I
The ribozymes were transcribed as previously discussed. The transcribed ribozymes were desalted by centrifugation using a membrane (3000 Dalton cut-off) three times. To ensure equal molar concentrations, the samples were concentrated and adjusted to the same concentration. These modified and native ribozymes were then digested with Snake Venom Phosphodiesterase I (0.001 U/μL, USB) in its buffer. Aliquots (3.5 μL each) were removed from the reaction mixture at the corresponding time points and quenched individually with the loading dye (3.5 μL) containing EDTA (100 mM), followed by placing on dry ice. The digestion reactions were analyzed by PAGE (Figure 5A).
FIGURE 5.
Se-Ribozyme resistance to the digestion of snake venom phosphodiesterase I. (A) The PAGE analysis of the digestion. (B) Snake venom phosphodiesterase I digestion of the modified and native ribozymes transcribed with NTP and UTPαSe I; these 32P-labeled ribozymes were digested after the use in their substrate cleavage reactions.
Se-Ribozyme (after Use in the RNA Substrate Cleavage) Resistance to the Digestion of Snake Venom Phosphodiesterase I
The 32P body-labeled ribozymes transcribed with NTPs and UTPαSe I were prepared as discussed previously in the transcription study section. The radioactive reagent, NTPs and salts were removed by centrifugation using a membrane (3000 Dalton cut-off) for three times. These modified and native ribozymes were used to digest the RNA substrate for 1 hr at 27 °C, as described in the RNA substrate digestion section. After the digestion, the ribozymes were recovered by centrifugation using a membrane (3000 Dalton cut-off) for three times. These modified and native ribozymes were then digested with Snake Venom Phosphodiesterase I (0.001 U/μL, USB) in its buffer. Aliquots (4.5 μL each) were removed from the reaction mixture at the corresponding time points and quenched individually with the loading dye (4.5 μL) containing EDTA (100 mM), followed by placing on dry ice. The digestion reactions were analyzed by PAGE (Figure 5B).
RESULTS
To investigate T7 RNA polymerase recognition of NTPαSe analogs and each diastereomer pairs, we separated two diastereomers of each NTPαSe by HPLC. The HPLC separation of UTPαSe diastereomers is shown as an example in Figure 2A, and the fast- and slow-moving peaks on HPLC were termed as NTPαSe I and II, respectively; both diastereomers of UTPαSe were also analyzed by HR-MS (Figure 2B). We then performed the in vitro transcription of the hammerhead ribozymes using each NTPαSe I and II diastereomer (Figure 3). The experimental results indicate that all NTPαSe I diastereomers are well recognized as substrates by T7 RNA polymerase, and all NTPαSe II diastereomers are neither substrates nor inhibitors (Figure 3B). Since all NTPαSe I and II diastereomers underwent the same treatment during purification, storage and transcription, it is unlikely that the observed RNA transcripts from NTPαSe to normal NTP (17) . As RNA transcripts with N+1 and even N+2 are often observed transcription and T7 RNA polymerase prefers to incorporate ATP as extra nucleotides in this non-templated incorporation, we also observed that ATPαSe at the the 3’ terminus. To further compare their efficiency as substrates with the natural NTPs, we also carried out the time-course transcription using all NTPαSe I analogs (Figure 3C). We found that T7 RNA polymerase recognizes NTPαSe I analogs as well as the natural NTPs, and the NTPαSe incorporation efficiency is at the same level of natural ones. The transcription yields of ATPαSe, CTPαSe, GTPαSe and UTPαSe, relative to the corresponding NTP, are 100%, 151%, 70% and 139%, respectively.
To study the catalytic activities of these Se-ribozymes transcribed with NTPαSe I diastereomers, we performed the catalytic reactions using a kinased 33 nt. RNA substrate (Figure 4). After the transcription, the catalytic investigation was done through comparison of the Se-modified ribozymes and the native in the presence of Mg2+ (10 mM) as the metal ion. The substrate digestion was initiated by addition of the native or modified ribozymes. Our experiment results reveal that the hammerhead ribozymes transcribed with UTPαSe I and CTPαSe I (Figure 4A & 4B) have the same level of efficiency as the native (98% and 76% of the native), the GTPαSe I-transcribed ribozyme has only 30% of native activity, while the ATPαSe I-transcribed ribozyme has very low activity (less than 0.1% of native activity). The activity of the ribozyme transcribed with ATPαSe I was accurately determined by extending the digestion time to 24 hours. However, the catalysis of ATPαSe-transcribed ribozyme can be rescued by nearly 200 fold when Mg2+ is replaced with Mn2+ in the reaction medium (Figure 4C and Supporting Material), while the activity enhancement of the GTPαSe-transcribed ribozyme is small (less than one fold) when Mg2+ is replaced with Mn2+ in the reaction medium (See Supporting Material).
Our previous MS study indicated that T7 RNA polymerase can incorporate the selenium modification into RNA (18), and the transcription difference (Figure 3B) between NTPαSe I and II diastereomers also suggests the incorporation of the phosphoroselenoate modification. To further confirm the presence of Se on the ribozymes, all ribozymes transcribed with NTPαSe I were subjected to digestion by snake venom phosphodiesterase I, which successively degrades both DNA and RNA from the 3’ to 5’ direction. We observed the reduction of the enzymatic digestion by 5−10 fold among the Se-modified RNAs (Figure 5A), suggesting the presence of the phosphoroselenoate groups in the RNAs. In addition, to confirm the stability of the selenium functionality in the RNA substrate cleavage, we also performed the enzymatic digestion after the cleavage reaction. We found that the Se-ribozyme, transcribed with UTPαSe I and used after the RNA substrate cleavage, showed the resistance to the enzymatic digestion (5 fold, Figure 5B) while the native showed no resistance. The digestion resistance of the Se-ribozyme before and after the substrate cleavage confirms the presence of the stable phosphoroselenoate modifications. This enzymatic digestion resistance is consistent with the resistance of DNA and RNA phosphoroselenoates (17, 18), phosphorothioates (25), and boranophosphates (26).
DISCUSSION
Our transcription experiments indicate that T7 RNA polymerase only recognizes NTPαSe I diastereomers, and all NTPαSe II diastereomers are neither substrates nor inhibitors (Figure 3), which suggests that separation of I and II diastereomers of each NTPαSe is not necessary for the transcription. In addition, because T7 RNA polymerase only recognizes the Sp diastereomer of 5’-(α-P-thio)triphosphates (22, 23), we tentatively assign Sp configuration to all NTPαSe I, and Rp configuration to all NTPαSe II. This selective recognition of the Sp and Rp diastereomers also indicates that the atom (O or Se) on the pro-Rp position probably involves in the polymerase interaction and/or catalysis while the atom on the pro-Sp center does not. The inversion of configuration at the phosphorous center upon the incorporation, reported in the literature (22-24), suggests that these phosphoroselenoate ribozymes have Rp configuration. Naturally, the transcribed Se-ribozymes are diastereomerically pure, which is an advantage over chemical synthesis of phosphoroselenoate nucleotides (19). As NTPαSe analogs behave as the same as the natural NTPs, large-scale transcription with NTPαSe analogs has demonstrated that it is possible to prepare large quantity (milligrams) for X-ray crystallography.
Our catalytic study using the selenium-modified ribozymes shows that the hammerhead ribozymes transcribed with UTPαSe and CTPαSe have the same level of activity as the native ribozyme. This is probably due to that the 5’-phosphate non-bridging pro-Rp oxygen atoms of the U and C located in the highly conserved sequences (U4 and C3 and C11.1, the underline sequences in Figure 3A) just contact with solvent water molecules, which is consistent with the determined structures (27, 28). On the other hand, due to the involvement of the 5’-phosphate pro-Rp oxygen atoms of As in the conserved sequences (A9, A13 and A14; 29, 30), the replacement of the oxygen with selenium results in over 1000 fold reduction in efficiency. The replacement of Mg2+ with Mn2+ in the reaction medium rescued the catalysis by nearly 200 fold (Figure 4C), suggesting the metal cation interaction with these specific sites. In the case of GTPαSe I, though there are three Gs in the highly conserved sequences, the transcribed ribozyme displayed an efficiency that is only 3 fold lower than that of the native. This minimum efficiency disruption and the Mn2+ non-rescuing property suggest that these 5’-phosphate pro-Rp oxygen atoms of the Gs are not involved in catalysis.
Our enzymatic digestion experiments of the Se-ribozymes, before and after the ribozyme catalysis, have clearly indicated the presence of the stable selenium substitution (phosphoroselenoate modification). The resistance is consistent with resistance of DNA and RNA phosphoroselenoates (17, 18), phosphorothioates (25), and boranophosphates (26). Clearly, this systematic and convenient modification of ribozyme with each NTPαSe I can assist the study and determination of its conserved sequences. This method is especially useful in elucidating the role of the pro-Rp oxygen on the 5’-phosphate of the modified nucleotide: whether they participate in catalysis. In addition, the most active Se-ribozymes, with the structure most similar to the native ribozyme, can be easily identified through a quick activity test, and will be the ideal candidates for X-ray crystal structure studies. This RNA derivatization strategy can also be applied in derivatization of RNA-protein complexes by labeling the nucleic acid molecules with selenium instead of the protein counterparts, as the derivatization and purification of nucleic acids are much quicker and easier than these of proteins.
In conclusion, we have shown that all NTPαSe I analogs are efficient substrates, as well as natural NTPs, for T7 RNA polymerase while the NTPαSe II analogs are not recognized. Since all II diastereomers are neither substrates nor inhibitors, separation of the mixture of diastereomer I and II is not necessary for the efficient transcription of the diastereomerically pure RNAs. More importantly, these hammerhead ribozymes transcribed using different NTPαSe analogs display catalytic activities at a variety of levels, which are consistent with the roles of the replaced pro-Rp oxygen atoms in the structure and catalysis. Therefore, this modification on the backbone with the phosphoroselenoate is useful in the catalytic mechanism study and in identification of highly conserved sequences of any functional RNA molecules. In addition, the most active ribozymes derivatized with Se can be identified via a simple and quick transcription and activity screening using different NTPαSe analogs. Obviously, this strategy of RNA derivatization with selenium also has great potential as a general approach for X-ray crystal structure studies of functional RNAs via MAD or single wavelength anomalous dispersion (SAD) phasing.
Supplementary Material
ACKNOWLEDGMENT
This work was partly supported by GSU Research Program, the U.S. National Institute of Health (GM069703), and National Science Foundation (MCB-0517092).
Footnotes
SUPPORTING INFORMATION AVAILABLE Synthesis and purification of NTPαSe analogs; Figure S1, catalysis of the modified and native hammerhead ribozymes using Mn2+ as the metal cation. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Storz G. An expanding universe of noncoding RNAs. Science. 2002;296:1260–1263. doi: 10.1126/science.1072249. [DOI] [PubMed] [Google Scholar]
- 2.Eddy SR. Non-coding RNA genes and the modern RNA world. Nat. Rev. Gen. 2001;2:919–929. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 3.Lathan MP, Brown DJ, McCallum SA, Pardi A. NMR methods for studying the structure and dynamics of RNA. ChemBioChem. 2005;6:1492–1505. doi: 10.1002/cbic.200500123. [DOI] [PubMed] [Google Scholar]
- 4.Blount KF, Uhlenbeck OC. The structure-function dilemma of the hammerhead ribozyme. Annu. Rev. Biophys. Biomol. Struct. 2005;34:415–440. doi: 10.1146/annurev.biophys.34.122004.184428. [DOI] [PubMed] [Google Scholar]
- 5.McKay DB. Structure and function of the hammerhead ribozyme: an unfinished story. RNA 2. 1996:395–403. [PMC free article] [PubMed] [Google Scholar]
- 6.Holbrook SR, Kim SH. RNA crystallography. Biopolymers. 1997;44:3–21. doi: 10.1002/(SICI)1097-0282(1997)44:1<3::AID-BIP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Egli M. Nucleic acid crystallography: current progress. Curr. Opin. Chem. Biol. 2004;8:580–591. doi: 10.1016/j.cbpa.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Ferre-D'Amare AR, Zhou Kaihong, Doudna JA. Crystal structure of a hepatitis delta virus ribozyme. Nature. 1998;395:567–574. doi: 10.1038/26912. [DOI] [PubMed] [Google Scholar]
- 9.Ke A, Doudna JA. Crystallization of RNA and RNA-protein complexes. Methods. 2004;34:408. doi: 10.1016/j.ymeth.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, Hendrickson WA, Crouch RJ, Satow Y. Structure of ribonuclease H phased at 2 A resolution by MAD analysis of the selenomethionyl protein. Science. 1990;249:1398–1405. doi: 10.1126/science.2169648. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickson WA. Synchrotron crystallography. Trends Biochem. Sci. 2000;25:637–643. doi: 10.1016/s0968-0004(00)01721-7. [DOI] [PubMed] [Google Scholar]
- 12.Carrasco N, Ginsburg D, Du Q, Huang Z. Synthesis of selenium-derivatized nucleosides and oligonucleotides for X-ray crystallography. Nucleosides Nucleotides Nucleic Acids. 2001;20:1723–1734. doi: 10.1081/NCN-100105907. [DOI] [PubMed] [Google Scholar]
- 13.Du W, Carrasco N, Teplova M, Wilds CJ, Egli M, Huang Z. Internal erivatization of oligonucleotides with selenium for X-ray crystallography using MAD. J. Am. Chem. Soc. 2002;124:24–25. doi: 10.1021/ja0171097. [DOI] [PubMed] [Google Scholar]
- 14.Teplova M, Wilds CJ, Wawrzak Z, Tereshko V, Du Q, Carrasco N, Huang Z, Egli M. Covalent incorporation of selenium into oligonucleotides for X-ray crystal structure determination via MAD: proof of principle. Biochimie. 2002;84:849–858. doi: 10.1016/s0300-9084(02)01440-2. [DOI] [PubMed] [Google Scholar]
- 15.Buzin Y, Carrasco N, Huang Z. Synthesis of selenium-derivatized cytidine and oligonucleotides for X-ray crystallography using MAD. Org. Lett. 2004;6:1099–1102. doi: 10.1021/ol0365077. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco N, Buzin Y, Tyson E, Halpert E, Huang Z. Selenium derivatization and crystallization of DNA and RNA oligonucleotides for X-ray crystallography using multiple anomalous dispersion. Nucleic Acids Res. 2004;32:1638–1640. doi: 10.1093/nar/gkh325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrasco N, Huang Z. Enzymatic synthesis of phosphoroselenoate DNA using thymidine 5'-(α-P- seleno)triphosphate and DNA polymerase for X-ray crystallography via MAD. J. Am. Chem. Soc. 2004;126:448–449. doi: 10.1021/ja0383221. [DOI] [PubMed] [Google Scholar]
- 18.Carrasco N, Caton-Williams J, Brandt G, Wang S, Huang Z. Efficient enzymatic synthesis of phosphoroselenoate RNA by using adenosine 5’-(α-P-seleno)triphosphate. Angew. Chem. 2006;2006;118:100–103. doi: 10.1002/anie.200502215. [DOI] [PubMed] [Google Scholar]; Efficient enzymatic synthesis of phosphoroselenoate RNA by using adenosine 5’-(α-P-seleno)triphosphate. Angew. Chem. Int. Ed. 45:94–97. doi: 10.1002/anie.200502215. [DOI] [PubMed] [Google Scholar]
- 19.Wilds CJ, Pattanayek R, Pan C, Wawrzak Z, Egli M. Selenium-assisted nucleic acid crystallography: use of phosphoroselenoates for MAD phasing of a DNA structure. J. Am. Chem. Soc. 2002;124:14910–19416. doi: 10.1021/ja021058b. [DOI] [PubMed] [Google Scholar]
- 20.Serganov A, Keiper S, Malinina L, Tereshko V, Skripkin E, Hobartner C, Polonskaia A, Phan AT, Wombacher R, Micura R, Dauter Z, Jaschke A, Patel DJ. Structural basis for Diels-Alder ribozyme-catalyzed carbon-carbon bond formation. Nat. Struct. Mol. Biol. 2005;12:218–224. doi: 10.1038/nsmb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig J, Eckstein F. Rapid and efficient synthesis of nucleoside 5'−0-(1-thiotriphosphates), 5'-triphosphates and 2',3'-cyclophosphorothioates using 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one. J. Org. Chem. 1989;54:631–635. [Google Scholar]
- 22.Burgers PM, Eckstien F. Absolute configuration of the diastereomers of adenosine 5’-O-(1-thiotriphosphate): consequences for the stereochemistry of polymerization by DNA-dependent RNA polymerase from Escherichia coli. Proc. Natl. Acad. Sci. 1978;75:4798–4800. doi: 10.1073/pnas.75.10.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths AD, Potter BV, Eperon IC. Stereospecificity of nucleases towards phosphorothioate-substituted RNA: stereochemistry of transcription by T7 RNA polymerase. Nucleic Acids Res. 1987;15:4145–4162. doi: 10.1093/nar/15.10.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein F, Gindl H. Polyribonucleotides containing a phosphorothioate backbone. Eur. J. Biochem. 1970;13:558–564. doi: 10.1111/j.1432-1033.1970.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein F, Gindl H. Polyribonucleotides containing a thiophosphate backbone. FEBS Lett. 1969;2:262–264. doi: 10.1016/0014-5793(69)80037-2. [DOI] [PubMed] [Google Scholar]
- 26.Hall AHS, Wan J, Shaughnessy EE, Shaw BR, Alexander KA. RNA interference using boranophosphate siRNAs: structure–activity relationships. Nucleic Acids Res. 2004;32:5991–6000. doi: 10.1093/nar/gkh936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pley HW, Flaherty KM, McKay DB. Model of an RNA tertiary interaction from the structure of an intermolecular complex between a GAAA tetraloop and an RNA helix. Nature. 1994;372:111–113. doi: 10.1038/372111a0. [DOI] [PubMed] [Google Scholar]
- 28.Scott WG, Finch JT, Klug A. The crystal structure of an all-RNA hammerhead ribozyme: a proposed mechanism for RNA catalytic cleavage. Cell. 1995;81:991–1002. doi: 10.1016/s0092-8674(05)80004-2. [DOI] [PubMed] [Google Scholar]
- 29.Peracchi A, Beigelman L, Scott EC, Uhlenbeck OC, Herschlag D. Involvement of a specific metal ion in the transition of the hammerhead ribozyme to its catalytic conformation. J. Biol. Chem. 1997;272:26822–26826. doi: 10.1074/jbc.272.43.26822. [DOI] [PubMed] [Google Scholar]
- 30.Ruffner DE, Uhlenbeck OC. Thiophosphate interference experiments locate phosphates important for the hammerhead RNA self-cleavage reaction. Nucleic Acids Res. 1990;18:6025–6029. doi: 10.1093/nar/18.20.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.