Abstract
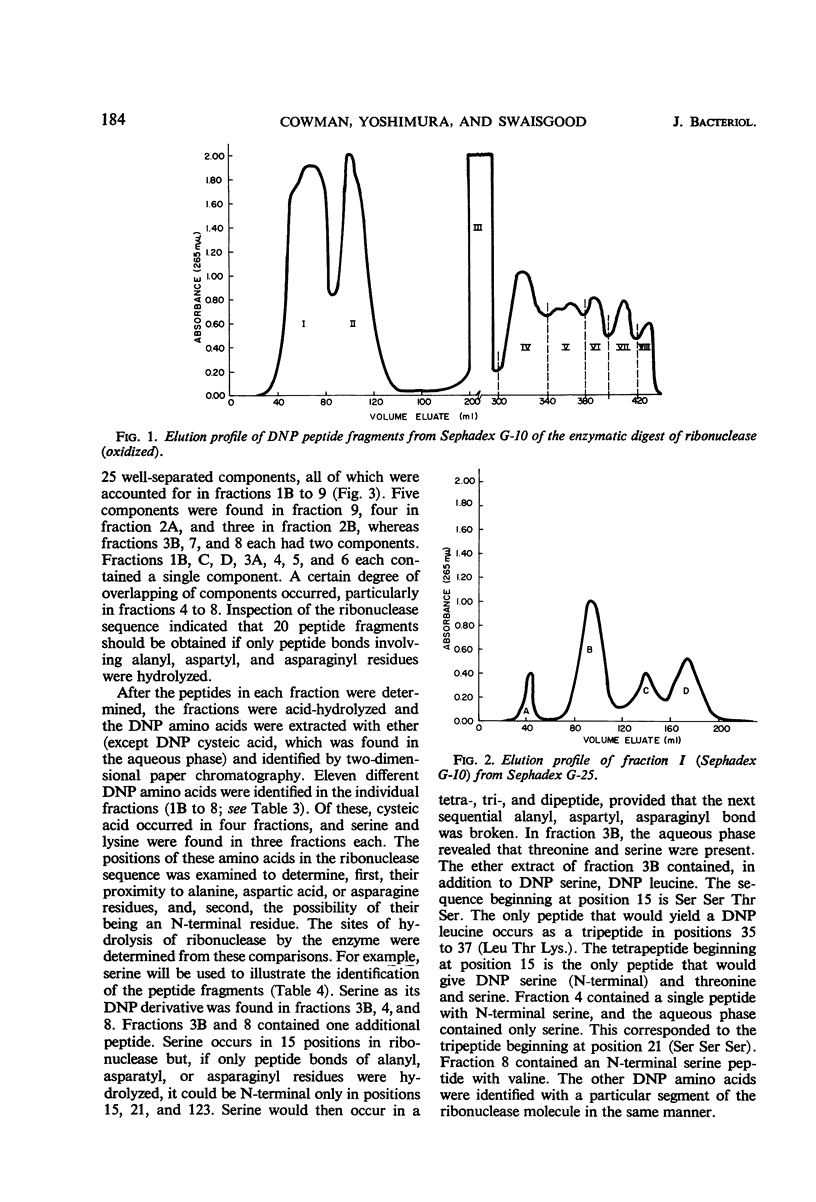
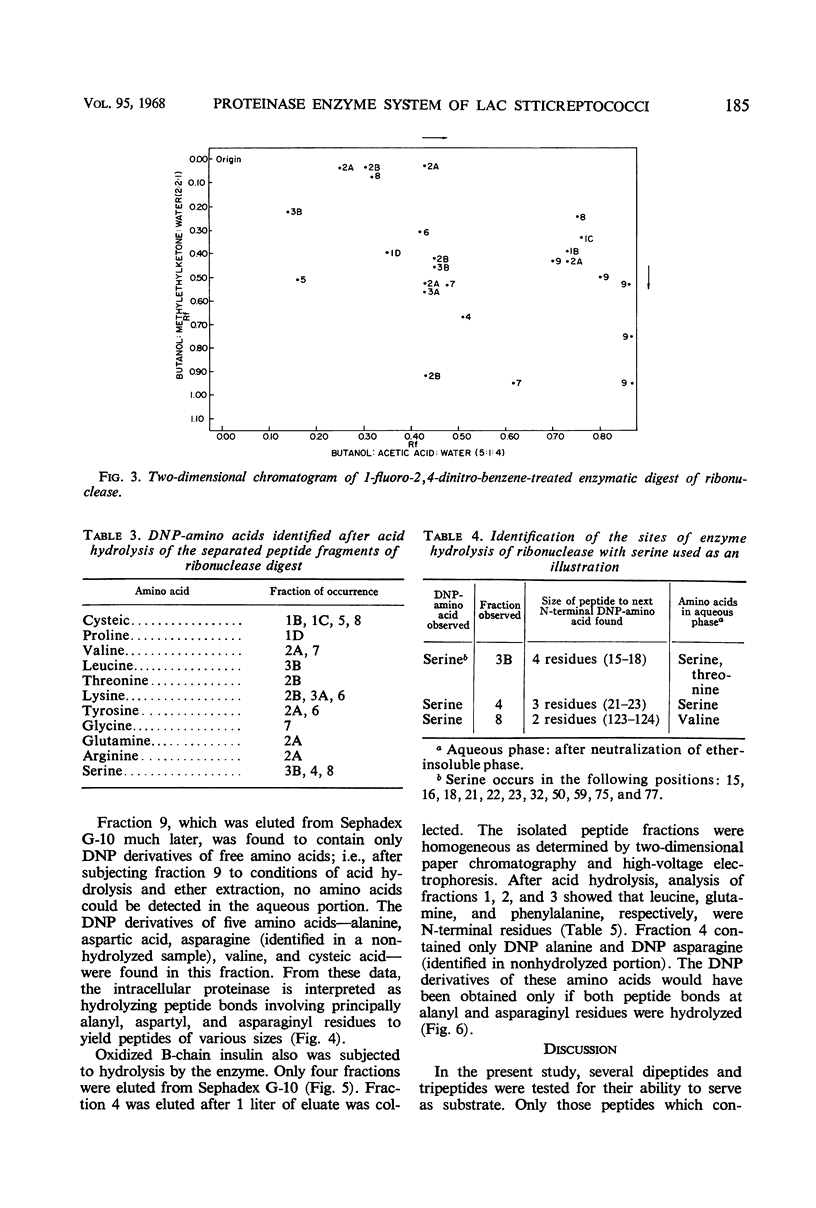
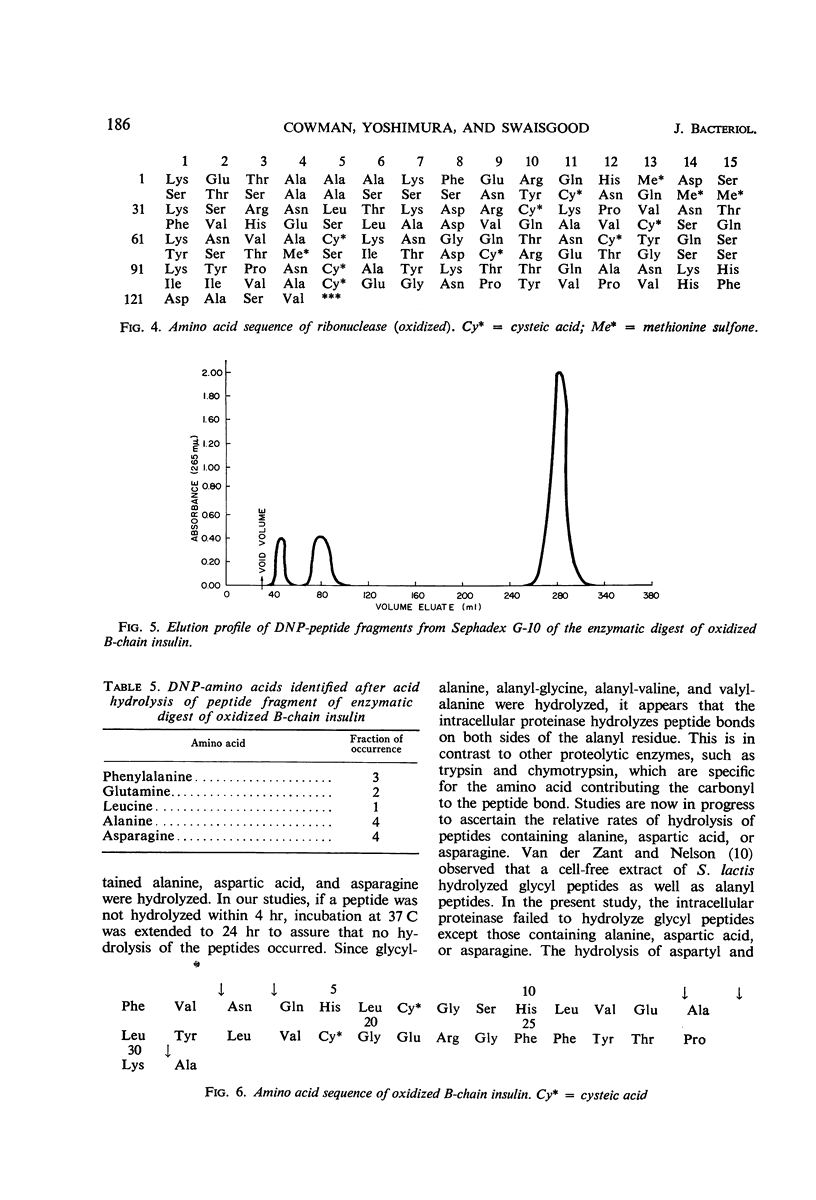
The substrate specificity of an intracellular proteinase from Streptococcus lactis was investigated in an effort to understand the role of the enzyme in the cell. Peptides in which the N-terminal residue was glycine were not hydrolyzed by the enzyme (exceptions were glycyl-alanine, glycyl-aspartic acid, and glycyl-asparagine), but the peptide was hydrolyzed if the N-terminal residue was alanine. The enzyme also showed activity toward peptides containing aspartic acid or asparagine. Hydrolysis of only the peptide bonds of alanyl, aspartyl, or asparaginyl residues was confirmed by the action of the enzyme on oxidized bovine ribonuclease A- and B- chain insulin. The N-terminal residues of the peptide fragments liberated were identified. The enzyme attacked both substrates only at alanyl, aspartyl, and asparaginyl residues, releasing these as free amino acids. In addition to alanine, aspartic acid, and asparagine, certain other amino acids were liberated from ribonuclease A, but these were accounted for by the relation of their position to alanine, aspartic acid, and asparagine residues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., REDFIELD R. R., CHOATE W. L., PAGE J., CARROLL W. R. Studies on the gross structure, cross-linkages, and terminal sequences in ribonuclease. J Biol Chem. 1954 Mar;207(1):201–210. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- HUSAIN I., MCDONALD I. J. Amino acids and utilization of sodium caseinate by lactic streptococci. Can J Microbiol. 1957 Apr;3(3):487–491. doi: 10.1139/m57-052. [DOI] [PubMed] [Google Scholar]
- de Koning P. J., van Rooijen P. J. Genetic variants of alpha-s1-casein; amino acid composition of the variants B, C and BC. Biochem Biophys Res Commun. 1965 Jul 26;20(3):241–245. doi: 10.1016/0006-291x(65)90353-0. [DOI] [PubMed] [Google Scholar]


