Abstract
A mixed-breed beef cow was presented with swelling of the front and hind left quarters of the mammary gland and mild depression. Direct examination and culture of the serosanguinous-like milk samples collected from these quarters were consistent with Bacillus anthracis infection.
Résumé
Mammite causée par Bacillus anthracis chez une vache. Une vache de boucherie de race croisée légèrement abattue a été présentée avec une tuméfaction des glandes mammaires avant et arrière gauches. L’examen microscopique des frottis et la culture des échantillons de lait d’apparence séro-sanguinolent collectés de ces glandes mammaires ont démontré une infection à Bacillus anthracis.
(Traduit par les auteurs)
In summer 2006, the Saskatchewan livestock industry dealt with an unprecedented outbreak of anthrax. The 1st case was diagnosed on June 27, 2006. As of November 3, 2006, the Canadian Food Inspection Agency (CFIA) had reported 804 animals dead, of which 493 were cattle, 254 bison, 33 sheep, 13 white-tailed deer and elk, 6 horses, 3 pigs, and 2 goats. A total of 155 premises positive for anthrax were quarantined (1,2) (Sandra Stephens, Canadian Food Inspection Agency, Saskatoon, personal communication). On July 25, 2006, an 8-year-old mixed-breed beef cow with severe mastitis in mid-lactation was examined on a farm that was under anthrax quarantine because the anthrax had been diagnosed on the premises approximately 10 d earlier. A few animals had died with anthrax, and subsequently, all the animals on the farm had been vaccinated with an anthrax live-attenuated spore vaccine (Colorado Serum Company, Denver, Colorado, USA), 1 mL, SC, approximately 1 wk prior to the 1st observation of the clinical signs of mastitis in this cow. There was tremendous swelling of unknown duration of the left front quarter of the udder; a presumptive diagnosis of coliform mastitis was made by the attending veterinarian (Dr. Pederson, Mohawk Animal Clinic). Milk samples were submitted to the bacteriology laboratory of Prairie Diagnostic Services Inc. (PDS) for examination and culture.
Case description
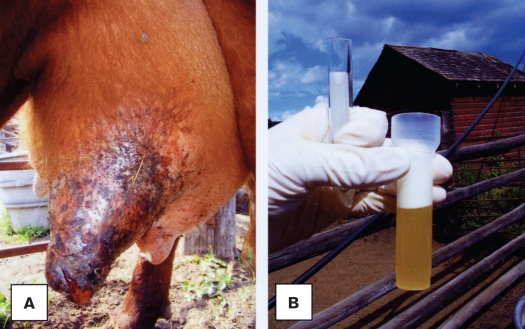
On physical examination, the cow was in moderate to poor body condition [score 2; reference range: 1 to 5; approximately 500 kg bodyweight (BW)] with signs of mild depression. The left front quarter of the udder was swollen and darkened towards the teat, with copious exudation of serosanguinous-like fluid from the teat (Figures 1A, 1B). The body temperature was 39.3°C (reference range: 37.8°C to 39.4°C). The other quarters appeared relatively normal; however, the milk collected from these quarters had a serum-like fluid appearance (Figure 1B).
Figure 1.
A — The swollen left front quarter of the udder darkened towards the teat. B — Watery and yellowish (serosanguinous-like) milk collected by compression of the quarter.
Left front and hind quarter milk samples were submitted for culture to the bacteriology laboratory. The distal part of the canal of the left front teat was opened to facilitate drainage. The cow was administered florfenicol (Nuflor 300 mg/mL; Schering-Plough Animal Health, Kirkland, Quebec), 20 mg/kg body weight (BW), IM, and flunixin meglumine (Banamine 100 mg/mL; Schering-Plough Animal Health), 2.5 mg/kg BW, IM.
Two days after initial presentation, the cow was still febrile (body temperature; 39.1°C) and depressed. There was moderate edema of the left front quarter of the udder. The udder was manually emptied of all milk and a scab on the distal part of the left front teat was removed to ensure further drainage of the quarter. The cow was administered a repeat dose of florfenicol, pending results of the bacteriologic culture.
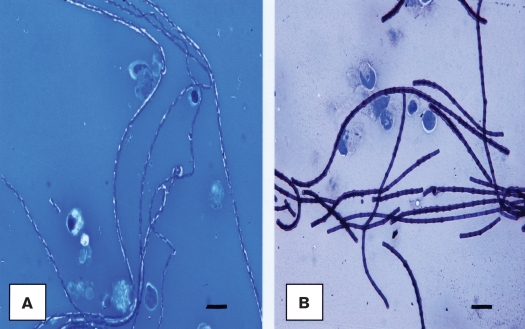
Direct microscopic examination of the gram-stained smears of the serosanguinous milk samples submitted to the laboratory revealed numerous gram-positive rods in chains, morphologically identical to Bacillus anthracis. Subsequently, additional smears were examined after staining with either polychrome methylene blue or Giemsa (3). On these stained smears, the organism revealed a pinkish or reddish capsular-like material, respectively, suggestive of the presence of B. anthracis (Figures 2A, 2B). A presumptive diagnosis of B. anthracis infection was made and the milk samples were cultured on blood agar plates in the presence of a penicillin disc (10 IU, Becton, Dickinson, Sparks, Maryland, USA) to assess the sensitivity of the organism, and on MacConkey agar plates. Cultures were incubated at 37°C for (blood agar plates) or 18 h in either an atmosphere of 5% CO2 a normal atmosphere (MacConkey agar plates).
Figure 2.
Direct examination of the polychrome-methylene blue (A) and Giemsa (B) stained smears of the serosanguinous milk samples from the affected quarters showed typical rods in long chains with respective pinkish and reddish capsular material, characteristic of Bacillus anthracis. Bar = 7 μm.
Cultures of the milk samples collected from both the left front and left hind quarters yielded pure cultures of large grayish colonies, characteristic of B. anthracis, on blood agar plates (3). There was no bacterial growth approximately 20 mm in diameter around the penicillin disc inserted on the culture, suggesting that the organism was sensitive to penicillin, as is observed for the majority of B. anthracis isolates (3). There was no bacterial growth on the MacConkey agar plates. These laboratory observations were sufficient to make a diagnosis of mastitis caused by B. anthracis. This was later confirmed by the Anthrax Reference Laboratory of the Canadian Food Inspection Agency in Lethbridge, Alberta, following a PCR test on the organism (Betty Golsteyn Thomas, CFIA Lethbridge, personal communication).
Six days after presentation, the cow was found to have a severe generalized edema of her udder, resulting in a noticeable increase in size compared with previous examinations. Pitting edema was present along the ventral midline of the cow from the brisket to the udder, but, otherwise, she appeared to be bright and alert.
For economical reasons, the owner declined further treatment of the cow and visits to the farm. However, on further communication, he informed the attending veterinarian that the cow died a few weeks after presentation, probably due to the infection. He had buried it on the premises in a similar manner to the cows that had died with anthrax.
Discussion
Bacillus anthracis is a spore forming, gram-positive organism, obligate pathogen within the genus Bacillus (3). The organism produces spores upon nutrient depletion and exposure to free atmospheric oxygen. When the carcass of a dead animal is opened in the field and not disposed of properly, these spores will contaminate the ground and can survive there for decades if the soil is alkaline or has a high organic matter content (4,5).
Animals become infected by ingestion of plant material containing spores from contaminated pastures; this is referred to as the intestinal form of the disease, which has an incubation period that is commonly from 3 to 7 d (6). The organism usually induces peracute or acute disease in infected cattle. Commonly, peracute disease results in death following a short course of illness (1 to 2 h). Acute anthrax may have a course of 48 h with signs of depression and fever (7). The disease can also manifest under cutaneous and respiratory forms (4,7).
Several virulence factors are responsible for the clinical manifestations induced by B. anthracis. The capsule induces bacterial resistance to phagocytosis. The edema and lethal factors induce edema and death, respectively. Toxins produced by the bacteria induce vascular permeability, leading to leakage of body fluids; death is usually due to shock and terminal anoxia (4,6,7).
Diagnosis of anthrax can be made by direct examination of polychrome methylene blue- or giemsa-stained smears of blood of infected animals. The presence of the organism can be confirmed by culture or PCR (3,8).
Mastitis appears to be an unusual presentation of the disease in cattle. Few cows had died with anthrax on the farm; the cow in question had been vaccinated at the same time as the other cows on the farm. It is possible that she was partially protected against anthrax, but contracted the disease from the heavily spore-contaminated soil within the premises and, therefore, developed environmental anthrax mastitis. However, since more than 1 quarter was infected, the cow may have ingested B. anthracis spores and experienced hematogenous spread of the organism within the body, but resisted systemic infection because of vaccination. The organism may have localized in the mammary gland and caused mastitis.
To the best of our knowledge, we have documented the 1st case of mastitis caused by B. anthracis. Aggressive treatment with penicillin could possibly have brought about recovery from the infection.
Acknowledgment
The authors thank Dr. Bobbie Lundquist (Canadian Food Inspection Agency, Saskatoon) for technical assistance. CVJ
Footnotes
Reprints will not be available from the authors.
Authors’ contributions
Dr. Pederson was the attending veterinarian with Dr. Hawkes, who was a final year student at the time. Dr. Ngeleka investigated the milk samples and made the diagnosis of anthrax.
References
- 1.Prairie Diagnostic Services [homepage on the Internet] Kerr R. Anthrax outbreak in Saskatchewan. Newsletter. [Last accessed May 1, 2008];Animal Health Perspective. 2006 Nov; Available from http://www.usask.ca/pds/Information/Perspectives%20-%20November%202006.pdf.
- 2.Prairie Diagnostic Services [homepage on the Internet] Ngeleka M. 2006 Saskatchewan anthrax outbreak. Newsletter. [Last accessed May 1, 2008];Animal Health Perspective. 2006 Nov; Available from http://www.usask.ca/pds/Information/Perspectives%20-%20November%202006.pdf.
- 3.Quin PJ, Carter ME, Markey BK, Carter GR. Bacillus spp. In: Quin PJ, editor. Clinical Veterinary Microbiology. London: Wolfe Publ, Year Book Europe Limited; 1994. pp. 178–183. [Google Scholar]
- 4.Turnbull PCB, Logan NA. Bacillus spp. and recently derived genera. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, et al., editors. Manual of Clinical Microbiology. 7. Washington, DC: ASM Pr; 1999. pp. 357–369. [Google Scholar]
- 5.Parkinson R, Rajic A, Jenson C. Investigation of an anthrax outbreak in Alberta in 1999 using a geographic information system. Can Vet J. 2003;44:315–318. [PMC free article] [PubMed] [Google Scholar]
- 6.Aiello SE, Mays A. Anthrax. In: Aiello S, editor. The Merck Veterinary Manual. 8. Whitehouse Station, New Jersey: Merck; 1998. pp. 432–435. [Google Scholar]
- 7.Radostits OM, Gay CC, Hinchcliff KW, Constable PD. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 10. St Louis, Missouri: Saunders Elsevier; 2007. Veterinary Medicine; pp. 815–819. [Google Scholar]
- 8.Berg T, Suddes H, Morrice G, Hornitzky M. Comparison of PCR, culture, and microscopy of blood smears for the diagnosis of anthrax in sheep and cattle. Lett Appl Microbiol. 2006;43:181–186. doi: 10.1111/j.1472-765X.2006.01931.x. [DOI] [PubMed] [Google Scholar]