Abstract
Ghrelin is a stomach hormone that stimulates growth hormone (GH) secretion, adiposity, and food intake. Gastric ghrelin production and secretion are regulated by caloric intake; ghrelin secretion increases during fasting, decreases with refeeding, and is reduced by diet-induced obesity. The aim of the present study was to test the hypotheses that 1) an increase in body adiposity will play an inhibitory role in the reduction of gastric ghrelin synthesis and secretion during chronic ingestion of a high-fat (HF) diet and 2) chronic ingestion of an HF diet will suppress the rise in circulating ghrelin levels in response to acute fasting. Adult male Sprague-Dawley rats were fed a standard AIN-76A (∼5–12% of calories from fat) or an HF (∼45% of calories from fat) diet. The effect of increased adiposity on gastric ghrelin homeostasis was assessed by comparison of stomach ghrelin production and plasma ghrelin levels in obese and nonobese rats fed the HF diet. HF diet-fed, nonobese rats were generated by administration of triiodothyronine to lower body fat accumulation. Our findings indicate that an increased fat mass per se does not exert an inhibitory effect on ghrelin homeostasis during ingestion of the HF diet. Additionally, the magnitude of change in plasma ghrelin in response to fasting was not blunted, indicating that a presumed, endogenous signal for activation of ingestive behavior remains intact, despite excess stored calories in HF-fed rats.
Keywords: gut hormones, regulation
the stomach hormone ghrelin is a 28-amino acid peptide that was discovered in extracts of the rat stomach as the endogenous ligand for the growth hormone (GH) secretagogue (GHS) receptor (GHS-R) (31, 46). The search for ghrelin resulted directly from the development of synthetic substances called GHSs and the subsequent discovery of a natural receptor, GHS-R, for these artificial ligands (8, 9, 21, 47). Systemic administration of ghrelin or GHSs evokes GH secretion from the pituitary by activation of GHS-R. GHSs are synthesized on the basis of the structure of enkephalins and do not resemble the hypothalamic hormone GH-releasing hormone, nor do they cause GH secretion by activation of GH-releasing hormone receptor.
Ghrelin exerts a variety of important metabolic actions. It stimulates GH secretion, food intake, body growth, and adiposity (17, 31, 42, 49, 53) and also influences gastric acid secretion and exerts a prokinetic action on the stomach (38). Ghrelin is the first endogenous peptide to be isolated with an n-octanol modification at Ser3 (31). The acyl and desacyl forms of ghrelin have been shown to exert biological activity (4, 6, 7, 10, 31). Although ghrelin is expressed in the pancreas, intestine, kidney, hypothalamus, heart, and placenta (51), the stomach epithelium is the primary site for synthesis of ghrelin immunoreactivity measured in the general circulation. Ghrelin is produced in gastric enteroendocrine X/A cells (14). Our laboratory and others have shown that gastrectomy in rats and humans will diminish plasma ghrelin levels ∼85% (5, 22).
Regulation of ghrelin secretion from the stomach is unique compared with other gastrointestinal hormones. In contrast to other gut hormones, plasma ghrelin levels increase in response to fasting and decrease on refeeding (22). Furthermore, plasma ghrelin levels are reduced by chronic intake of high-calorie diets and obesity in humans (50). In rodents, prolonged exposure to high-fat (HF) diets will result in a positive energy balance, obesity, and a reduction of stomach production and secretion of ghrelin (33); however, little is known about the extent to which the increased adiposity itself exerts an inhibitory influence on stomach ghrelin production and secretion.
The purpose of the present study, therefore, was to investigate the role of the increased body adiposity on ghrelin production and secretion during ingestion of an HF diet. To diminish the influence of the increased adiposity during the HF diet, we treated HF-fed rats with triiodothyronine (T3) to “clamp” their body weights and adiposity at levels comparable to those measured in control rats.
METHODS AND MATERIALS
Animals
All animal experiments were conducted in accordance with mandated standards of humane care and were approved by the Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats were maintained in an air-conditioned and light-regulated room (lights on from 0600 to 1800) and given access to food and water ad libitum.
Chemicals and Peptides
All chemicals were obtained from Sigma Chemical (St. Louis, MO). Synthetic peptides were purchased from Phoenix (Belmont, CA).
Experiments
Experiment 1.
Six groups of rats (n = 8–10 rats/group) were studied. Two groups (AIN + VEH and HF + VEH) were fed a commercial AIN-76A (∼5% of calories from fat by weight) or an HF (∼45% of calories from fat) diet for 8 wk (BioServ, Frenchtown, NJ) and treated with vehicle (VEH). Two groups (AIN + T3 and HF + T3) were fed the AIN-76 and HF diets and treated concurrently with T3 (100 μg/kg sc, every other day). T3 was administered to avert body fat accumulation in rats fed the HF diet. Thyroid hormones (thyroxine and T3) are the predominant regulators of basal metabolic rate, and circulating levels of thyroid hormones are directly correlated with energy expenditure and calorie loss (35). T3 was prepared as described previously (23). Because T3 has been reported to stimulate food intake (32, 36) and food intake affects stomach ghrelin homeostasis, a group of rats (HFPFHF + VEH + T3) were treated with T3 and pair fed an HF diet according to the daily caloric intake of rats fed the HF diet but not treated with T3. We pair fed another group of rats (HFPFAIN + VEH + VEH) the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet to determine the extent to which a higher percentage of ingested dietary fat is a key signal in reduction of stomach ghrelin production and blood ghrelin levels. Daily food intake was monitored in AIN + VEH and HF + VEH groups throughout the experiment. Food intake was measured in AIN + T3 and HF + T3 groups during the last 2 wk of the experiment. At the end of the 8-wk treatment period, body weight was recorded, and the rats were killed. Plasma was harvested for measurement of plasma ghrelin, insulin, leptin, and triglyceride levels. The stomach-fundal mucosa was collected for extraction of total cellular RNA and ghrelin peptide. Total body fat was measured by quantitative nuclear magnetic resonance using an MRI Whole Body Composition Analyzer (Echo Medical Systems, Houston, TX) (37). Plasma triglyceride levels were measured using a commercial kit (Sigma-Aldrich). The compositions (approximate percentage of calories) of the commercial AIN-76A biscuit and HF diets are as follows: 5% fat, 20% protein, and 72% carbohydrate for the AIN-76A biscuit and 48% fat (beef tallow), 16% protein, and 34% carbohydrate for the HF diet. Two percent of the fat calories in the beef tallow-containing diet were derived from safflower oil to supply essential fatty acids. All rats had unrestricted access to water. A preweighed amount of food was provided daily, and food intake was determined daily from the amount of food remaining in the food bowl + waste below the cage. Pair-fed rats were given an additional 10% of food to accommodate for loss due to spillage.
Experiment 2.
Two groups of adult male Sprague-Dawley rats (n = 10 rats/group) were fed the AIN-76A (∼12% of calories from fat) or HF (∼40–45% of calories from fat) diet for 5 wk. All rats had unrestricted access to water. Plasma was collected from ad libitum-fed and fasted (∼24 h) groups of AIN-76A- and HF-fed rats for measurement of ghrelin levels by immunoassay. Food intake was determined for rats fed the AIN-76A and HF diets for 2 wk as described for experiment 1.
Ghrelin, Insulin, and Leptin Assays
For experiment 1, plasma acyl-ghrelin was measured using a commercial ELISA kit that detects acyl-ghrelin specifically (ALPCO, Salem, NH). The sensitivity for this assay is 3.9 pg/ml. An anticoagulant (EDTA) and a protease inhibitor (p-hydroxymercuribenzoic acid, 1 mM in the final sample volume) were added at the time of blood collection. After separation of plasma, 100 μl of 1 N HCl was immediately added per milliliter of collected plasma, and the sample was centrifuged briefly. Supernatants were saved for assay. Blood and plasma samples were kept on ice until frozen. A double-antibody radioimmunoassay procedure was used to measure plasma ghrelin in experiment 2 and stomach tissue ghrelin in experiment 1 as described in detail previously by our laboratory (33). The ghrelin antiserum was produced in rabbits by immunization with a synthetic carboxy-terminal fragment of rat ghrelin. This ghrelin antibody recognizes acyl- and desacyl-ghrelin equally; hence, ghrelin levels reflect total ghrelin (acyl- + desacyl-ghrelin). The sensitivity and ID50 (50% inhibition of bound radio-iodinated ligand) were 0.01 and 0.2 ng/tube, respectively.
Stomach ghrelin peptide was extracted from stomach tissue specimens as reported earlier (33). For experiment 2, plasma ghrelin was extracted from rat plasma (1.2 ml/sample) by use of C18 Sep Pak cartridges (Waters, Milford, MA). Samples were assayed in duplicate. Plasma insulin was measured as described previously (24). The sensitivity and ID50 for this insulin assay were 0.04 and 4.0 ng/ml, respectively. A double-antibody radioimmunoassay procedure was used to measure plasma leptin. The sensitivity and ID50 for this leptin assay were 1.0 and 6.0 ng/tube, respectively. All assays used specific antibodies that do not recognize structurally unrelated pancreatic or gastrointestinal peptides. For all assays, the intra- and interassay coefficients of variation were ∼3–5.0 and ∼8–12%, respectively.
RNA Purification and Northern Blot Analysis
Fresh tissues were homogenized immediately in 4 M guanidinium isothiocyanate containing 25 mM sodium citrate (pH 7.0), 0.5% sodium N-lauroylsarcosine, and 0.1 M β-mercaptoethanol (33). Extracts were frozen at −80°C until purification by ultracentrifugation over a cesium chloride cushion (2 ml, 5.7 M). RNA samples were separated on a 1% agarose gel (30 μg/lane) in a 20 mM MOPS running buffer system (33) and then transferred to a nylon membrane and subjected to Northern hybridization. 32P-labeled riboprobes prepared from Strip-EZ RNA kits (catalog no. 1366, Ambion, Austin, TX) were used for Northern hybridizations. Ribosomal 18S was used to normalize for variations in RNA loading and transfer. Expression levels of ghrelin or the 18S genes were quantitated by phosphoimaging.
Statistics
Values are means ± SE. Data were evaluated using an ANOVA followed by Newman-Keuls test where appropriate. Statview software (Cary, NC) was used for data analysis.
RESULTS
Experiment 1
The purpose of experiment 1 was to examine the role of body adiposity in the reduction of stomach ghrelin production and plasma ghrelin levels that occur in response to a long-term HF diet and to determine the extent to which a higher percentage of ingested dietary fat is a signal to reducing stomach ghrelin production and blood ghrelin levels. An HF diet, i.e., diet-induced obesity, will suppress ghrelin synthesis and secretion in rodents, and obesity will lower circulating ghrelin levels in humans (33, 50). To isolate the influence of increased body fat on stomach ghrelin production and secretion, T3 treatment was given to rats fed an HF diet to prevent body fat accumulation. Because T3 administration has been reported to stimulate food intake (32, 36) and food intake influences stomach ghrelin homeostasis, a group of rats (HFPFHF+VEH + T3) were T3 treated and pair fed an HF diet according to the daily caloric intake of rats fed the HF diet but not given T3. Another group of rats (HFPFAIN +VEH + VEH) were pair fed the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet to determine the extent to which a higher percentage of ingested dietary fat is a signal to reducing stomach ghrelin production and blood ghrelin levels.
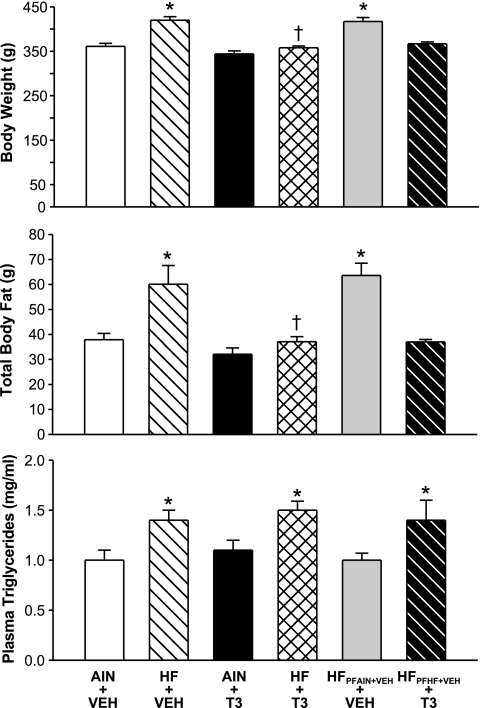
The body weights of rats given the HF diet plus vehicle injections (HF + VEH), but not the body weights of HF + T3 rats, were significantly higher (P < 0.05) compared with rats given the AIN-76A diet plus vehicle injections (AIN + VEH) (Fig. 1). The body weights of AIN + VEH and AIN + T3 rats did not differ significantly (P > 0.05). The body weights of rats (HFPFAIN+VEH + VEH) pair fed the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet were significantly higher when compared with rats given the AIN-76A diet (AIN + VEH). The body weights of rats (HFPFHF+VEH + T3) given T3 and pair fed an HF diet according to the daily caloric intake of HF rats but not given T3 (HF + VEH) did not differ significantly compared with rats given the AIN-76A diet.
Fig. 1.
Influence of a high-fat (HF) diet in combination either with vehicle (VEH) or triiodothyronine (T3) treatments on body weight, total body fat, and plasma triglyceride levels in rats. Groups of rats pair fed (PF) according to the daily caloric intakes of AIN-76A diet (AIN) + VEH and HF + VEH are included (HFPFAIN+VEH + VEH; HFPFHF+VEH + T3); the latter group was given T3. Control rats were fed an AIN-76A diet. Rats were fed AIN-76A (5% fat) or HF (40–45% fat) diets for 8 wk. Body weights, total body fat, and plasma triglyceride levels were measured as described in the text. *P < 0.05 vs. AIN + VEH; +P < 0.05 vs. HF + VEH. n = 8–10 rats/group.
Body composition analyses indicated that the body fat contents of HF + VEH but not HF + T3 rats were significantly greater than the body fat contents of AIN + VEH rats (Fig. 1). The body fat contents of HF + T3 rats were reduced significantly compared with HF + VEH rats. The body fat contents of AIN + T3 and AIN + VEH rats did not differ significantly. The body fat contents of HFPFAIN+VEH + VEH rats were increased significantly compared with AIN rats and did not differ significantly compared with HF + VEH rats. The body fat contents of HFPFHF+VEH + T3 rats were similar to those of AIN + VEH rats and significantly lower compared with HF + VEH rats.
With one exception, plasma triglyceride levels were significantly higher in rats fed the HF diet (HF + VEH; HF + T3; HFPFHF+VEH + T3) compared with rats fed the AIN-76A diet. In rats given HFPFAIN+VEH + VEH, plasma triglyceride levels did not differ compared with AIN + VEH rats.
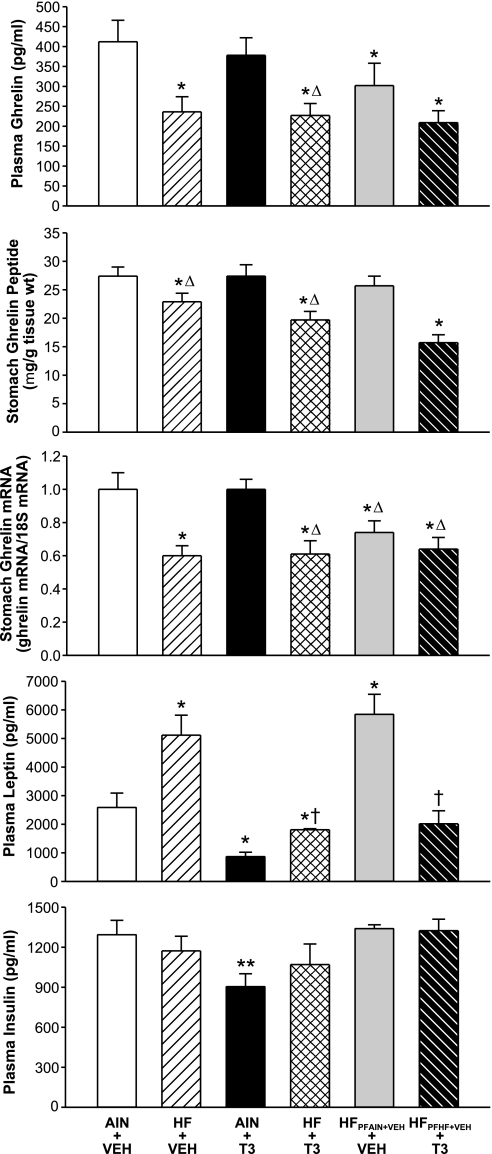
Plasma ghrelin levels were reduced significantly in rats given HF + VEH or HF + T3 compared with rats given AIN + VEH or AIN + T3 (Fig. 2). Compared with AIN + VEH rats, plasma ghrelin levels were reduced significantly in rats (HFPFAIN+VEH) pair fed the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet (AIN + VEH). Compared with AIN + VEH rats, plasma ghrelin levels were reduced significantly in rats (HFPFHF+VEH + T3) that were T3 treated and pair fed an HF diet according to the daily caloric intake of HF + VEH rats.
Fig. 2.
Role of adipose tissue in suppression of stomach ghrelin production and secretion in obese rats. As described in the text and legend of Fig. 1, rats were fed an AIN-76A or HF diets for 8 wk. Groups of AIN- and HF-fed rats were given vehicle or T3. Groups of rats pair fed according to the daily caloric intakes of AIN + VEH and HF + VEH are included (HFPFAIN+VEH + VEH; HFPFHF+VEH + T3); the latter group was given T3. At the end of the treatment period, plasma was collected for measurements of plasma ghrelin, leptin, and insulin levels, and stomachs were harvested and extracted for measurements of stomach ghrelin mRNA and peptide levels. *P < 0.05 vs. AIN + VEH; ΔP < 0.05 vs. AIN + T3. †P < 0.05 vs. HF + VEH; **P < 0.05 vs. all other groups. n = 8–10 rats/group.
In addition, stomach ghrelin mRNA levels were decreased significantly in rats given HF + VEH or HF + T3 compared with rats given AIN + VEH or AIN + T3. In rats given HFPFAIN+VEH + VEH or HFPFHF+VEH + T3, stomach ghrelin mRNA levels were decreased significantly compared with rats given AIN + VEH or AIN + T3.
Stomach ghrelin peptide levels in HF + VEH and HF + T3 rats were reduced significantly compared with stomach ghrelin peptide levels in AIN + VEH or AIN + T3 rats. Stomach ghrelin peptide levels of rats (HFPFAIN+VEH + VEH) pair fed the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet were not altered significantly compared with AIN-fed rats. Stomach ghrelin peptide levels in HFPFHF+VEH + T3 rats were reduced significantly compared with AIN + VEH rats.
Plasma leptin levels in HF + VEH rats were significantly higher compared with plasma leptin levels in AIN + VEH, AIN + T3, HF + T3, or HFPFHF+VEH + T3 rats. In HF + T3 and HFPFHF+VEH + T3 rats, plasma leptin levels were reduced significantly compared with HF + VEH rats. Plasma leptin levels in HFPFAIN+VEH + VEH rats were increased significantly compared with AIN-76A-fed rats.
Plasma insulin levels in AIN + T3 rats were significantly lower compared with all other groups. Plasma insulin levels in AIN + VEH, HF + VEH, HF + T3, HFPFAIN+VEH + VEH, and HFPFHF+VEH + T3 rats did not differ significantly.
Table 1 shows that daily food intakes of AIN +VEH and HF + VEH rats did not differ significantly. Daily food intakes of rats given AIN + T3 were significantly higher compared with AIN + VEH rats.
Table 1.
Average daily food intake of rats given AIN-76A or HF diets with vehicle or T3 treatments
| Group |
Food Intake |
|
|---|---|---|
| Weight, g | kCal | |
| AIN + VEH | 20.2±1.2 | 69.8±4.2 |
| AIN + T3 | 26.2±1.7* | 90.6±5.7* |
| HF + VEH | 18.2±1.6 | 82.1±7.1 |
| HF + T3 | 19.4±1.2 | 87.7±5.4 |
Values are means ± SE; daily food intakes for the last 2 wk of experiment 1. VEH, vehicle; T3, triiodothyronine; AIN, AIN-76A diet; HF, high-fat diet.
P < 0.05 vs. AIN + VEH.
Experiment 2
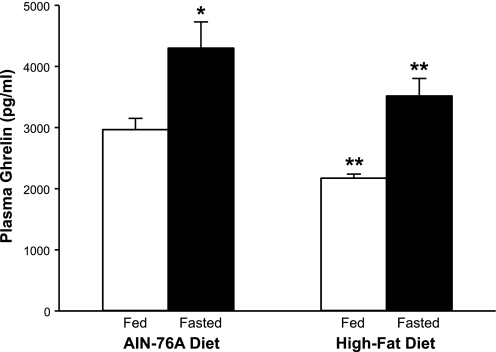
The aim of experiment 2 was to determine the extent to which plasma ghrelin levels increase in response to acute fasting in rats fed a long-term HF diet. Control rats were fed the AIN-76A diet (12% fat). Interestingly, in rats fed an HF diet, plasma ghrelin levels increased significantly in response to fasting (Fig. 3). The plasma ghrelin increments in response to fasting were similar in rats fed HF (∼1,300 pg) or AIN-76A (∼1,400 pg) diets. Food intake was measured for a 2-wk period in rats fed AIN-76A and HF diets. Daily food intake, in terms of grams·day−1·rat−1 in rats fed the AIN-76A diet was significantly greater compared with rats fed the HF diet (Table 2). In terms of kcal·day−1·rat−1, the daily food intakes did not differ significantly.
Fig. 3.
HF diet does not inhibit the stomach ghrelin secretory response to acute fasting. Rats were fed AIN-76A (12% fat) or HF (40–45% fat) diet for 5 wk. Plasma was collected from ad libitum-fed (fed) and 24-h-fasted (fasted) rats for measurement of plasma ghrelin levels. *P < 0.05 vs. fed. **P < 0.05 vs. AIN-76A diet-fed.
Table 2.
Average daily food intake of rats given AIN-76A or HF diets
| Group |
Food Intake |
|
|---|---|---|
| Weight, g | kCal | |
| AIN-76A | 21.7±0.4 | 72.6±1.3 |
| HF | 15.8±0.4* | 71.6±1.7 |
Daily food intakes were monitored for a 2-wk period in experiment 2.
P < 0.05 vs. AIN-76A.
DISCUSSION
The primary aim of these experiments was to investigate the hypotheses that 1) the increased body adiposity in rats fed an HF diet will exert an inhibitory influence on gastric ghrelin production and systemic ghrelin levels, 2) a higher percentage of dietary fat is a signal to reduce stomach ghrelin production and blood ghrelin levels, and 3) an increased body adiposity will suppress the rise in circulating ghrelin levels in response to acute fasting. In the present studies, T3 was used to avert body fat accumulation in rats fed the HF diet. It is well known that the thyroid hormones thyroxine and T3 are the predominant regulators of basal metabolic rate and that circulating levels of thyroid hormones have a direct correlation with energy expenditure and calorie loss (35). T3 administration may stimulate food intake (32, 36); it is possible, therefore, that stomach ghrelin homeostasis and plasma ghrelin levels are influenced partly by this T3-induced increase in food intake. Therefore, stomach ghrelin homeostasis and secretion were measured in a group of rats treated with T3 and pair fed the HF diet according to the daily caloric intake of rats fed the HF diet but not treated with T3. We pair fed another group of rats the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet to determine the extent to which a higher percentage of ingested dietary fat plays a role in the reductions of stomach ghrelin production and blood ghrelin levels during ingestion of an HF diet.
It is well documented that chronic ingestion of HF diets and the ensuing obesity will reduce stomach ghrelin production and plasma ghrelin levels in rodents; plasma ghrelin levels are also reduced in obese humans (33, 50). The source and identity of the inhibitory signal on ghrelin homeostasis during increased adiposity, however, are not known. One potential source of inhibitory signals is the increased body fat mass that accrues from regular ingestion of excess calories. Possible inhibitory signals secreted by adipose cells include several adipokines, such as leptin, apelin, adiponectin, resistin, and others (48). Studies in humans and rodents show that circulating levels of leptin and ghrelin can be inversely correlated (12, 50): in the isolated perfused rat stomach, leptin infusion will inhibit ghrelin secretion (28). Together, these earlier findings suggest that leptin may participate in the lowering of ghrelin homeostasis during ingestion of an HF diet and obesity. In the present study, plasma ghrelin levels were reduced in two groups of rats (HF + VEH and HFPFAIN + VEH + VEH) with elevated plasma leptin levels; both of these groups were fed the HF diet. However, plasma ghrelin levels were also reduced in rats without elevated plasma leptin levels (HF + T3 and HFPFHF + VEH + T3). These latter findings indicate that the reduced plasma ghrelin levels in rats fed an HF diet are not dependent on an elevation of circulating leptin levels and, furthermore, suggest that the inhibitory signal during ingestion of an HF diet is most likely not leptin. Alternatively, if the inhibitory signal includes leptin, leptin is a partial component. The lower plasma leptin levels in the HF + T3 and HFPFHF + VEH + T3 groups are consistent with the reduced body fat content of these groups. In nonobese rats fed the HF diet (HF + T3 and HFPFHF + VEH + T3), the amount of body adiposity was not significantly different from the amount measured in the lean AIN + VEH group. The influence of other adipokines, such as apelin, adiponectin, and resistin, on gastric ghrelin production and secretion has not been investigated.
Another potential inhibitor of plasma ghrelin levels during ingestion of an HF diet is insulin, since circulating insulin levels can be elevated during ingestion of chronic HF diets and obesity (15, 34, 39), and, in the isolated perfused rat stomach, insulin infusion will inhibit ghrelin secretion (28). Additionally, in humans, plasma ghrelin levels decrease in response to an insulin infusion or a meal, suggesting that insulin is a physiological inhibitor of ghrelin secretion (13, 18). It should be pointed out that other studies indicate that insulin does not affect ghrelin secretion (3). In the present report, plasma insulin levels were unaltered by the various treatment combinations, indicating that alterations in plasma ghrelin levels during ingestion of HF diets are not influenced by plasma insulin.
An elevated fat mass will also result in elevated systemic levels of several cytokines, including TNF-α and IL-6 (20, 27). The effects of exogenous TNF-α and IL-6 on gastric ghrelin production and secretion have not been investigated, although administration of LPS, an activator of IL-6 and TNF-α secretion (29, 30, 43, 44), will lower ghrelin secretion in rats (52).
Our results indicate that the reduction of gastric ghrelin homeostasis during ingestion of an HF diet is not associated primarily with an inhibitory signal from adipose tissue. Reductions of stomach ghrelin homeostasis and plasma ghrelin levels appear to be related to ingestion of the HF diet: it is an enteric signal. In nonobese rats fed the HF diet (HF + T3 and HFPFHF + VEH + T3), the amount of body adiposity is equal to that measured in the lean AIN + VEH group. Despite normal body adiposity, gastric ghrelin production and secretion remain reduced.
In the present studies, we pair fed a group of rats (HFPFAIN + VEH + VEH) the HF diet according to the daily caloric intake of rats fed ad libitum the AIN-76A diet to determine the extent to which a higher percentage of ingested dietary fat signals reduction of stomach ghrelin production and blood ghrelin levels. Interestingly, plasma ghrelin and stomach ghrelin mRNA levels were reduced in these rats. However, these rats also have elevated body fat contents; therefore, it is impossible to discern the influence of a fat-calorie-dense diet on stomach ghrelin homeostasis and plasma ghrelin levels apart from other nutrition-associated effectors. However, the reduced plasma ghrelin and stomach ghrelin mRNA levels in the HFPFAIN + VEH + VEH group indicate that the inhibitory mechanism does not involve intake of excess calories, since calories were restricted. We did not expect to find increased body weight and body fat content in rats fed a calorie-restricted HF diet. These findings, however, agree with and extend earlier data showing that HF diets produce obesity partly because they produce a metabolically efficient state (41, 45, 54).
T3 treatment has been reported to stimulate food intake in rats (32, 36). Interestingly, our findings show that food intake increased in the AIN + T3 group compared with the AIN + VEH group, whereas food intake did not increase significantly in the HF + T3 group compared with the HF + VEH group. To determine the extent to which this possible T3-enhanced food intake plays a role in lowering stomach ghrelin production and secretion, we fed a group of rats (HFPFHF + VEH + T3) the HF diet and treated them with T3 but restricted the daily amount of ingested calories to an amount consumed by rats fed the HF diet but not treated with T3. In the HFPFHF + VEH + T3 group, stomach ghrelin production and secretion were reduced. Furthermore, T3 treatment of rats fed the HF diet does not enhance food intake. Together, these findings indicate that the reduced stomach ghrelin production and secretion in the HF + T3 group is not due to an enhanced food intake. Additionally, the persistent reductions in stomach ghrelin production and secretion in the HFPFHF + VEH + T3 group along with a normal body weight and total body fat content further support the idea that increased body fat does not have a role in the reduction of stomach ghrelin production and secretion in rats fed the HF diet. Although speculative, the marginal but insignificant reduction in plasma ghrelin levels of the AIN + T3 group may be attributed to the increased food intake.
Our results imply that the inhibitory signal on gastric ghrelin production and secretion during HF diet consumption is an enteric or pancreatic hormone, ingested nutrients, or both. As expected (11), the present study shows that HF diet consumption results in hyperlipidemia (accumulation of triglycerides in plasma). Because blood-borne nutrients can depress ghrelin secretion, the increased levels of blood lipids may act on the systemic side of ghrelin cells to reduce ghrelin secretion. The identity of a possible inhibitory enteric hormone during consumption of HF diets or obesity is not known, but it is expected to act systemically on ghrelin cells. Interestingly, our laboratory has reported that gastric lavage of soybean trypsin inhibitor, a secretagogue for release of intestinal cholecystokinin (40), will lower ghrelin secretion in fasted rats (22), implying that cholecystokinin might act to inhibit ghrelin secretion. Because physiological changes occur in muscle and liver tissue during consumption of HF diets and obesity (2, 16, 19), a factor(s) from these tissues might conceivably affect ghrelin homeostasis.
Ghrelin is a potent activator of food intake, and its secretion from the stomach is sensitive to caloric intake (22, 33). Our findings show that the acute elevation in secretion of gastric ghrelin in response to fasting in rats fed the HF diet is unaffected by an excess of stored calories, i.e., obesity. Furthermore, the plasma ghrelin increment in rats fed the HF diet was comparable to that measured in rats fed the AIN-76A diet: ∼1,400 and ∼1,300 pg/ml in AIN-76A- and HF-fed rats, respectively.
Stomach ghrelin is synthesized and circulates as acylated and desacylated variants (1, 26). Levels of desacyl-ghrelin predominate over acyl-ghrelin in the rat stomach, and plasma desacyl-ghrelin levels are ∼4- to 10-fold higher than acyl-ghrelin levels (26, 56; unpublished observations). Although both ghrelin variants have multiple biological activities, only acyl-ghrelin stimulates food intake (31).
The enzyme that acylates ghrelin has been described recently (25, 55). Substrate for this enzyme is medium-chain, primarily C8 and C10, fatty acids. In our experiments, the HF diet consists of long-chain fatty acids. In experiment 1, plasma levels of acyl-ghrelin (C8) are measured. Although speculative, the decreased plasma acyl-ghrelin levels measured in rats fed an HF diet may reflect, in part, the composition of the diet and an inadequate level of enzyme substrate. In contrast, in rats fed the AIN-76A diet, a diet with little fat and a reduced caloric density, the likelihood of β-oxidation and generation of medium-chain fatty acids for ghrelin acylation is greater.
Perspectives and Significance
Ghrelin is an exceptionally intriguing stomach hormone, since data indicate that luminal and systemic nutrient signals influence ghrelin secretion. Exactly how excess caloric intake and obesity exert a negative regulation over stomach ghrelin production and secretion is enigmatic. One very likely source of inhibitory signals is the elevated body fat mass. However, our results clearly indicate that adipose mass does not feed back to inhibit gastric ghrelin homeostasis. This is a primary finding of the present study. Another important finding is that the inhibitory signal is enteric in nature: it may be a gastrointestinal or pancreatic hormone or specific ingested nutrients. It is most likely a combination of multiple inhibitory signals. Future studies should address and elucidate identities of inhibitory signals.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-61614.
Acknowledgments
The authors thank Eileen Figueroa and Steve Schuenke for preparation of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Kangawa K. Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab 90: 6–9, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression is altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294: E918–E927, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Angeloni SV, Glynn N, Ambrosini G, Garant MJ, Higley JD, Suomi S, Hansen BC. Characterization of the rhesus monkey ghrelin gene and factors influencing ghrelin gene expression and fasting plasma levels. Endocrinology 145: 2197–2205, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, Kangawa K, Nakao K. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology 146: 355–364, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol 159: 1029–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43: 4370–4376, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bowers CY Unnatural growth hormone-releasing peptide begets natural ghrelin. J Clin Endocrinol Metab 86: 1464–1469, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Casanueva FF, Dieguez C. Growth hormone secretagogues: physiological role and clinical utility. Trends Endocrinol Metab 10: 30–38, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Cassoni P, Papotti M, Ghe C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E, Muccioli G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 86: 1738–1745, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Coenen KR, Hasty AH. Obesity potentiates development of fatty liver and insulin resistance, but not atherosclerosis, in high-fat diet-fed agouti LDLR-deficient mice. Am J Physiol Endocrinol Metab 293: E492–E499, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology 124: 1532–1535, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Eberhart GP, West DB, Boozer CN, Atkinson RL. Insulin sensitivity of adipocytes from inbred mouse strains resistant or sensitive to diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 266: R1423–R1428, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Engl J, Sturm W, Sandhofer A, Kaser S, Tschoner A, Tatarczyk T, Weiss H, Tilg H, Patsch JR, Ebenbichler CF. Effect of pronounced weight loss on visceral fat, liver steatosis and adiponectin isoforms. Eur J Clin Invest 38: 238–244, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Englander EW, Gomez GA, Greeley GH Jr. Alterations in stomach ghrelin production and in ghrelin-induced growth hormone secretion in the aged rat. Mech Ageing Dev 125: 871–875, 2004. [DOI] [PubMed] [Google Scholar]
- 18.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab 87: 2984, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Frick F, Hume R, Robinson IC, Eden S, Oscarsson J. Hepatic and adipose tissue depot-specific changes in lipid metabolism in late-onset obese (LOB) rats. Lipids 43: 313–324, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83: 847–850, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ghigo E, Arvat E, Muccioli G, Camanni F. Growth hormone-releasing peptides. Eur J Endocrinol 136: 445–460, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Gomez G, Englander EW, Greeley GH Jr. Nutrient inhibition of ghrelin secretion in the fasted rat. Regul Pept 117: 33–36, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Greeley GH, Jahnke G, Nicholson GF, Kizer JS. Decreased serum 3,5,3′-triiodothyronine and thyroxine levels accompanying acute and chronic ritalin treatment of developing rats. Endocrinology 106: 898–904, 1980. [DOI] [PubMed] [Google Scholar]
- 24.Greeley GH, Thompson JC. Insulinotropic and gastrin-releasing action of gastrin-releasing peptide (GRP). Regul Pept 8: 97–103, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105: 6320–6325, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279: 909–913, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept 119: 77–81, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Jang SI, Kim YJ, Chung HT, Yun YG, Kang TH, Jeong OS, Kim YC. Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia 75: 261–266, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Kim DH, Baek SH, Lee HJ, Kim MR, Kwon HJ, Lee CH. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-κB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem Pharmacol 71: 1198–1205, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ, Bloom SR. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology 145: 5252–5258, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 143: 185–190, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenstein AH, Schwab US. Relationship of dietary fat to glucose metabolism. Atherosclerosis 150: 227–243, 2000. [DOI] [PubMed] [Google Scholar]
- 35.LiVolsi VA Pathology of the Thyroid. Philadelphia: Saunders, 1989.
- 36.Luo L, MacLean DB. Effects of thyroid hormone on food intake, hypothalamic Na/K ATPase activity and ATP content. Brain Res 973: 233–239, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem 279: 19832–19838, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 276: 905–908, 2000. [DOI] [PubMed] [Google Scholar]
- 39.McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 293: E1746–E1755, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Miyasaka K, Guan DF, Liddle RA, Green GM. Feedback regulation by trypsin: evidence for intraluminal CCK-releasing peptide. Am J Physiol Gastrointest Liver Physiol 257: G175–G181, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Mobbs CV, Mastaitis J, Yen K, Schwartz J, Mohan V, Poplawski M, Isoda F. Low-carbohydrate diets cause obesity, low-carbohydrate diets reverse obesity: a metabolic mechanism resolving the paradox. Appetite 48: 135–138, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Park SY, Ji GE, Ko YT, Jung HK, Ustunol Z, Pestka JJ. Potentiation of hydrogen peroxide, nitric oxide, and cytokine production in RAW 264.7 macrophage cells exposed to human and commercial isolates of Bifidobacterium. Int J Food Microbiol 46: 231–241, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem 281: 33019–33029, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44: 645–651, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, Alexander G, Chenard MP, Rio MC. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 119: 395–405, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Torsello A, Luoni M, Schweiger F, Grilli R, Guidi M, Bresciani E, Deghenghi R, Muller EE, Locatelli V. Novel hexarelin analogs stimulate feeding in the rat through a mechanism not involving growth hormone release. Eur J Pharmacol 360: 123–129, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27: 762–778, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes 50: 707–709, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Lee HM, Englander E, Greeley GH Jr. Ghrelin—not just another stomach hormone. Regul Pept 105: 75–81, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, Tache Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol 291: G611–G620, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Wei W, Qi X, Reed J, Ceci J, Wang HQ, Wang G, Englander EW, Greeley GH Jr. Effect of chronic hyperghrelinemia on ingestive action of ghrelin. Am J Physiol Regul Integr Comp Physiol 290: R803–R808, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132: 387–396, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K, Nakao K. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol 13: 2748–2752, 2002. [DOI] [PubMed] [Google Scholar]