Abstract
Young rabbits are nursed every 24 h for a period of 3–5 min. As a consequence, pups are synchronized to this nursing event; this synchronization is characterized by increased locomotor activity and a peaking of core temperature and plasma corticosterone in anticipation of the daily meal. Ghrelin is a hormone suggested to play a role in meal initiation and to promote food intake. The present study explored the role of ghrelin in food-entrained conditions. Newborn rabbits were maintained in constant darkness and nursed once daily at 1000 by the lactating dam. On postnatal day 7, rabbits were killed at six different time points to complete a 24-h cycle. All pups developed locomotor rhythms entrained by mealtime and exhibited anticipatory activity. Food-entrained rhythms in plasma corticosterone and free fatty acids were observed even if two meals were omitted. In contrast, daily food-driven rhythms in stomach weight, plasma glucose, liver glycogen, and ghrelin did not persist when two meals were omitted. Peak ghrelin levels were observed at the moment in the cycle when the stomach weight was lowest, i.e., before initiation of anticipation. The present data are in agreement with previous data from rabbit pups maintained in light-dark conditions and provide evidence that 7- to 9-day-old rabbits in constant darkness can exhibit metabolic and hormonal rhythms mainly driven by the restricted daily nursing.
Keywords: food entrainment, corticosterone, ghrelin, development, circadian rhythms
the rabbit is a natural model of food entrainment, because does, in the field (3) and in laboratory conditions (15), nurse their pups for one brief (<5-min) period every day. This behavior is a circadian rhythm, because even if the mother has free access to her pups in laboratory conditions, she visits the nest and nurses them only once a day with a period of every 24 h (16, 17). As a consequence, pups are synchronized to this event and exhibit behavioral rhythms associated with the daily meal. At 2–3 h before nursing, pups emerge from the nest material and show a dramatic increase in their locomotor activity in preparation for the dam's visit (16, 17). When the mother arrives, the pup readily grasps a nipple and, in <5 min, sucks large amounts of milk, up to 35% of its body weight on postnatal day (P) 7 (P7) (4). The mother terminates the nursing by jumping out of the nest and leaving the burrow or nesting cage. The pups remain alone, huddled in the nest, mostly quiescent, until they start to increase their locomotor activity again 2–3 h before the dam arrives again (15). This behavioral pattern persists even if the doe fails to arrive 1 day and is present again on the following day at the expected nursing time; such behavior indicates that nursing is driven by the circadian system (16, 17).
Physiological parameters also are synchronized to this daily nursing event. Core body temperature shows a significant increase of 0.4–0.6°C at ∼2.5–3.5 h before nursing and persists after a 48-h period of fasting (18). Previously we reported that in light-dark (L:D) conditions the brief daily visit of the doe for nursing coincides with a sharp increase of the adrenal hormone corticosterone (Cort) in the pup's plasma. This sharp increase is followed by a steady decrease to a nadir 16 h after nursing, when Cort increases again in anticipation of the forthcoming daily nursing event (27). This finding was surprising, because extensive experiments in other altricial mammals, such as rats and mice, have well established that pups are in a stress-hyporesponsive state during the first 2 wk of life (21, 34).
The above-mentioned studies support the assumption that the anticipatory behavior of rabbit pups is similar to food anticipatory activity (FAA) seen in adult rodents under a restricted feeding schedule (31). They also indicate that periodic nursing is a potent zeitgeber for the anticipatory behavior of pups. Because of the robust changes in Cort at this age, it is possible that other hormones related to food intake may also undergo cyclic changes associated with the feeding daily cycle, since rabbit pups seem to experience a circadian cycle of hunger and satiety every 24 h. Here we aimed to describe the daily rhythm of ghrelin in the rabbit pups. Ghrelin is a hormone produced and released primarily by the oxyntic cells in the gut (9) and, to a lesser extent, in the rat's hypothalamus (7). Several studies suggest a role for this hormone in meal initiation. Indeed, ghrelin levels in plasma rise before feeding in rats (2), sheep (32), and humans (6), and it has been suggested that ghrelin plays a central role, along with other hormones, such as leptin, in promotion of food intake and maintenance of energy balance (for review see Ref. 30). Nothing is known about ghrelin in the neonatal rabbit. It is possible that plasma ghrelin levels fluctuate daily, with maximal secretion during the hours of anticipatory arousal to promote feeding, similar to Cort secretion. To better understand the physiological mechanisms involved in periodic arousal and food intake in the rabbit, we analyzed ghrelin and Cort plasma levels in undisturbed daily nursed rabbit pups maintained in constant darkness. Furthermore, to eliminate the influence of milk ingestion on the daily rhythms, we studied the same hormones under conditions of fasting. Finally, we also measured some metabolic parameters, inasmuch as they exhibit daily cycles (13) associated with the expression of FAA in rodents (11) and rabbit pups maintained in an L:D condition (12).
METHODS
Animals and housing.
New Zealand White female rabbits bred in our colony (Laboratorio de Biología de la Reproducción, IIB, Universidad Veracruzana) were maintained under controlled lighting (12:12-h L:D cycle, lights on at 0700) and stable temperature (23 ± 2°C), with rabbit pellets (Purina) and water provided ad libitum. Females were mated and housed individually in stainless steel cages inside the rabbit colony. On day 28 of gestation, females were transferred to circadian activity-recording cages and monitored daily until delivery and then during daily lactation. Each cage has two compartments, one for the doe and one for the nest. The compartments are connected by a tunnel with two doors on opposite sides of the tunnel, which allowed us to keep the pups in constant darkness. Mother and nest compartments are 0.60 m wide × 0.50 m long × 0.40 m high, and the tunnel is 0.25 m wide × 0.50 m long × 0.40 m high. Before parturition, the doe had free access to the nest compartment, where she built a nest with straw and fur from her belly. On the day of parturition, which always occurs in the nest compartment, a sliding door between the tunnel and the mother's cage was locked, forcing the doe to remain in the nest until parturition was completed. The pup's cage was maintained in continuous darkness, but the mother was mainly exposed to an L:D cycle. In the ceiling of the nest box, a thermal detector sensitive to infrared radiation (5- to 14-μm wavelength) was used to monitor continuously locomotor activity of the pups.
All experimental procedures were approved and conducted according to the “Statement of Assurance With Standards for Humane Care and Use of Laboratory Animals” approved by the National Institutes of Health (NIH) for the Universidad Veracruzana.
Experimental groups.
The day of parturition was designated P0. Litters were adjusted to four to six pups in each litter and were left undisturbed in the dark for the rest of the experiment. Every day at 1000 starting at P1, the sliding door was opened, and immediately the doe jumped into the nest to nurse the pups for <5 min. Then she jumped out of the nest and returned to her compartment. Immediately the sliding door was locked and was reopened 24 h later.
Basal Cort and total ghrelin levels were determined in 36 pups that were randomly killed at P7 starting at 0 h just before their scheduled time of nursing and then at 4-h intervals (n = 6 per time point) at 4, 8, 12, 16, and 20 h after nursing, including no more than two pups from each litter at the same time point. Persistence of oscillations was determined in another 42 pups that were fasted for 2 consecutive days and killed 48, 52, 56, 60, 64, 68, and 72 h after the last nursing event.
Locomotor activity was monitored and stored in 15-s bins, from which double-plotted actograms and periodograms were obtained with use of the circadian recording system SPAD9 (OMNIALVA), which automatically generates actograms and periodograms.
Blood sampling and Cort and ghrelin assays.
Pups were anesthetized with an overdose of pentobarbital sodium (20 mg ip per pup), and blood was collected from the left ventricle of the heart with a 21-gauge needle attached to a 3-ml syringe and then transferred to microcentrifuge tubes placed on crushed ice. All procedures from anesthesia to blood collection lasted 2.5–3 min. Samples were centrifuged for 10 min at 2,500 rpm at 4°C. Serum was transferred to labeled 1.5-ml tubes and stored at −20°C for further analysis of metabolites. Immediately after blood sampling, the stomach and liver were removed. Serum Cort and ghrelin were determined by RIA using commercial kits; standards were run in triplicate and samples in duplicate. For Cort (ICN Biomedicals, Costa Mesa, CA), intra-assay variation was 6.42 μl/dl and sensitivity was 0.125 μl/dl. For ghrelin (Linco Research, St. Charles, MO), intra-assay variability was 9.5% and sensitivity was 7.8–2,000 pg/ml. The protocol was run exactly as the manufacturer recommended; i.e., the serum was not diluted.
Metabolic measurements.
Serum aliquots were thawed and processed using spectophotometric methods as previously described for rabbit pups (12). Glucose was estimated from a 10-μl sample with use of a commercial colorimetric kit (catalog no. 70428, Hycel) that is based on enzymatic glucose oxidase reactions and measured at 500 nm. Free fatty acids were estimated from 100-μl samples incubated in the presence of Co(NO3)2 and α-nitroso-β-naphthol following the method reported by Novák (26) and measured at 500 nm. Liver glycogen was determined from a 1-g sample of liver dissolved in hot 30% KOH and then precipitated with 95% ethanol and hydrolyzed with HCl following protocols described by Hassid and Abraham (14). The extracted glucose was determined using the same standard kit by Hycel. Metabolite concentrations were estimated from reaction-product absorbance using a spectrophotometer (Jenway) set to the appropriate wavelength for each product.
Data analysis.
Data were analyzed using a one-way ANOVA to determine effects in time followed by Tukey's post hoc test (SigmaStat version 3.5). Values are means ± SE. Statistical significance was accepted at P < 0.05.
RESULTS
Locomotor activity.
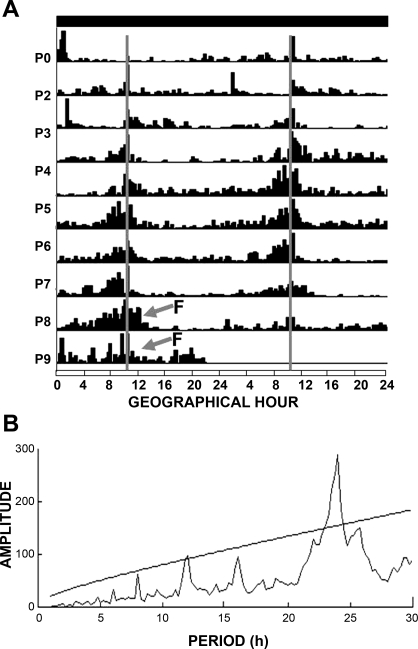
All litters developed a 24-h rhythm of activity associated with daily nursing (Fig. 1). On P0–P2, pups showed low nursing-related activity; from P3 to P4, most litters developed increased activity before scheduled nursing. For all litters, this nursing anticipatory activity was evident at P5 and thereafter. This activation sharply declined immediately after nursing, but when pups were left in a fasting condition (P8–P9), they remained active for a longer period after the expected nursing time (Fig. 1A). The periodogram indicates a significant rhythm of 24 h (P < 0.001, by χ2 test; Fig. 1B).
Fig. 1.
Locomotor activity of neonatal rabbits from postnatal days 1 to 9 (P1–P9). A: double-plotted actogram from 1 representative litter (n = 6 pups); nursing was scheduled to occur at 1000 (gray vertical line). Intensity of activity is represented by black vertical lines. From P0 to P4, synchronization to nursing develops, and anticipatory rhythm is well established at P5. At P8 and P9, the doe was not permitted to nurse (F, fasting). Horizontal black bar at top represents constant darkness for the pups. B: periodogram of activity indicates a circadian component (P < 0.001, χ2 test).
Stomach weight.
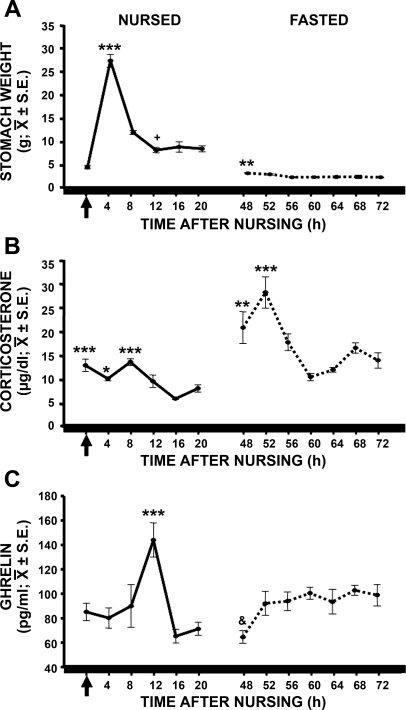
One-way ANOVA revealed a significant exogenous rhythm in stomach weight in nursed [F(5,35) = 92.880, P < 0.001] and fasted [f(6,41) = 5.591, P < 0.001] pups (Fig. 2A). In nursed pups, stomach weight was lowest just before nursing and highest 4 h after nursing (P < 0.001 vs. other time point). Although stomach emptying was slow and continuous, it was clear that the first two-thirds of the stomach content were evacuated in the first 12 h after nursing and the remaining one-third was evacuated in the next 12 h. In fasted pups, residuals of milk were found in the stomach 48 and 52 h after the last nursing event, but not at subsequent time points. Stomach weight at 48 h was significantly different from stomach weight at 56, 60, 64, 68, and 72 h (P < 0.01).
Fig. 2.
Temporal profiles of stomach weight (A) and serum concentrations of corticosterone (B) and ghrelin (C) in nursed and fasted pups. Pups were scheduled to nurse at 1000, and blood samples were collected every 4 h starting at the time of the expected hour of nursing. Fasted pups were not permitted to suck milk during 2 consecutive periods of scheduled nursing. Arrow represents time of nursing. Horizontal black bar at bottom represents constant darkness for the pups. Values are means ± SE. Significant difference between highest and lowest values: *P < 0.05; **P < 0.01; ***P < 0.001. +P < 0.05 vs. 4 and 8 h. &P < 0.05 vs. 60 and 68 h.
Corticosterone.
One-way ANOVA indicated a significant effect of time in nursed [F(5,35) = 10.939, P < 0.001] and fasted [F(6,41) = 8.726, P < 0.001] pups (Fig. 2B). In nursed pups, Cort values at the time of nursing and during the next 8 h were significantly increased, whereas the lowest values were observed 16 and 20 h after nursing. Cort values at 0 h (P < 0.001), 4 h (P < 0.02), and 8 h (P < 0.001) were statistically different from Cort at 16 h after nursing, whereas Cort values at 0 and 8 h were statistically different from Cort at 20 h after nursing (P < 0.001). Even though fasted pups did not suckle milk during two consecutive periods, they showed a circadian rhythm, with a temporal pattern similar to that observed during nursing, but with increased amplitude due to fasting. After 48 h from the last nursing event, Cort was higher and significantly different from Cort at 60 and 64 h after nursing (P < 0.01 in both cases). At 52 h after the last nursing event, Cort increased even further and was significantly higher than all remaining Cort values in fasted pups (P < 0.001 in all cases). However, at 60 h after the last nursing event, Cort was markedly decreased, but it began to increase steadily in advance of the next period of nursing.
Ghrelin.
In nursed rabbit pups [F(5,35) = 11.055, P < 0.001], ghrelin exhibited a daily rhythm (Fig. 2C), and the maximal level was reached 12 h after nursing and then sharply decreased to reach a nadir. This maximal value was significantly different from all remaining time points (P < 0.001). The ghrelin peak observed in nursed pups disappeared, and from 52 to 72 h after the last nursing event, ghrelin remained elevated and similar in magnitude to the level in the fasting condition. ANOVA revealed a significant variation across time [F(6,41) = 2.937, P < 0.020]. Only at 48 h were ghrelin levels significantly lower than at 60 and 68 h after nursing.
Glucose.
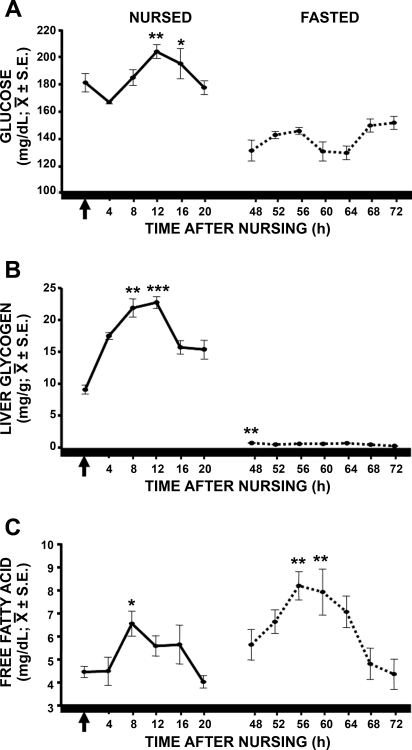
Glucose serum concentration in nursed pups showed a rhythm [F(5,35) = 4.126, P < 0.01], with the lowest values at 4 h after nursing and the highest values at 12 and 16 h after nursing (P < 0.01, 4 vs. 12 h; P < 0.05, 4 vs. 16 h; Fig. 3A). In fasted pups, one-way ANOVA indicated a significant effect in time [F(6,41) = 3.324, P < 0.01]; however, the temporal pattern was different from that of the nursed rabbits, and the post hoc test did not indicate statistical difference among time points.
Fig. 3.
Temporal profiles of serum concentration of glucose (A), liver glycogen (B), and serum free fatty acids (C) in nursed and fasted pups. Pups were scheduled to nurse at 1000, and blood samples were collected every 4 h starting at the time of the expected hour of nursing. Fasted pups were not permitted to suck milk during 2 consecutive periods of scheduled nursing. Arrow represents time of nursing. Horizontal black bar at bottom represents constant darkness for the pups. Values are means ± SE. Significant difference between highest and lowest values: *P < 0.05; **P < 0.01; ***P < 0.001.
Liver glycogen.
Liver glycogen in nursed pups showed a robust daily rhythm [F(5,35) = 21.414, P < 0.001]: liver glycogen levels were lowest at the time of nursing and increased steadily to reach the highest levels 8 and 12 h after nursing (Fig. 3B), which were significantly different from liver glycogen levels 0, 4, 16, and 20 h after nursing (P < 0.01). In fasted pups, although liver glycogen was <1 mg/g, one-way ANOVA also indicated significant effects in time [F(6,41) = 12.977, P < 0.001], and liver glycogen at 48 h was significantly different from that at 68 and 72 h (P < 0.001).
Free fatty acids.
One-way ANOVA indicated that serum concentration of free fatty acids in nursed pups has a rhythm [F(5,35) = 3.236, P < 0.01]: serum free fatty acid concentration was highest 8 h after nursing and lowest 20 h after nursing (Fig. 3C). Serum free fatty concentration at 8 h was significantly different from that at 4 and 20 h after nursing. In fasted pups, free fatty acids also exhibited a rhythm [F(6,41) = 4.515, P < 0.002], with a temporal pattern similar to that observed in nursed rabbits: free fatty acid concentration was highest at 56 h after nursing (8 h after the expected nursing) and lowest at 72 h after the last nursing event. Free fatty concentrations at 56 and 60 h were significantly different from free fatty acid concentrations at 68 and 72 h after nursing (P < 0.01).
DISCUSSION
Neonatal rabbits showed anticipatory locomotor activity entrained to scheduled nursing that was well established by P5. At P7, pups showed hormonal rhythms of Cort and ghrelin. However, high levels of Cort, but not ghrelin, were observed at the moment the pups were anticipating the daily nursing. Present data confirm that, even under constant darkness, circadian rhythm of Cort serum concentration of young rabbits appears to be entrained by the scheduled nursing rhythm of the mother, as evidenced during fasting; this finding is in agreement with our previous finding (27). With respect to ghrelin and contrary to our hypothesis, we were not able to detect an elevation before nursing during the anticipatory period, when pups endured a long interval of fasting and were assumed to be hungry. However, we detected a daily rhythm with a sharp increase at the middle of the cycle, 12 h after nursing, which disappeared in fasting conditions, during which values remained elevated without an apparent cyclic change.
In our previous study, in which young rabbits were maintained in L:D conditions (27), we detected elevated Cort values 46 and 48 h after the last nursing opportunity. These values were greater than the corresponding values observed 24 h previously. Our present results confirm a dramatic Cort elevation 48 h after the last nursing event, a further increase 4 h later, and a decline 8 h later. Despite lack of food, Cort did not remain elevated during the entire fasting interval. If the increase had been related only to stress due to the fasting condition, Cort would have remained high throughout the fasting interval. This persistence confirms that neonatal rabbits maintain a circadian rhythm of Cort in the absence of the entraining signal.
Given the significant two- and threefold elevations in Cort at 48 and 52 h of fasting, respectively, and that these elevations persist at 56 h of fasting, it is possible to hypothesize that these high levels of Cort would lead to a more persistent negative feedback of the hypothalamic-pituitary-adrenal axis and, thus, prevent the expected rise in Cort. Elevated serum levels of Cort have been reported in young rats after food removal 2 h before the onset (19 h) of the dark period, resulting in threefold increases in Cort plasma levels compared with control nonfasted pups (8). Similar enhanced Cort secretion in response to fasting has been found in rats at 12 (35) and 20 (29) days of age. However, this increase remains controlled in a circadian manner, since even when food is not available throughout the dark period, Cort levels drop at lights on (1, 8) at the expected nadir hours, similar to our results. The above-mentioned evidence indicates that even when the normal response of the hypothalamic-pituitary-adrenal axis is upregulated during fasting, it tends to follow a circadian rhythm (8).
The present results do not support our hypothesis that ghrelin increases during the anticipatory period and before daily milk ingestion. Several studies have suggested that ghrelin signals hunger and meal initiation, inasmuch as ghrelin plasma levels show a preprandial rise and a subsequent fall after feeding in several mammals, such as rats (20), sheep (32), cows (23), and humans (6), with fixed meal times. Such studies have suggested a correlation of the empty stomach with the release of ghrelin as a peripheral signal of hunger. It is well established that the action of this hormone is mediated by the growth hormone secretagogue receptor (19); however, it is controversial whether it is an essential orexigenic factor, since appetite, growth, and development are similar in ghrelin hormone secretagogue receptor-null mice and their wild-type littermates (33).
There are discrepancies between secretory daily peaks of ghrelin in rats under ad libitum food conditions and L:D cycles. In one study, ghrelin peaked at the middle of the light period during the rest phase (2); in another study, two peaks were reported: one at 1500 (in the middle of the light period) and another at 0600 (into the dark period) (25). In this latter study (25), the two peaks coincide with the lowest and highest volumes, respectively, of gastric content. This finding indicates that ghrelin plasma secretion is more complex than initially reported. Moreover, ghrelin secretion can also be modulated by the ingested food; thus the macronutrient composition of ingested food affects the expression of ghrelin in fasting and refed pups in the rat (10, 20, 24), as in other mammals (for review see Ref. 19). High levels have been reported in rats after intake of a fat-rich meal, but not after a sucrose carbohydrate meal (28).
We hypothesize that the ghrelin peak concentration we observed 12 h after nursing can be related to the fat-rich meal provided by the maternal milk, which in the rabbit is well known to be extremely rich in lipids (5), and to the significant and abrupt emptying of the stomach, which occurred ∼4 h before the peak of ghrelin was detected. We found that two-thirds of the stomach content was evacuated 12 h after last nursing and the remaining one-third in the next 12 h; these findings coincide with previous results in rabbit pups (12). We speculate that the peak of ghrelin was a consequence of the almost-empty stomach, which led to arousal and prepared the individual for the next milk intake opportunity.
During fasting, after two cycles of nursing were omitted, ghrelin remained high for most of the sampling period. This result, which is consistent with data from several mammalian species during fasting (for review see Ref. 19), is interpreted as pups being hungry and confirms the relationship of high levels of ghrelin with an empty stomach. The loss of oscillation in fasting also points out that, in young rabbits, ghrelin is not driven by an endogenous time-keeping system but, rather, is related to the metabolic state of the pups.
Interestingly, free fatty acids exhibited a robust daily rhythm that persisted in fasting, thus confirming their relationship with an endogenous clock. A previous study in rats reported that free fatty acids peak at the moment when the animals are exhibiting FAA and that such a temporal pattern persists in fasting (22). We previously reported that young rabbits regularly fed free fatty acids do not show a daily rhythm, but a fasting event apparently unmasked a daily rhythm with a peak in anticipation of the expected meal (12). In our previous study, pups were handled and stimulated to urinate daily under an L:D cycle, in contrast to the present study, in which pups were undisturbed and maintained in constant darkness, which is more similar to their natural conditions. Our present data indicate that, in rabbit pups kept in constant darkness, this endogenous oscillation is expressed and is maintained under fasting conditions, but with a different phase angle to feeding time, which points out the relevance of the L:D cycle in setting the phase angle with mealtime for this metabolic oscillation.
Stomach weight and liver glycogen exhibited daily rhythms associated with the nursing time and did not show oscillations during fasting, indicating a depletion of liver glycogen and an empty stomach after a prolonged interval without ingestion. It is worth pointing out that the stomach seems to function as a slow emptying reservoir that, because of the slow emptying rate, allows the individual to receive nutrients along the 24-h fasting interval. As observed in Fig. 2A, at the moment of nursing the stomach still contained some milk and was only observed to be empty after one nursing event was omitted. However, the stomach was almost empty during fasting.
In conclusion, the present study provides evidence that young rabbit pups exhibit daily metabolic and hormonal rhythms associated with the daily scheduled nursing. All variables showed significant daily oscillations under regular nursing conditions; however, only oscillations of Cort and free fatty acids persisted after two cycles of fasting, suggesting that these oscillations are driven by an internal time-keeping mechanism. Oscillations of other variables did not persist in fasting, indicating that they are dependent on the daily nursing event. Present results provide evidence that 7- to 9-day-old rabbits can exhibit metabolic and hormonal rhythms mainly driven by the restricted daily nursing.
Perspectives and Significance
Neonatal rabbits are unusual among mammals, inasmuch as they seem to have a circadian rhythm of Cort at an age when rodents are in a stress-hyporesponsive state. Moreover, this and other circadian and exogenous rhythms seem to depend on the particular natural strategy of feeding once a day, which should be confirmed in future studies.
GRANTS
This work was supported by Fogarty/National Institutes of Health Grant R01 TW-006636 (M. Caba).
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology 135: 1125–1134, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Bodosi B, Gardi J, Hadju I, Szentirmai E, Obal F, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol 287: R1071–R1079, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Broekhuizen S, Mulder JL. Differences and similarities in nursing behaviour of hares and rabbits. Acta Zool 174: 61–63, 1983. [Google Scholar]
- 4.Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Dev Brain Res 143: 119–128, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cowie AT Variations in the yield and composition of the milk during lactation in the rabbit and the galactopoietic effect of prolactin. J Endocrinol 44: 437–450, 1969. [DOI] [PubMed] [Google Scholar]
- 6.Cummins DE, Purnell JQ, Frayo RS, Schmidova K, Wiesse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KI, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman MI, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Hovarth TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37: 649–661, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, sequelae. Endocrinology 140: 4015–4023, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept 116: 101–107, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Escobar C, Díaz-Muñoz M, Encinas F, Aguilar-Roblero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am J Physiol Regul Integr Comp Physiol 274: R1309–R1316, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Escobar C, Hudson R, Martínez-Gómez M, Aguilar-Roblero R. Metabolic correlates of the circadian pattern of suckling-associated arousal in young rabbits. J Comp Physiol [A] 186: 33–38, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Friedman MI, Stricker EM. The physiological psychology of hunger: a physiological perspective. Psychol Rev 83: 409–431, 1976. [PubMed] [Google Scholar]
- 14.Hassid WZ, Abraham S. Chemical procedures for analysis of polysaccharides. In: Methods in Enzymology, edited by Collowick SP and Kapan C. New York: Academic, 1957, vol. III, p. 34–50.
- 15.Hudson R, Distel H. Temporal pattern of suckling in rabbit pups: a model of circadian synchrony between mother and young. In: Development of Circadian Rhythmicity and Photoperiodism in Mammals, edited by Reppert SM. Ithaca, NY: Perinatology, 1989, p. 83–102.
- 16.Jilge B The ontogeny of circadian rhythms in the rabbit. J Biol Rhythms 8: 247–260, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Jilge B Ontogeny of the rabbit's circadian rhythms without an external zeitgeber. Physiol Behav 58: 131–140, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Jilge B, Kuhnt B, Landers W, Rest S. Circadian thermoregulation in suckling rabbit pups. J Biol Rhythms 15: 329–335, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 85: 495–522, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine and dietary manipulations. Endocrinology 143: 185–190, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Levine S The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann NY Acad Sci 746: 275–288, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Merlos MT, Angeles-Castellanos M, Díaz-Muñoz M, Aguilar-Roblero R, Mendoza J, Escobar C. Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Endocrinol 181: 53–63, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Miura H, Tsuchiya N, Sasaki I, Kikuchi M, Kojima M, Kangawa K, Hasegawa Y, Ohnami Y. Changes in plasma ghrelin and growth hormone concentrations in mature Holstein cows and three-month-old calves. J Anim Sci 82: 1329–1333, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab 88: 5510–5514, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Murakami N, Hayashida T, Kuroiwa T, Ankara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 174: 283–288, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Novák M Colorimetric ultramicro method for the determination of free fatty acids. J Lipid Res 6: 431–433, 1965. [PubMed] [Google Scholar]
- 27.Rovirosa MJ, Levine S, Gordon MK, Caba M. Circadian rhythm of corticosterone secretion in the neonatal rabbit. Dev Brain Res 158: 92–96, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez J, Oliver P, Palou A, Picó C. The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology 145: 5049–5055, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Okimoto DK, Dent GW, Gordon MK, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the 20-day-old rat. Consequences of laboratory weaning. J Neuroendocrinol 14: 450–457, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Stanley SM, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev 85: 1131–1158, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Stephan FK Circadian rhythm dissociation induced by periodic feeding in rats with suprachiasmatic lesions. Behav Brain Res 7: 81–98, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Sugino T, Yamaura J, Yamagishi M, Ogura A, Hayashi R, Kurosa Y, Kojima M, Kangawa K, Hasegawa Y, Teraschima Y. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem Biophys Res Commun 298: 785–788, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Wang P, Zhen H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101: 4679–4684, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker CD, Anand K, Plotsky PM. Development of the hypothalamic-pituitary-adrenal axis and the stress response. In: Handbook of Physiology. Coping With the Environment, edited by McEwen BS. New York: Oxford University Press, 2001. p. 237–270.
- 35.Ward G, Xing HC, Carnide N, Slivchak J, Wainwright P. Adrenocortical response to stress in fasted and unfasted artificially reared 12-day-old rat pups. Dev Psychobiol 45: 245–250, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.