Abstract
Intraoral infusions of bitter tastants activate expression of the immediate-early gene c-Fos in neurons located in the medial third of the rostral nucleus of the solitary tract (rNST). The distribution of these neurons is distinct from that activated by sour or sweet stimuli. Bitter stimuli are also distinctive because of their potency for eliciting gaping, an oral reflex that functions to actively reject potentially toxic substances. Glossopharyngeal nerve transection profoundly reduces, whereas decerebration spares, the bitter-evoked Fos-like immunoreactivity (FLI) pattern and gaping, implicating the medial rNST as a substrate for the sensory limb of oral rejection. The present experiment tested this hypothesis using microstimulation (100 Hz, 0.2 ms, 5–40 μA) to activate the rNST in awake rats. NST microstimulation elicited licking and gaping, and gaping was evoked from a restricted rNST region. The results indicated some topographic organization in sites effective for evoking gaping, but, in direct conflict with the hypothesis, lateral sites farther from bitter-evoked FLI were more effective than medial sites centered closer to FLI-expressing neurons. The gape-effective sites resemble locations of bitter-responsive neurons recently observed in neurophysiological recordings. These results indicate that bitter-responsive rNST neurons critical for triggering gaping may not express FLI and imply an alternate function for bitter-responsive neurons that do.
Keywords: oromotor reflexes, taste reactivity, chemotopy, bitter Fos
primary afferent nerves carrying taste information make their initial synapse in the rostral portion of the nucleus of the solitary tract (rNST), terminating in an overlapping topography. The chorda tympani (CT) and greater superficial petrosal branches of the VIIth nerve, which innervate taste buds on the anterior tongue and palate, make their densest terminations more rostrally and laterally than the IXth nerve, which innervates taste buds located on the posterior tongue (25, 63, 67). Neurophysiological responses to taste stimulation demonstrate convergence from different oral regions onto individual neurons, but an orotopic organization is maintained, such that neurons responsive to stimulation of the anterior tongue and/or hard palate are concentrated more rostrally and laterally than those responsive to the soft palate and/or the foliate or circumvallate papillae (22, 23, 63). Compared with this clear orotopy, there is only limited evidence for a topographic organization of taste quality in the rNST.
The most consistent support for chemotopy comes from using Fos immunohistochemistry following gustatory stimulation in awake rats. These data demonstrate that bitter stimuli elicit Fos-like immunoreactivity (FLI) in neurons concentrated in the medial third of the rNST; sweet and sour tastants result in FLI distributed more laterally and diffusely (6, 26, 31, 32, 34, 60–62). Although the FLI data suggest a distinction between the locations of bitter-responsive and other neurons, it is unlikely that these FLI-expressing neurons comprise the entire population of bitter-responsive cells. Bilateral transection of the CT nerve minimally impacts bitter FLI (34) but does impair operant tasks of taste discrimination, including those involving a bitter stimulus (15, 38, 48, 49, 51), suggesting that neurons not expressing FLI are most crucial for these behaviors.
In contrast, elimination of input from the IXth nerve has only minimal impact on operant behaviors involving bitter (and other) discriminations (51) but significantly reduces the number and abolishes the distinctive topographic pattern of bitter-induced Fos neurons of the NST (32–34). Despite minimal effects on operant behavior, IXth nerve transection profoundly impairs the unconditioned oral rejection reflex (gaping), a behavior preferentially elicited by bitter stimuli (19, 33, 57). These parallel behavioral and anatomic effects of IXth nerve cuts suggest the possibility that bitter-activated Fos neurons in the medial third of the NST have a preferential role in eliciting gaping. The present experiment tested this hypothesis by observing oral movements in awake rats during delivery of constant-frequency microampere levels of current through microelectrodes to achieve spatially specific activation of discrete NST regions.
MATERIALS AND METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Thirty-five male Sprague-Dawley rats (250–450 g initial body wt) were housed individually, given ad libitum access to rat chow and water, and maintained on a 12:12-h light-dark cycle. Experiments were performed during the light phase.
Surgical Procedures
After overnight food deprivation, rats were anesthetized with pentobarbital sodium (Nembutal; 50 mg/kg ip) and given supplemental doses when needed to maintain a state of areflexia. Body temperature was monitored and regulated at ∼37°C with a heating pad. After an incision overlying the skull, a pair of intraoral cannulas were implanted to allow controlled delivery of tastants (17). The animal's head was placed in a stereotaxic device, a hole was drilled in the left parietal bone posterior to lambda, and the dura was removed to reveal the cerebellum above the gustatory NST. An etched tungsten microelectrode (Frederick Haer; ∼22 mm long, 2.5 × 105–1.5 × 106 Ω impedance) was angled 4° in the posterior direction.
Standard extracellular recording techniques were used to determine locations for electrode implantation. The goal was to place approximately half of the electrodes in the medial third of the rNST (i.e., centered in the region with the densest bitter-elicited FLI) and the other half farther lateral. More placements were targeted caudally, because bitter-elicited Fos-expressing cells are most numerous and the medial distribution is most distinctive just rostral to the point at which the NST abuts the IVth ventricle (6, 26, 61). In addition, the smaller size of the NST at the rostral pole presents a challenge for differentiation of stimulation sites along the medial-lateral axis. Nevertheless, since bitter stimuli elicit FLI medially along the entire rostral-caudal axis, some electrodes were placed rostrally. All electrodes were implanted unilaterally in or around the left NST. The typical strategy was to identify the rostral pole of the rNST, characterized by responses to application of 0.3 M NaCl to the anterior tongue, and then to adjust the coordinates and/or electrode angle to reach other regions of the nucleus. Initial coordinates for the rostral pole were ∼4 mm posterior and 1.8 mm lateral to lambda and ∼6–7 mm below the cerebellar surface. If the electrode was intended for more caudal levels, the electrode entry point on the cerebellar surface was marked, and the electrode was angled an additional 8°, to position it ∼1 mm caudal to the original track without having to remove any more parietal bone. Potential implant sites were tested for responsiveness to gustatory stimulation of the anterior tongue and innocuous mechanical stimulation of the oral mucosa. Although stimulation of the foliate and circumvallate papillae with tastants would have been informative, it was not practical because of the risk of fluid aspiration. However, our previous studies showed that neurons responsive to posterior tongue taste stimulation almost always have posterior tongue mechanical receptive fields as well (22, 63). Throughout most of the rNST, responsiveness to mechanical stimulation of the circumvallate and foliate papillae was indicative of a more medial placement (Fig. 1). After the initial preparations, we determined that the optimal procedure was to implant the electrode 100–200 μm above the dorsal border of the nucleus to accommodate the ventral movement that often occurred after surgery. Except in one instance, sites outside the nucleus were not intentionally targeted, because shifting of the electrode tips in the dorsal-ventral axis provided an adequate number of control placements. The electrode and intraoral cannulas were secured to the skull and metal screws using dental acrylic, the incision was closed with wound clips, and a topical antibiotic-analgesic was applied. Ampicillin (135 mg/kg) was administered at the end of the surgery and injected for 3 days postoperatively. Animals were also given a one-time injection of an analgesic-anti-inflammatory, carpofen (5 mg/kg), immediately after surgery. Throughout the 7-day recovery period, animals were weighed daily, and a diet of powdered rat chow and pure vegetable oil (Crisco) was given to encourage weight gain.
Fig. 1.
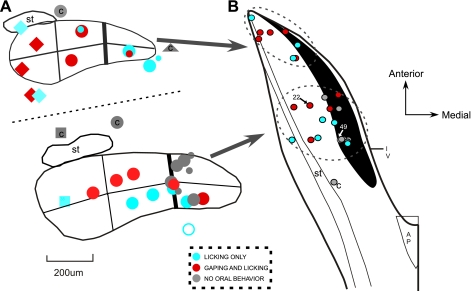
Coronal (A) and horizontal (B) schematic diagrams of electrode placements in the nucleus of the solitary tract (NST). Stimulation sites are color coded according to oromotor behaviors exhibited after electrical stimulation: licking only, licking and gaping, and no response. A: coronal diagram showing the ventral extent of all electrode tracks, except 1 placement in the caudal NST: 25 in the rostral NST (rNST), 3 in the vestibular nucleus, 5 in the reticular formation, and 1 medial to the nucleus. Top: sites near rostral pole of the NST; bottom: placements ∼1 mm farther caudally. C, “control” placement (i.e., a site not in rNST or the subjacent reticular formation). Different-shaped symbols indicate dominant sensory responses recorded in the NST on the implant track: anterior tongue taste (⧫), foliate and/or circumvallate mechanical (•), other mechanical (▪), and jaw stretch (▴). Smaller symbols indicate weak responses. ○, Uncertain response. Final position of the electrode was sometimes above or below the NST response recorded during the implant. Lines dividing the nucleus into sixths indicate “subfields” used previously for analysis of Fos-like immunoreactivity (FLI); these studies indicated that bitter-evoked FLI was most prominent in the medial third of the NST (34). Behavioral effects of sites on and medial to this border (heavy vertical line) were compared with those farther laterally. B: horizontal diagram of only the NST electrode placements (n = 26). Darkened area indicates medial quinine hydrochloride (QHCl) FLI. Color coding indicates behavioral responses as described in A; sensory responses are not shown. Solitary tract (ST) is outlined laterally, and location of the fourth ventricle (IV) and area postrema (AP) are marked. Small arrows and associated numbers indicate locations of stimulation sites depicted in Fig. 7.
Testing Procedures
Chemical and electrical stimulation.
During recovery, the animals (n = 35) were adapted to the Plexiglas testing chamber for ∼1 h on ≥2 days. Throughout this adaptation period, the electrode impedance was measured to ensure proper functioning during the test sessions. On the final day of adaptation, 50 μl of distilled water were infused twice through each intraoral cannula, with each delivery followed by six consecutive water rinses, to mimic parameters used for water and tastant trials during the test sessions (see below).
Each test session began with ≥30 min of chamber adaptation. The animals were subjected to microstimulation during all test sessions, but chemical stimulation was only tested for 2 days. Most (30 of 35) of the animals were tested with two fluid stimuli, water and 3 mM quinine hydrochloride (QHCl). QHCl was usually randomly assigned to day 1 or 2 of testing, whereas water was tested during both sessions. During a given test session, the animals were subjected to two to three fluid stimulation trials. A trial comprised a stimulus (QHCl or water) followed 1 min later by six water rinses, each separated by 5 s. Each stimulus and rinse delivered 50 μl of fluid in 1.4 s.
For the first 2 days, microstimulation consisted of biphasic pulses delivered at a frequency of 100 Hz, train durations of 1.4 s (to parallel the duration of the taste solution delivery), and pulse durations at 0.2 ms (models S88 and PSIU6, Grass Instruments). Stimulation frequency and current amplitude were chosen to be within the range of previous microstimulation studies performed to restrict spatial spread of current (1, 14, 47) and were also based on initial pilot experiments conducted to assess the efficacy of stimulation. Day 1 tested an ascending current series with two trials per intensity (0, 5, 10, 20, 40, and 80 μA). Day 2 used a descending series of the same currents. For some (11 of 30) sites, 80-μA current could not be tested, because of vestibular effects, such as marked, rapid turning behavior, indicating that the current had spread to the vestibular nuclei or activated vestibular fibers passing through the NST. Therefore, the 80-μA data were qualitatively inspected but not included in the statistical analyses. On day 3 of testing, a majority (30 of 35) of the stimulation sites were examined using ascending and descending series of varying train durations (0.1, 0.3, 0.9, 2.7, 8.1, and 24.3), and pulse duration and frequency were equivalent to days 1 and 2 of stimulation. For the train duration series, the initial 15 animals were tested with the lowest effective current, usually 20 or 40 μA, that evoked licking or gaping behavior on a majority of trials during the current series. If no behavior was evoked during the current series, the higher current was used. The remaining 15 animals were tested with 20- and 40-μA currents. The minimum effective current was used for all analyses. The beginning of each chemical or electrical stimulation trial was separated by a 2-min period before the next stimulation. Behavioral responses were videotaped (20 frames/s) for later analysis. Fluid and electrical stimulation were computer controlled using commercial analog-to-digital hardware and software (Spike 2, Cambridge Electronics Design), which also synched the video to stimulus delivery.
Immunohistochemistry
After behavioral testing, we used procedures previously established in the laboratory (6, 26, 61) to stimulate rats with QHCl to elicit FLI. After the animals were adapted to the chamber for 1 h, 7 ml of 30 mM QHCl were delivered at a rate of 0.223 ml/min for 30 min to elicit FLI. After QHCl infusion, the animal was left undisturbed for ∼45 min and then deeply anesthetized (Nembutal, 150 mg/kg) and perfused transcardially with PBS followed by 4% paraformaldehyde-lysine-periodate. After perfusion, the brain was removed and placed in 0.1 M phosphate buffer (PB) with 20% sucrose or, if necessary, postfixed in 4% paraformaldehyde-lysine-periodate for 3 h and then transferred to 20% sucrose PB. On the following day, the brain stem was cut on a freezing microtome into two series of 52-μm sections. The first series was immediately mounted on gelatin-subbed slides for cresyl violet staining, and the second series was stored in a cryoprotectant solution for Fos immunohistochemistry.
Immunohistochemistry for Fos was accomplished using a standard protocol employed extensively in our laboratory (26). For each reaction, a few NST sections from previously successful FLI experiments were processed along with tissue from the present study to serve as a positive control. Each step of the reaction was separated by rinses in PBS or PB and carried out at room temperature unless otherwise noted. Processing commenced with a rinse in PBS followed by incubation in 1% sodium borohydride, 5% H2O2, and then 10% sheep serum. The tissue was placed in the rabbit anti-c-fos antibody (catalog no. sc-52, Santa Cruz Biotechnology) diluted to 1:10,000 in PB and 0.4% Triton (PBTx) for 48–72 h at 4°C. After the primary incubation, the tissue was subjected to successive 90-min incubations in biotinylated goat anti-rabbit IgG (catalog no. BA1000, Vector Laboratories) diluted 1:600 with 15 ml of 0.4% PBTx and 0.015 g of BSA followed by a mixture of avidin-biotin-peroxidase (ABC kit, Vector Laboratories) and 0.02 g of BSA in 20 ml of 0.1 M PB. The tissue was then placed in 5% 3,3′-diaminobenzidine (0.5 mg/ml of PB) with 5% nickel ammonium sulfate for 15 min, and, finally, 20 μl of 1.5% H2O2 were added. The oxidation proceeded for ∼2–4 min and concluded with the appearance of neurons with brown-black-stained nuclei in the NST. Sections were rinsed with 0.1 and 0.05 M PB, mounted on gelatin-subbed slides, air-dried overnight, dehydrated, and cleared, and coverslips were applied using Permount.
Data Analysis
Histology.
Electrode placements were determined by location of the cresyl- and Fos-stained sections that contained the ventralmost extent of the electrode track using a light microscope (model E600, Nikon; ×40–400). Digital photomicrographs (DXM-1200 camera, ACT-1 software, Nikon) were taken, and files were imported into Canvas X (Deneba). Cresyl violet-stained sections were used to outline NST borders and measure the location of the electrode tip as a proportion of its distance along the lateral-medial and dorsal-ventral axes. Sections containing the rostral and caudal (where the NST leaves the IVth ventricle) limits of the rNST were also identified to provide localization along this axis. Electrode placements were plotted on summary diagrams containing coronal and horizontal sections of the NST (Fig. 1).
The ventral extents of the electrode tracks in relation to neurons expressing FLI after QHCl stimulation were also determined. Similar to previous findings, Fos neurons appeared clustered in the medial third of the NST, particularly dorsally (6, 26, 61). The densest concentration of the medial cluster of QHCl/Fos neurons on the section with the ventral extent of the electrode track was outlined, and the distance from the tip of the electrode to the center of the outlined FLI was measured.
Behavioral analysis.
A frame-by-frame (20 frames/s) analysis was performed for the first licking and gaping bout(s) in a given trial. Behaviors consisting of a minimum of three consecutive mouth movements, lateral tongue protrusions and/or tongue protrusions were classified as licking. Higher-amplitude, triangular mouth openings lasting at least two frames were considered gapes (17). Five parameters were measured: latency to lick, duration of the first lick bout, latency to gape, duration of the first gape bout, and number of gapes. A licking or gaping bout was considered to end when a 1-s pause followed the behavior. Interlick and intergape intervals (ILI and IGI, respectively) were also calculated for some individual trials for comparison of chemically to electrically evoked oromotor behaviors.
Stimulation sites were classified by oromotor behaviors on the basis of the four trials of the current series. The train duration data were not used to classify sites, because there were only two trials and because 5 of 35 sites were not included in this phase of testing. Because licking is frequently observed in the absence of stimulation, a criterion was necessary to separate stimulus-linked licking from spontaneously occurring licking. To be classified as a stimulus-bound lick, the behavior was required to commence within 1 s of the end of the stimulus train (i.e., latency ≤2.4 s) and to last for ≥0.5 s. The duration criterion ensured that the bout contained at least two to three mouth movements. (An exception to the latency requirement for licking was made if gaping occurred within 2.4 s, and licking immediately followed.) In contrast to licking, gaping was rarely observed in the absence of stimulation; consequently, all gapes were considered stimulus bound. For a given site to be classified as effective for eliciting licking or gaping, the behavior was required to occur on a majority (3 of 4) of trials at a particular current intensity. To ascertain the internal validity of these criteria, we determined how sites would be classified on the basis of control trials (i.e., trials where no chemical or electrical stimulation was applied). Only one (of 35) site (<3%) met the criteria for licking; none met the criteria for gaping. This was considered to be an acceptable false-positive rate.
Statistical analysis.
All analyses were performed using the statistical program Systat (versions 11 and 12). Statistical significance was defined as P ≤ 0.05. Two-way ANOVAs were conducted for latency and duration of lick and gape bouts, with location (medial vs. lateral and rostral vs. caudal) as a between-groups factor and stimulus (current intensity/train duration) as a repeated-measures factor. One-way repeated-measures ANOVAs were performed to assess differences in licking and gaping behavior as a function of stimulus parameters. Significant ANOVAs were followed by two-sample or paired t-tests (without Bonferroni's adjustment). Paired t-tests were also used to evaluate differences for water- and QHCl-evoked, as well as chemically vs. electrically elicited, licking and gaping. Pearson's correlations (r) were conducted to examine relationships between licking and gaping and the anatomic locations of the electrode tips. χ2 tests were performed to compare the number of sites eliciting licking and gaping behavior in the NST or reticular formation (RF) vs. control placements medial to or above the NST and to compare rostral sites with farther-caudal sites.
RESULTS
Overview of Electrode Placements
Thirty-five animals were used: 25 with placements in the rNST, 5 with placements in the RF, and 5 controls with placements above (n = 3), medial to (n = 1), or caudal to (n = 1) the rNST (Fig. 1). A majority of the stimulation sites were in the caudal half of the rNST (n = 17; Fig. 1A, top), and a smaller number were in the rostral third (n = 8). The dominant sensory responsiveness of the NST along a given track, as assessed by recording at the time of the implant, is also depicted in Fig. 1. Many sites, particularly those in the medial and middle third of the nucleus, were responsive to stroking of the circumvallate and foliate papillae. More laterally, at caudal levels, sites tended to be responsive to mechanical stimulation of other oral regions (e.g., the hard and/or soft palate), whereas at the rostral lateral pole, the NST was responsive to gustatory stimulation of the anterior tongue.
General Behavioral Characteristics (All Sites)
On the basis of the current series, stimulation at 66% (23 of 35) of the sites evoked consistent licking, whereas gaping was only elicited at 31% (11 of 35) of the placements. Gaping was never an isolated event. If gaping was evoked at a particular stimulation site, then licking was also elicited. These proportions appeared relatively stable with longer stimulus trains, despite a nonsignificant trend for longer trains to evoke more gaping (P > 0.1 by χ2 test). For the 21 animals tested with varying train lengths at 40 μA, 48% gaped with the longer trains and only 33% gaped with the standard-length (1.4-s) train.
Several analyses are presented below, including contrasts among different anatomic locations and between electrically evoked and fluid-elicited behaviors. The anatomic comparisons are most extensive and comprise those in the NST/RF vs. control placements outside these regions, sites in the medial one-third vs. lateral two-thirds of the rNST, and sites in the caudal vs. rostral rNST.
Microstimulation: NST/RF vs. Control
For evaluation of the specificity of NST/RF microstimulation in eliciting oromotor behaviors, animals with placements in the NST or RF (n = 30) were compared with control placements 60–150 μm medial to or above the NST (n = 5). Placements in the NST and RF were grouped for comparison with controls, because RF stimulation was also expected to elicit oromotor behaviors on the basis of its known connectivity and functional characteristics (13, 55, 58). Stimulation at 77% (23 of 30) of NST/RF sites consistently evoked licking and/or gaping for at least one current intensity (0–40 μA), but none (0 of 5) of the control placements did (P < 0.01 by χ2 test). The proximity of control sites to the NST provides evidence for limited current spread, suggesting that ≤40-μA currents directly activated neurons within a radius of only 150 μm.
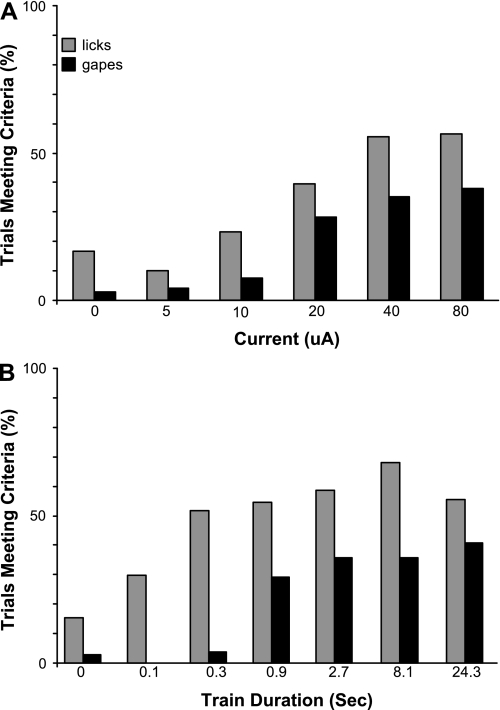
A systematic relationship between current intensity and oral behavior was apparent for placements in the NST/RF (Fig. 2, A and B). One-way ANOVAs revealed a significant decrease in the average latency to lick [F(4,112) = 13.09, P < 0.001] and gape [F(4,112) = 14.86, P < 0.001] with higher currents. Similarly, although less dramatic, a one-way ANOVA revealed main effects for current for average lick [F(4,112) = 3.36, P = 0.012] and gape [F(4,112) = 9.81, P < 0.001] duration. Similar relationships were seen with varying train lengths (Fig. 2, C and D). The average latency to lick [F(6,156) = 6.23, P < 0.001] or gape [F(6,156) = 13.41, P < 0.001] decreased, and bout duration increased with longer trains [lick bout duration: F(6,156) = 5.22, P < 0.001; gape bout duration: F(6,156) = 6.95, P < 0.001]. Because the latency functions were steeper than the duration functions, they provided the most sensitive index for threshold determination. Sites in the NST/RF shared the same current threshold for the two behaviors. Paired t-tests revealed that stimulation with 20- and 40-μA currents resulted in significantly shorter average latencies to lick (20 μA: T = 2.4029, P = 0.023; 40 μA: T = 4.6628, P < 0.001) and gape (20 μA: T = 4.1529, P < 0.001; 40 μA: T = 4.6428, P < 0.001) than no (0 μA) stimulation (Fig. 2A). However, licking could be elicited with shorter trains than gaping. A significant decrease in lick latency occurred with stimulation trains as short as 0.3 s (T = 4.2226, P < 0.001), but gape latency did not decrease until the trains were lengthened to 0.9 s (T = 3.8526, P = 0.01; Fig. 2C).
Fig. 2.
Relationships between current intensity or train duration and response latency or duration, averaged across stimulation sites in the NST/RF. *Significant decrease in latency or increase in response duration compared with control condition (0 μA). Higher currents (A and B) and longer trains (C and D) produced decreases in latency (A and C) and increases in bout duration (B and D) for licking and gaping. Open boxes mark licking and gaping thresholds, determined using latency measures. Licking and gaping shared the same current threshold, but licking could be elicited with shorter trains than gaping.
The succeeding analyses use these average measures of latency and bout duration derived from all subjects and trials for comparison of different NST regions. Inspection of individual trials suggested that both measures are strongly influenced by response incidence; i.e., since the averages include animals and trials where no behavior occurred, decreases in latency strongly reflect an increase in the proportion of trials where stimulation elicited oral behavior (see below).
Figure 3 depicts proportions of individual trials meeting the criteria for stimulus-bound licking or gaping and includes 80 μA for the current series, an intensity tested in just a subset of animals. The incidence of licking and gaping was clearly related to current intensity (Fig. 3A) and train duration (Fig. 3B). The largest increments, particularly for gaping, occurred as threshold was exceeded.
Fig. 3.
Proportion of individual trials where licks or gapes meeting the criterion for stimulus-bound behavior occurred. To derive the proportion, data were collapsed across all animals, and the number of trials meeting criteria was divided by the number of total trials for a given current or train duration. Higher currents (A) and longer trains (B) led to a clear increase in response incidence. Abrupt increases in gaping occurred at 20 μA and for 0.9-s trains. Clear increases for licking occurred at 20 μA and for 0.3-s trains. Although licks apparently occurred on 10–20% of individual control (no current) trials, this did not occur reliably across control trials in individual animals. On very rare occasions (n = 3 trials), gapes occurred during control trials, but only when real stimulation trials eliciting gapes preceded the control trial.
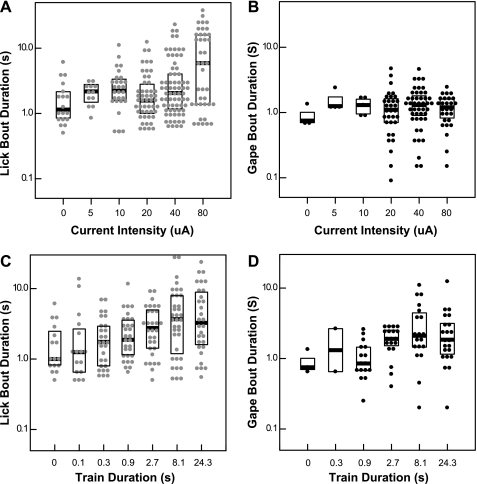
On individual trials where stimulus-bound behavior occurred (see Behavioral analysis), latencies were markedly shorter and gape bouts somewhat longer than for the averaged data for all animals/trials. Licks were elicited with median latencies <1 s and had little relationship to current intensity (not shown); however, lick bouts tended to be longer at the highest current (Fig. 4A). The median duration with 20 μA was 1.6 s; at 80 μA, it was 5.9 s. Similarly, above threshold, median gape latencies were <1 s, regardless of current intensity. However, higher currents did not prolong gaping (Fig. 4B). Similar relationships were apparent for train length. In contrast to the averaged data, there was little effect of train length on lick or gape latency. Similar to the averaged data, however, longer trains elicited longer lick and gape bouts (Fig. 4, C and D). In sum, in the subsequent analyses, shorter latencies as a function of higher currents and longer train durations mainly reflect an increased response incidence. The longer bout durations with higher currents and longer train lengths similarly reflect response incidence but also incorporate some influence of bout length.
Fig. 4.
Box-dot density plots depicting durations of licking or gaping as a function of current intensity or train length only for those trials meeting the criterion for stimulus-bound behavior. •, Individual trials; heavy lines, median. Boxes enclose the central 50% of the values. At >20 μA (threshold), increases in current intensity are associated with longer median and maximum lick (A) but not gape (B) bout durations. Above the train length threshold for licking (0.3 s), lick bout duration increases with train length (C). Above the train length threshold for gaping (0.9 s), gape durations tend to be longer (D).
Placements in the NST: Medial vs. Lateral
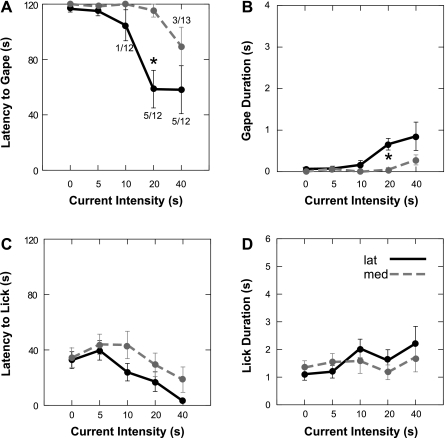
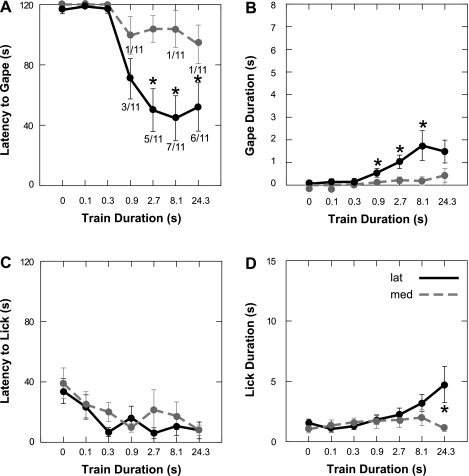
Previous studies demonstrated that bitter-elicited FLI is densest in the medial third of the NST (6, 26, 32, 34, 61). To evaluate whether this medial region was more effective for eliciting gaping, we compared placements in the medial one-third (n = 13 for current series, n = 11 for train duration) with those in the lateral two-thirds (n = 12 for current series, n = 11 for train duration) of the nucleus. In direct opposition to our hypothesis, gaping was evoked by stimulation at a higher proportion (7 of 12) of sites (58%) in the lateral two-thirds of the NST than in medial sites (3 of 13, 23%). This was reflected in shorter average latencies and longer durations for the current intensity (Fig. 5, A and B) and train duration (Fig. 6, A and B) tests.
Fig. 5.
Oromotor behaviors evoked from electrical stimulation of the medial third (n = 13) of the NST compared with sites farther lateral (n = 12). Results are from the current intensity series only (0–40 μA). ANOVAs (see text) revealed that lateral sites were significantly more effective for eliciting gaping than medial sites, as reflected in latency and duration measures (A and B). *Significantly different (P ≤ 0.05) from medial. Numbers at data points in A denote proportion of stimulation sites that elicited gaping at a given current. In contrast to gaping, there were no significant differences between medial and lateral placements for licking behavior (C and D).
Fig. 6.
Licking and gaping evoked by electrical activation of the medial third of the NST (n = 11) vs. the lateral NST (n = 11) using stimulus trains of different lengths. For the current series, stimulation of the lateral NST with various train durations evoked more gaping than activation of the sites near the medial Fos (A and B), as reflected in latency and duration of gaping. *P ≤ 0.05 for a given train duration. Numbers adjacent to data points denote proportion of stimulation sites that elicited gaping at a given train duration. Although there were no significant differences between medial and lateral placements for latency to lick (C), lateral sites displayed longer licking bouts at 24.3 s (D).
Two-way ANOVAs for gape latency and duration revealed significant main effects for location for the current [gape latency: F(1,22) = 8.68, P = 0.007; gape duration: F(1,22) = 7.84, P = 0.01] and train duration series [gape latency: F(1,20) = 10.10, P = 0.005; gape duration: F(1,20) = 13.63, P = 0.017], as well as interactions between location and current intensity [gape latency: F(4,88) = 4.70, P = 0.002; gape duration: F(4,88) = 3.98, P = 0.005] or train duration [gape latency: F(6,120) = 4.89, P < 0.001; gape duration: F(6,120) = 3.55, P = 0.003]. There was less evidence for a medial-lateral difference for eliciting licking. Lateral sites tended to be more effective, but only the analysis of lick bout duration vs. train length revealed a significant location-by-train interaction [F(6,120) = 3.58, P = 0.001].
This comparison of medial and lateral sites required segregation of varied stimulation sites into just two groups. To better incorporate the fact that stimulation sites were continuously distributed, Pearson's correlations were calculated between gaping and each site's medial/lateral coordinates. This analysis supported the conclusions from the ANOVAs. Proximity to the medial NST border was inversely correlated with gape duration (r = −0.554, P = 0.004) and positively correlated with gape latency (r = 0.530, P = 0.006), confirming that lateral placements were more effective. Correlations with gaping were also calculated as a function of the distance from a stimulation site to the center of the densest concentration of FLI on that same section. Gape latency was inversely correlated with distance from the FLI (r = −0.504, P = 0.01), and duration was positively correlated (r = 0.530, P = 0.006). These results directly suggest that NST sites more distant from cells expressing bitter-evoked FLI are more effective for eliciting gaping. Similar analyses for other anatomic axes and for licking yielded no additional significant correlations.
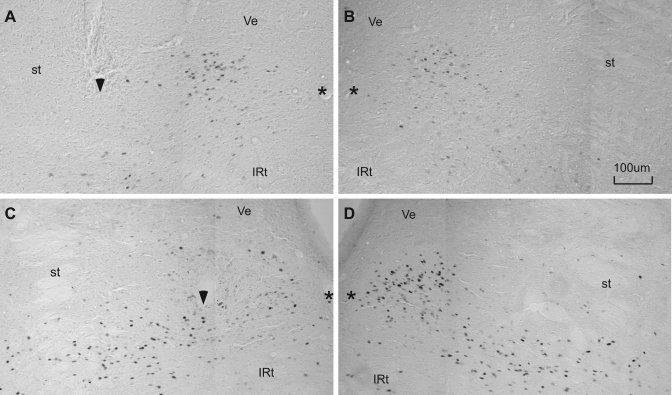
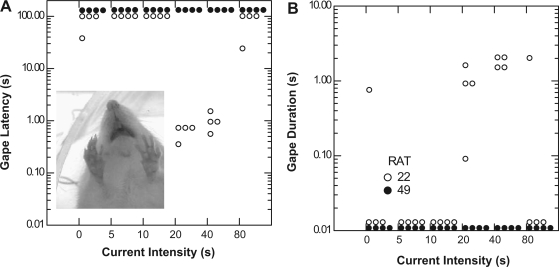
Figure 7 shows two stimulation sites in the caudal rNST at different medial/lateral coordinates. Site 22 (Fig. 7A) was in the lateral two-thirds of the NST, ∼315 μm from the center of the medial cluster of QHCl-elicited FLI. Stimulation with 20 and 40 μA evoked consistent gaping (Fig. 8). Similarly, gaping was observed during the train duration series (not shown). Activation of this site also evoked licking (not shown), and, interestingly, whereas gaping was dominant at current intensities of 20 and 40 μA, stimulation with 80 μA primarily produced licking. In contrast, stimulation at site 49, situated in the center of the densest QHCl FLI (Fig. 7C), elicited neither licking nor gaping (Fig. 8). In fact, each of the seven rNST sites that were ineffective in eliciting any type of oromotor behavior were in this vicinity, i.e., close to the densest FLI, in the medial third of the caudal rNST.
Fig. 7.
Two stimulation sites in sections immunohistochemically stained for QHCl-elicited FLI. A–D: composites of 2 side-by-side photos of the NST. Both stimulation sites were in the caudal half of the rNST, but at different medial-lateral coordinates. Position of these sites is indicated by arrows in Fig. 1B. Stimulation site 22 was effective for eliciting gaping (see Fig. 8) but was located ∼315 μm lateral to the center of the medial QHCl-stimulated Fos-enriched region (A). B: symmetrical FLI on the contralateral side. Site 49 was ineffective for eliciting any oromotor behavior (Fig. 8) and was located more caudally and far enough medially to fall within the Fos cluster (C); FLI expression appeared disrupted near the electrode tip compared with the denser FLI apparent in the analogous location on the opposite side (D). st, Location of the solitary tract; *, medial border of the nucleus; arrowhead, ventral extent of the electrode track; IRt, intermediate zone of the reticular formation; Ve, vestibular nucleus.
Fig. 8.
Latency (A) and duration (B) of gaping behavior elicited by electrical stimulation during the current intensity series (0–80 μA) at stimulation sites 22 (○) and 49 (•), as depicted in Fig. 7. Latency and duration are plotted on a log10 scale. Stimulation at site 22, located ∼315 μm from the QHCl-stimulated Fos-enriched region, elicited consistent gaping at 20- and 40-μA currents (A and B); at 80 μA, gaping was only evoked on 1 trial, and licking became the dominant behavior (not shown). Licking and gaping were also elicited during train duration testing (not shown). Stimulation at site 49, located within the medial QHCl-stimulated Fos-enriched region, did not elicit consistent licking or gaping behavior when stimulated with 5- to 80-μA currents (A and B) or when train duration was varied from 0.1 to 24.3 s (not shown). Inset: video frame of electrically evoked gaping resulting from stimulation of site 22.
Placements in the rNST: Rostral vs. Caudal
Stimulation in the caudal rNST (n = 17 for current series, n = 15 for train duration) was compared with stimulation in the rostral rNST (n = 8 for current series, n = 7 for train duration). Two-way ANOVAs revealed no significant main effects of location for licking or gaping latency or duration (P > 0.1) or any significant interactions between stimulus and location for the current or train duration tests (P > 0.1). However, on the basis of the classification of stimulation sites, the rostral rNST was more effective for evoking oromotor behavior in general. All 8 sites in the rostral rNST were effective for eliciting licking or gaping, compared with only 10 of 17 more-caudal sites (P = 0.03 by χ2 test). Nevertheless, rostral sites were just nominally more effective for evoking gaping (5 of 8) than caudal sites (5 of 17, P > 0.1 by χ2 test).
Electrical vs. Fluid Stimulation
Fluid stimulation produced behaviors similar to those originally reported by Grill and Norgren (17). Rats typically ingested water but rejected 3 mM QHCl. The latencies and durations of licking and gaping evoked by 40-μA/1.4-s trains of electrical stimulation were compared with fluid-evoked behaviors in rats with effective electrode placements using just the trials that met the criteria.
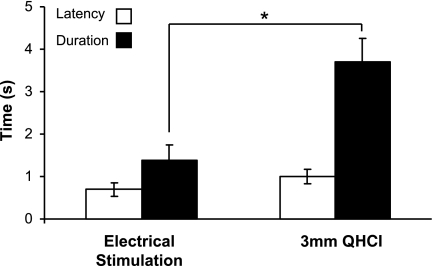
In animals with placements that elicited gapes, 40-μA current and 3 mM QHCl produced gapes at similar, short latencies (T = 1.427, P = 0.2; Fig. 9, left). However, QHCl elicited gape bouts that were two to three times longer than electrical stimulation (T = 3.87, P = 0.01; Fig. 9, right). In addition, 3 mM QHCl was sometimes effective for eliciting other components of the rejection sequence, such as chin rubbing, rearing, or paw flailing, but these other behaviors were never observed after electrical stimulation.
Fig. 9.
Latencies and durations of gape bouts elicited by electrical stimulation (left) or stimulation with 3 mM QHCl (right). Averages were calculated only from trials on which stimulus-bound behaviors occurred. Although electrical and chemical (QHCl) stimulation evoked gaping behavior with similar latencies, significantly longer gaping bouts were elicited by QHCl stimulation than by electrical activation. Values are means ± SE. *P ≤ 0.05.
To more closely examine chemically vs. electrically evoked oromotor behaviors, ILIs and IGIs were calculated. The mean ILI for water-elicited licking was 0.17 s, and that for electrical stimulation was 0.18 s (T = 0.1366, P = 0.896). This corresponds to a frequency of 6–7/s, consistent with previous reports of intraorally evoked (17, 59) or appetitive licking (10, 21, 65, 69). The mean IGI for QHCl-elicited gaping was 0.30 s. Electrically elicited gaping had a slightly shorter mean IGI, 0.25 s, but this difference only approached significance (T = −2.3027, P = 0.055). In either case, gape frequency (3–4/s) fell within the previously reported range (17, 59). Furthermore, the IGIs for electrically evoked and QHCl-elicited gaping were significantly longer than the water-elicited ILIs in these same animals (P < 0.0005).
DISCUSSION
The present study is the first to clearly demonstrate that focal electrical stimulation of the rNST in awake rats can evoke licking and gaping. Electrical stimulation thus seems to at least partially mimic natural primary afferent input to this nucleus, i.e., gustatory input from the facial and glossopharyngeal nerves and intraoral somatosensory signals from the trigeminal nerves. Electrical stimulation of the RF immediately subjacent to the NST similarly elicited licking and gaping, consistent with its known connectivity and functional characteristics; i.e., the parvocellular and intermediate RF receives direct projections from the NST (4, 24, 28, 54), projects to the oromotor nuclei (58, 64), is neurophysiologically active during licking and gaping (55), and is required for these behaviors (7, 8).
Electrical or chemical stimulation can also elicit or prolong licking when applied to other central nervous system gustatory nuclei, including the parabrachial nucleus (PBN) (14, 43) and the ventrobasal thalamus (2, 44). Rhythmic jaw movements, including licking, lapping, or chewing, can similarly be evoked by electrical stimulation of the “masticatory” cortex and its output pathways (40, 56), the lateral hypothalamus (30, 41), the amygdala (30), and the mesencephalic reticular formation (27). Artificially evoked gaping has not been as clearly documented, but probable instances exist in the literature. Di Lorenzo and colleagues (11, 12) reported a significant decrease in licking after NST stimulation when pulse trains mimicked the firing pattern of a quinine-responsive neuron, and although gaping was not explicitly described, it had been mentioned in preliminary reports (unpublished observations, 2006 Meeting of the Association for Chemoreception Sciences). In addition, early experiments that stimulated sites close to the gustatory thalamus reported “aversive behavior” characterized by “higher-amplitude mouth movements” (2, 44). In contrast, neither electrical nor glutamatergic stimulation of any of several PBN subnuclei elicited gaping (14, 43).
Electrically stimulated licks and gapes were distinct and resembled natural responses to fluid stimulation. Licking elicited by electrical stimulation had frequency characteristics identical to those in response to intraoral infusions of tastants or water (17, 59) and to drinking from a sipper spout (10, 21, 65, 69). QHCl-elicited and electrically evoked gapes were characterized by lower-frequency (3- to 4-Hz), triangular, wide-mouthed movements. Gape bouts elicited by electrical stimulation, however, were shorter than those evoked by 3 mM QHCl and lacked other components of the rejection response, similar to less concentrated bitter stimuli (17).
We tested the hypothesis that the NST region, where the immediate-early gene Fos is most densely expressed after stimulation with bitter tastants, comprises an afferent trigger zone for gaping. As documented previously (6, 26, 32–34, 60–62, 64), neurons expressing FLI after QHCl appeared to be distributed preferentially in the medial third of the rNST. The hypothesis that this region would be a gape trigger zone was based on the correlation between Fos expression and gaping. The preferential medial distribution of FLI is unique for bitter vs. other taste qualities (26, 61) and is preserved with decerebration (60), a manipulation that also preserves gaping (18). In contrast, bilateral transections of the IXth nerve greatly attenuate the medial FLI and result in a profound reduction in gaping (19, 33, 34, 57). Moreover, when the IXth nerve is allowed to regenerate, FLI and gaping recover in parallel (32, 33). However, this correlation did not prove to be causal. Instead, stimulation of the lateral rNST was significantly more effective for eliciting gaping. Furthermore, although most (18 of 25) rNST sites could evoke licking or gaping, only a minority (4 of 11) were in the caudal/medial rNST. That stimulation in the caudal/medial rNST is relatively ineffective in eliciting the oral rejection response is consistent with a recent study suggesting that activation of these neurons is not essential for gaping. Intriguingly, in the absence of the IXth nerve, cross-regenerating the CT into the posterior tongue led to recovery of QHCl-induced gaping, but not recovery of FLI in the caudal/medial NST (33).
Each rNST site that failed to elicit an oromotor behavior was in the caudal/medial rNST. This finding suggests that this region is functionally distinct from the more lateral and rostral sites, which were more effective for eliciting gapes and licks. On the caudal implant tracks made through the middle and medial thirds of the nucleus, similar sensory responses, i.e., stroking the circumvallate and foliate papillae, were obtained. Nevertheless, in agreement with earlier observations (23), responses tended to be weaker medially (Fig. 1). Single-unit recordings have similarly revealed only scattered taste neurons in the medial third of the rNST (16, 63). In common with the laterally adjacent rNST, the medial third receives IXth nerve input, but terminations taper off medially (25, 42). Thus the weaker behavioral and neurophysiological responses may reflect sparser primary afferent input. On the other hand, the Fos data suggest that, at least in awake animals, the medial third of the NST can be potently activated by bitter taste stimuli via the IXth nerve (32, 34).
Anatomic and neurochemical data suggest varied functions for the medial third of the caudal rNST. Dorsally, neurons in the central subnucleus project to the second-order taste relay, the PBN (24, 62, 66). In fact, many QHCl/Fos cells are intermingled among PBN projection neurons, although only a minority project to the PBN (62). Ventrally, nitric oxide synthase-containing neurons comprising the rostral extent of the dorsal motor nucleus of the vagus invade the medial rNST (39) and are juxtaposed to neurons expressing bitter-induced FLI (64). Thus stimulation in the medial third of the caudal rNST may fail to elicit oromotor behavior because it primarily activates ascending circuits and/or pathways for visceral, not somatic, reflexes. Previous data indicate that taste stimuli can indeed modulate gastric functions controlled by the dorsal motor nucleus of the vagus; e.g., bitter tastants and posterior oral cavity stimulation can inhibit gastric emptying (35, 36, 68).
Although the present data strongly suggest that the caudal/medial rNST does not comprise a specialized trigger zone for gaping, the possibility that stimulation parameters were inadequate must be considered. Previous studies have demonstrated that bitter-selective neurons have slower conduction velocities (9, 16) and are smaller (46) than those optimally responsive to other taste qualities. Because smaller neurons require higher current intensities (45, 52, 53) and/or longer pulse durations (52) for activation, it is possible that more gaping would have been elicited medially with stronger stimulation. However, a higher current, 80 μA, was tested at 11 placements in the medial rNST, and additional gaping occurred only once. Similarly, classifications of stimulation sites changed for just a single site near the bitter FLI when train lengths were increased to 24.3 s. These results suggest that an inadequate amount of current is not the culprit.
The possibility that particular temporal patterns of stimulation might be required also should be considered. Previous work by Di Lorenzo's group (11, 12) demonstrated differential effects of electrical stimulation in rNST depending on the temporal pattern of pulses. In particular, irrespective of NST location, when patterns mimicking neurophysiological responses to QHCl were employed, licking was inhibited, but with patterns mimicking sucrose responses, no suppression occurred. Thus it is conceivable that use of such patterns might have revealed different results.
Unexpectedly, stimulation in the lateral two-thirds of the NST was more effective for eliciting gaping. Indeed, there was an inverse correlation between gaping and distance from the center of the densest QHCl-evoked FLI. This implies that neurons lateral to those that express FLI after bitter stimulation may be more important in comprising the afferent limb of the gape reflex. This suggestion agrees with recent neurophysiological data showing that bitter-selective neurons were located in the middle third of the nucleus just lateral to the medial focus of bitter FLI (16). As discussed previously (61), only a subset of neurons neurophysiologically activated by taste stimuli express FLI; therefore, it seems likely that those bitter-activated neurons are more important in triggering gaping. The greater efficacy of more lateral regions in eliciting gaping and their tendency to be more generally effective for eliciting oral motor behavior are also consistent with the preferential location of NST/RF projection neurons in the lateral and ventral subnuclei (24, 62).
Although not statistically significant, licking also tended to be elicited more readily from lateral sites. Furthermore, sites eliciting licking and gaping were intermingled. Regardless of the predicted relationship to the FLI, it was somewhat surprising that gaping was not segregated as a function of location or sensory responsiveness. Nerve transection studies indicate that the IXth nerve, which innervates the posterior tongue and terminates farther caudally and medially in the NST, is more crucial for gaping than the CT branch of the VIIth nerve, which innervates the anterior tongue and terminates farther laterally and rostrally (19, 25, 34, 42, 57, 67). Nevertheless, the rostral rNST was just as effective for eliciting gaping. In fact, gaping was evoked at a majority of the few rostral/lateral sites responsive to anterior tongue taste stimulation. However, the present study did not attempt to compare VIIth with IXth nerve responsive sites. Instead, even rostrally, most sites were posterior-tongue responsive and located in the medial two-thirds of the nucleus, similar to the distribution of glossopharyngeal-evoked responses (20). Thus additional topography might be evident with further mapping. In fact, in the cross-regeneration study mentioned above, when gaping recovered after the CT was rerouted to the posterior tongue, bitter-elicited FLI recovered in the rostral/medial rNST, despite its failure to recover more caudally (33). Thus it remains possible that the rostral extent of the medial, Fos-rich region could be especially effective for triggering gaping. However, using electrical stimulation, this proposition would be difficult to test conclusively because of the small size of the NST rostrally, coupled with the fact that IXth nerve fibers enter the solitary tract rostral and lateral to their termination sites (25). Pharmacological manipulation would ameliorate the fibers-of-passage problem but probably would not provide the requisite spatial resolution.
Despite the lack of a lick-gape topography, only about half as many sites elicited gaping. Moreover, all sites that elicited gaping also elicited licking. This resembles the typical motor sequence evoked by aversive tastants, which usually includes both behaviors (17, 59). The fact that fewer sites evoked gaping also resembles natural behavior. Licking can be evoked by multiple stimuli, but gaping is preferentially elicited by bitter tastants (17, 59). These results imply some specificity in the neural circuitry engaged by stimulation. Gape-effective sites may be those at which stimulation activates a critical proportion of neurons optimally responsive to bitter stimuli. On the other hand, stimulation at all sites may engage a similar, varied mix of cells, but gape-effective sites may be those at which stimulation simply has more potent effects. This latter possibility would be consistent with the observation that the gape threshold is higher than the recognition threshold for bitter-tasting stimuli (17, 37).
Although our study focused on motor behaviors, it is likely that electrical stimulation also engaged ascending motivational and perceptual circuits. An earlier investigation demonstrated that rats were motivated to self-stimulate through rNST electrodes (5). In addition, DiLorenzo's group (11) demonstrated that rNST stimulation using a temporal pattern mimicking a particular sucrose response elicited a sensation that most closely resembled the sweet quality. It would be interesting to determine whether activation of NST locations that produce gapes, licks, or no behavior is differentially effective for supporting self-stimulation or eliciting different gustatory qualities.
Perspectives and Significance
The present study is the first to demonstrate a functional spatial organization of the rostral NST on the basis of experimental activation. Lateral sites were more effective than medial sites in evoking oromotor behavior, particularly gaping. Thus, similar to the caudal, viscerosensory NST (3, 29, 50, 71), the rostral NST exhibits regional specificity in controlling reflexive behavior. The poor efficacy of the medial NST in eliciting oromotor rejection was unexpected on the basis of the robust bitter-induced FLI in this region. However, similar results were reported in the second-order gustatory relay, the PBN. Electrical and glutamatergic stimulation in the external subnuclei did not elicit oromotor responses (14), despite strong bitter-evoked FLI in this region (31, 70). The mismatch between bitter-evoked FLI and gaping supports a specialized but unknown role for brain stem gustatory neurons that express this immediate-early gene. The proximity of bitter-evoked FLI expression to the vagal representations in the NST and PBN is suggestive that these neurons may underlie interactions between the special and general visceral senses.
GRANTS
This research was supported by National Institutes of Health Grants R01 DC-00416 (S. P. Travers) and T32 DE-0014320 (N. Kinzeler).
Acknowledgments
We thank Drs. Joseph Travers and Laura Geran for helpful comments on the manuscript and Ken Herman, Ji-Eun Yoo, and Kevin Li for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature 442: 692–695, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Andersson B, Jewell PA. Studies on the thalamic relay for taste in the goat. J Physiol 139: 191–197, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barraco RA, el-Ridi MR. Cardiorespiratory responses following electrical stimulation of caudal sites in the rat medulla. Brain Res Bull 23: 299–310, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Carter DA, Phillips AG. Intracranial self-stimulation at sites in the dorsal medulla oblongata. Brain Res 94: 155–160, 1975. [DOI] [PubMed] [Google Scholar]
- 6.Chan CY, Yoo JE, Travers SP. Diverse bitter stimuli elicit highly similar patterns of Fos-like immunoreactivity in the nucleus of the solitary tract. Chem Senses 29: 573–581, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol 285: R68–R83, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. Am J Physiol Regul Integr Comp Physiol 280: R1085–R1094, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cho YK, Li CS, Smith DV. Gustatory projections from the nucleus of the solitary tract to the parabrachial nuclei in the hamster. Chem Senses 27: 81–90, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992. [PubMed] [Google Scholar]
- 11.Di Lorenzo PM, Hallock RM, Kennedy DP. Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci 117: 1423–1433, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo PM, Hecht GS. Perceptual consequences of electrical stimulation in the gustatory system. Behav Neurosci 107: 130–138, 1993. [DOI] [PubMed] [Google Scholar]
- 13.DiNardo LA, Travers JB. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. J Neurosci 17: 3826–3839, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvin KE, King CT, King MS. Stimulation of specific regions of the parabrachial nucleus elicits ingestive oromotor behaviors in conscious rats. Behav Neurosci 118: 163–172, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Geran LC, Garcea M, Spector AC. Transecting the gustatory branches of the facial nerve impairs NH4Cl vs. KCl discrimination in rats. Am J Physiol Regul Integr Comp Physiol 283: R739–R747, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279, 1978. [DOI] [PubMed] [Google Scholar]
- 18.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res 143: 281–297, 1978. [DOI] [PubMed] [Google Scholar]
- 19.Grill HJ, Schwartz GJ, Travers JB. The contribution of gustatory nerve input to oral motor behavior and intake-based preference. I. Effects of chorda tympani or glossopharyngeal nerve section in the rat. Brain Res 573: 95–104, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Hallock RM, Di Lorenzo PM. Effects of electrical stimulation of the glossopharyngeal nerve on cells in the nucleus of the solitary tract of the rat. Brain Res 1113: 163–173, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Halpern BP, Tapper DN. Taste stimuli: quality coding time. Science 171: 1256–1258, 1971. [DOI] [PubMed] [Google Scholar]
- 22.Halsell CB, Travers JB, Travers SP. Gustatory and tactile stimulation of the posterior tongue activate overlapping but distinctive regions within the nucleus of the solitary tract. Brain Res 632: 161–173, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol 78: 920–938, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–197, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 222: 560–577, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res 711: 125–137, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto N, Katayama T, Ishiwata Y, Nakamura Y. Induction of rhythmic jaw movements by stimulation of the mesencephalic reticular formation in the guinea pig. J Neurosci 9: 2887–2901, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu HH, Travers JB, Travers S. Subnuclear location of orosensory neurons in the nucleus of the solitary tract. Soc Neurosci Abstr 23: 1036, 1997. [Google Scholar]
- 29.Jean A Brainstem organization of the swallowing network. Brain Behav Evol 25: 109–116, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Kaku T Functional differentiation of hypoglossal motoneurons during the amygdaloid or cortically induced rhythmical jaw and tongue movements in the rat. Brain Res Bull 13: 147–154, 1984. [DOI] [PubMed] [Google Scholar]
- 31.King CT, Deyrup LD, Dodson SE, Galvin KE, Garcea M, Spector AC. Effects of gustatory nerve transection and regeneration on quinine-stimulated Fos-like immunoreactivity in the parabrachial nucleus of the rat. J Comp Neurol 465: 296–308, 2003. [DOI] [PubMed] [Google Scholar]
- 32.King CT, Garcea M, Spector AC. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci 20: 8426–8434, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King CT, Garcea M, Stolzenberg DS, Spector AC. Experimentally cross-wired lingual taste nerves can restore normal unconditioned gaping behavior in response to quinine stimulation. Am J Physiol Regul Integr Comp Physiol 294: R738–R747, 2008. [DOI] [PubMed] [Google Scholar]
- 34.King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci 19: 3107–3121, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobashi M, Koga T, Mizutani M, Matsuo R. Neural mechanism and possible role of inhibition of gastric motility induced by superior laryngeal afferents. Chem Senses 30 Suppl I: I72–I73, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Kobashi M, Mizutani M, Matsuo R. Water stimulation of the posterior oral cavity induces inhibition of gastric motility. Am J Physiol Regul Integr Comp Physiol 279: R778–R785, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Koh SD, Teitelbaum P. Absolute behavioral taste thresholds in the rat. J Comp Physiol Psychol 54: 223–229, 1961. [DOI] [PubMed] [Google Scholar]
- 38.Kopka SL, Geran LC, Spector AC. Functional status of the regenerated chorda tympani nerve as assessed in a salt taste discrimination task. Am J Physiol Regul Integr Comp Physiol 278: R720–R731, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol 377: 49–69, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Lund JP, Enomoto S. The generation of mastication by the mammalian central nervous system. In: Neural Control of Rhythmic Movements in Vertebrates, edited by Cohen A, Rossignol S, and Grillner S. New York: Wiley, 1988.
- 41.Mary Christopher S, Butter CM. Consummatory behaviors and locomotor exploration evoked from self-stimulation sites in rats. J Comp Physiol Psychol 66: 335–339, 1968. [DOI] [PubMed] [Google Scholar]
- 42.May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J Comp Neurol 497: 658–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morganti JM, Odegard AK, King MS. The number and location of fos-like immunoreactive neurons in the central gustatory system following electrical stimulation of the parabrachial nucleus in conscious rats. Chem Senses 32: 543–555, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Norgren R Behavioral correlates of the thalamic gustatory area. Brain Res 22: 221–230, 1970. [DOI] [PubMed] [Google Scholar]
- 45.Ranck JB Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975. [DOI] [PubMed] [Google Scholar]
- 46.Renehan WE, Jin Z, Zhang X, Schweitzer L. Structure and function of gustatory neurons in the nucleus of the solitary tract. II. Relationships between neuronal morphology and physiology. J Comp Neurol 367: 205–221, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature 346: 174–177, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol Regul Integr Comp Physiol 263: R169–R176, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Spector AC, Markison S, St. John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol Regul Integr Comp Physiol 272: R1210–R1218, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Spencer SE, Talman WT. Modulation of gastric and arterial pressure by nucleus tractus solitarius in rat. Am J Physiol Regul Integr Comp Physiol 250: R996–R1002, 1986. [DOI] [PubMed] [Google Scholar]
- 51.St. John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci 18: 4353–4362, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swadlow HA Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J Neurosci Methods 79: 131–141, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Tehovnik EJ Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods 65: 1–17, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Travers JB Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 457: 1–11, 1988. [DOI] [PubMed] [Google Scholar]
- 55.Travers JB, DiNardo LA, Karimnamazi H. Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp Brain Res 130: 78–92, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Travers JB, DiNardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev 21: 631–647, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis in the rat. Behav Brain Res 25: 233–246, 1987. [DOI] [PubMed] [Google Scholar]
- 58.Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 220: 280–298, 1983. [DOI] [PubMed] [Google Scholar]
- 59.Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci 100: 544–555, 1986. [DOI] [PubMed] [Google Scholar]
- 60.Travers JB, Urbanek K, Grill HJ. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 277: R384–R394, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Travers SP Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 282: R1798–R1810, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Travers SP, Hu H. Extranuclear projections of rNST neurons expressing gustatory-elicited Fos. J Comp Neurol 427: 124–138, 2000. [PubMed] [Google Scholar]
- 63.Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol 73: 2144–2162, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Travers SP, Travers JB. Taste-evoked Fos expression in nitrergic neurons in the nucleus of the solitary tract and reticular formation of the rat. J Comp Neurol 500: 746–760, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Weijnen JA, Wouters J, van Hest JM. Interaction between licking and swallowing in the drinking rat. Brain Behav Evol 25: 117–127, 1984. [DOI] [PubMed] [Google Scholar]
- 66.Whitehead MC Subdivisions and neuron types of the nucleus of the solitary tract that project to the parabrachial nucleus in the hamster. J Comp Neurol 301: 554–574, 1990. [DOI] [PubMed] [Google Scholar]
- 67.Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol 220: 378–395, 1983. [DOI] [PubMed] [Google Scholar]
- 68.Wicks D, Wright J, Rayment P, Spiller R. Impact of bitter taste on gastric motility. Eur J Gastroenterol Hepatol 17: 961–965, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Wiesenfeld Z, Halpern BP, Tapper DN. Licking behavior: evidence of hypoglossal oscillator. Science 196: 1122–1124, 1977. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto T, Shimura T, Sako N, Sakai N, Tanimizu T, Wakisaka S. c-Fos expression in the parabrachial nucleus after ingestion of sodium chloride in the rat. Neuroreport 4: 1223–1226, 1993. [DOI] [PubMed] [Google Scholar]
- 71.Yang RH, Igarashi Y, Wyss JM, Chen YF. Dopamine D2 receptors in the posterior region of the nucleus tractus solitarius mediate the central pressor action of quinpirole (LY171555). Brain Res Bull 24: 97–103, 1990. [DOI] [PubMed] [Google Scholar]