Abstract
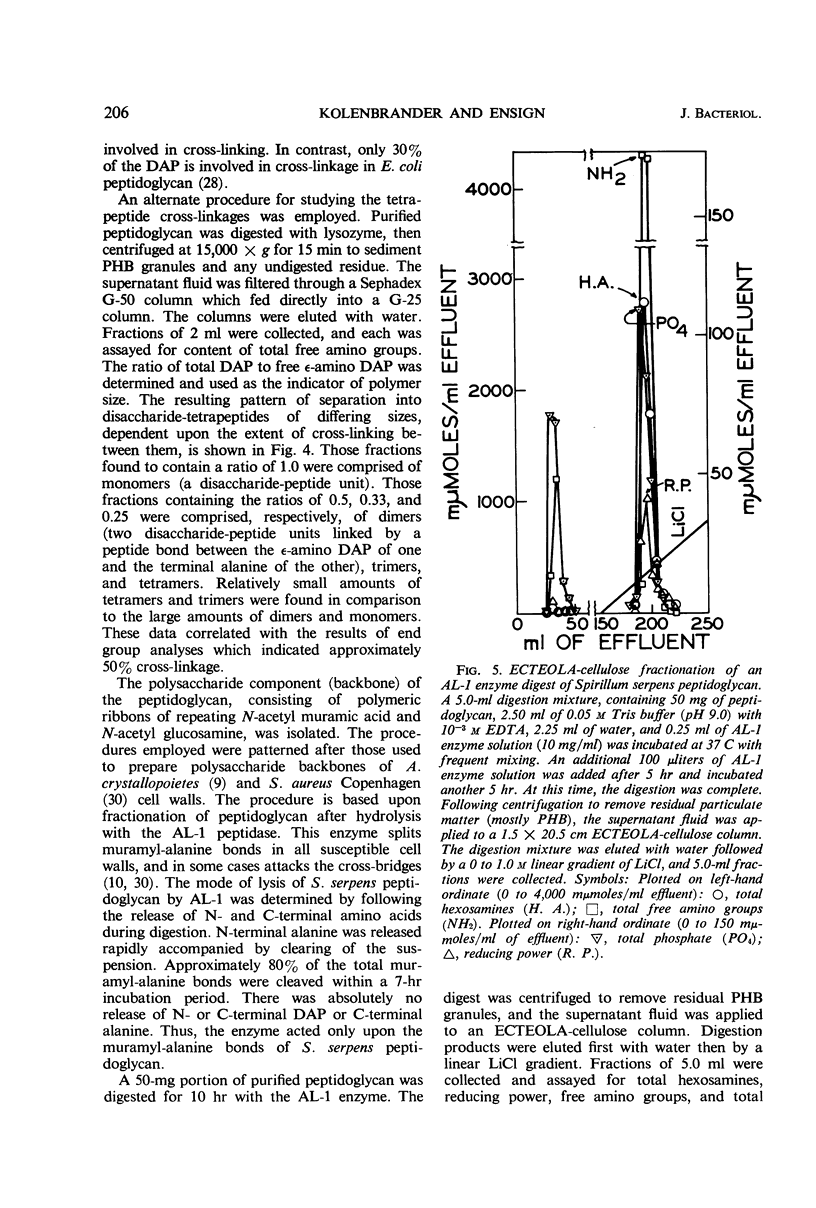
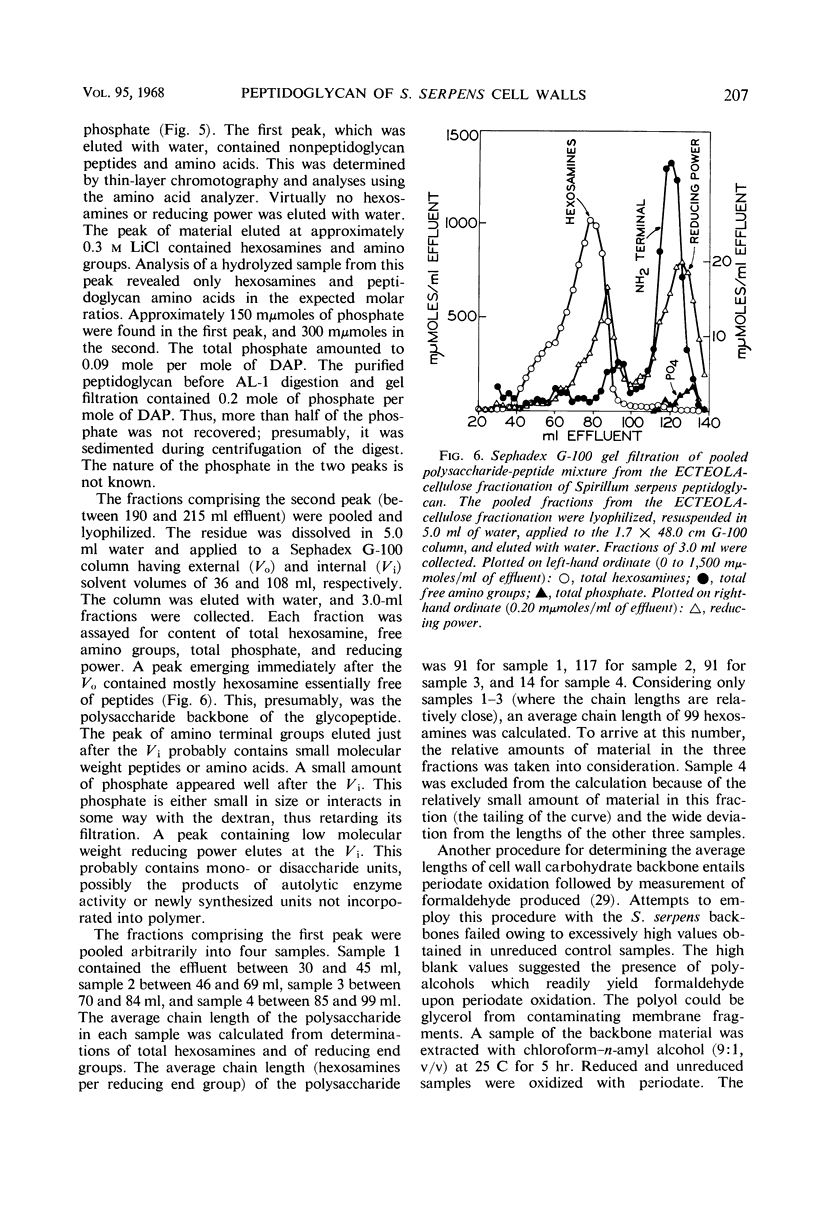
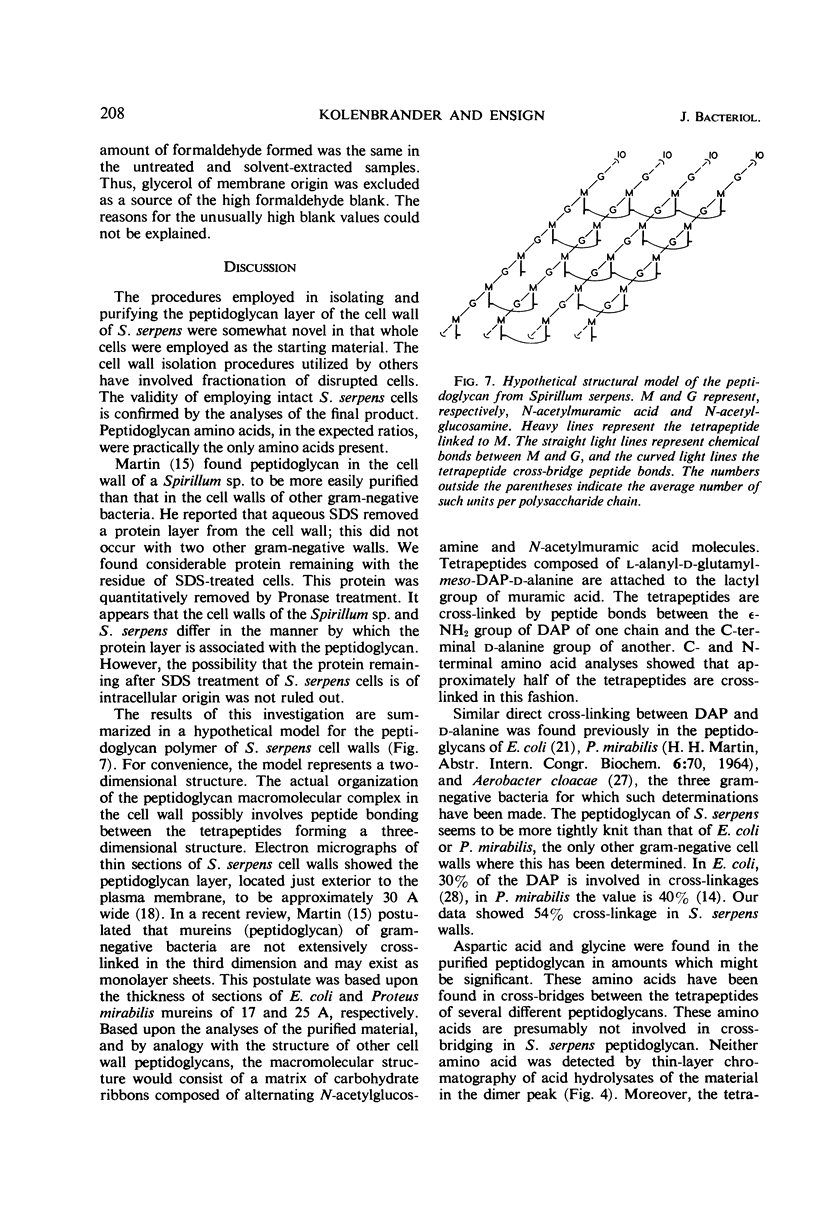
The peptidoglycan layer of Spirillum serpens cell walls was isolated from intact cells after treatment with sodium dodecylsulfate and digestion with Pronase. The isolated peptidoglycan contained glucosamine, muramic acid, alanine, glutamic acid, and meso-diaminopimelic acid in the approximate molar ratio of 1:1:2:1:1. Aspartic acid and glycine were the only other amino acids found in significant quantities. N-terminal amino acid analyses of the tetrapeptide amino acids in the peptidoglycan revealed that 54% of the diaminopimelic acid molecules are involved in cross-linkage between tetrapeptides. This amount of cross-linkage is greater than that found in the peptidoglycan of previously studied cell walls of gram-negative bacteria. The polysaccharide backbone was isolated, after myxobacter AL-1 enzyme digestion of the peptidoglycan, by fractionation with ECTEOLA-cellulose and Sephadex G-100. An average length of 99 hexosamines for the polysaccharide chains was found (ratio of total hexosamines to reducing end groups).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ENSIGN J. C., WOLFE R. S. LYSIS OF BACTERIAL CELL WALLS BY AN ENZYME ISOLATED FROM A MYXOBACTER. J Bacteriol. 1965 Aug;90:395–402. doi: 10.1128/jb.90.2.395-402.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., TIPPER D. J., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. IV. THE TEICHOIC ACID-GLYCOPEPTIDE COMPLEX. Biochemistry. 1965 Mar;4:474–485. doi: 10.1021/bi00879a016. [DOI] [PubMed] [Google Scholar]
- Heath E. C., Mayer R. M., Edstrom R. D., Beaudreau C. A. Structure and biosynthesis of the cell wall lipopolysaccharide of Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):315–333. doi: 10.1111/j.1749-6632.1966.tb52374.x. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- MARTIN H. H. ZUSAMMENSETZUNG DER STUETZMEMBRAN IN DER ZELLWAND VON NORMALEN ZELLEN UND PENICILLIN-SPHAEROPLASTEN VON PROTEUS MIRABILIS. Zentralbl Bakteriol Orig. 1963 Dec;191:409–414. [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- SCHOCHER A. J., BAYLEY S. T., WATSON R. W. Composition of purified mucopeptide from the wall of Aerobacter cloacae. Can J Microbiol. 1962 Feb;8:89–98. doi: 10.1139/m62-012. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]