Abstract
Chronic administration of anorexigenic substances to experimental animals by injections or continuous infusion typically produces either no effect or a transient reduction in food intake and body weight. Our aim here was to identify an intermittent dosing strategy for intraperitoneal infusion of peptide YY(3-36) [PYY(3-36)] that produces a sustained reduction in daily food intake and adiposity in diet-induced obese rats. Rats (665 ± 10 g body wt, 166 ± 7 g body fat) with intraperitoneal catheters tethered to infusion swivels had free access to a high-fat diet. Vehicle-treated rats (n = 23) had relatively stable food intake, body weight, and adiposity during the 9-wk test period. None of 15 PYY(3-36) dosing regimens administered in succession to a second group of rats (n = 22) produced a sustained 15–25% reduction in daily food intake for >5 days, although body weight and adiposity were reduced across the 9-wk period by 12% (594 ± 15 vs. 672 ± 15 g) and 43% (96 ± 7 vs. 169 ± 9 g), respectively. The declining inhibitory effect of PYY(3-36) on daily food intake when the interinfusion interval was ≥3 h appeared to be due in part to an increase in food intake between infusions. The declining inhibitory effect of PYY(3-36) on daily food intake when the interinfusion interval was < 3 h suggested possible receptor downregulation and tolerance to frequent PYY(3-36) administration; however, food intake significantly increased when PYY(3-36) treatments were discontinued for 1 day following apparent loss in treatment efficacies. Together, these results demonstrate the development of a potent homeostatic response to increase food intake when PYY(3-36) reduces food intake and energy reserves in diet-induced obese rats.
Keywords: gastrointestinal, peptide, intraperitoneal administration, anorexia, body composition
an important early step in the development of obesity drugs is determining whether chronic administration of anorexigenic substances, either alone or in combination, can produce a sustained reduction in daily food intake, body weight, and adiposity in obese experimental animals. Methods of administration generally include either daily injections or insertion of an osmotic minipump beneath the skin or into the peritoneal cavity to deliver substances continuously for a week or more. Such methods typically produce only transient reductions in daily food intake and weight gain. Reasons include development of a compensatory increase in food intake between injections, receptor downregulation, and tolerance (tachyphylaxis) to continuous or frequent administration of the anorexigenic substances and redundancy and plasticity in the energy regulatory system (17, 25, 31).
We have developed a novel experimental model that permits precise intravenous or intraperitoneal administration of anorexigenic substances to rats tethered via infusion swivels to computer-controlled pumps. Rats are free to move, eat, and drink within their individual cages, and their indwelling catheters remain functional for many months. Measurement of food bowl weight, recorded by computer every 20 s, permits daily assessment of the instantaneous effects of infused substances on food intake. Adjustments in dosing pattern can be performed daily to define a dosing strategy that minimizes both compensatory hyperphagia between doses and tolerance.
We have used this model to demonstrate several important properties of the effects of acute and chronic administration of the gut hormone peptide YY(3-36) [PYY(3-36)] on food intake, body weight, and adiposity in lean and diet-induced obese rats. First, in lean rats, 3-h intravenous infusion of PYY(3-36) (3–30 pmol·kg−1·min−1) at dark onset dose dependently reduces short-term food intake (6). Second, in lean rats, intermittent 3-h intravenous infusion of PYY(3-36) at 30 pmol·kg−1·min−1 during 0–3 and 6–9 h of the dark period for 7 days produces a sustained reduction in food intake within and across infusion intervals with no apparent loss of sensitivity to the peptide. However, rats develop a compensatory increase in food intake between PYY(3-36) infusions, such that there is no net decrease in daily food intake by the 7th day of infusion. Third, in lean rats, intermittent 1-h intravenous infusion of PYY(3-36) at 30 pmol·kg−1·min−1 every other hour for 10 days produces a sustained reduction in daily food intake of ∼20% and prevents weight gain (5). Fourth, in diet-induced obese rats (>25% body fat) with free access to both a high-fat solid diet and a high-fat liquid diet, intermittent intraperitoneal infusion of PYY(3-36) at 10–30 pmol·kg−1·min−1 during 0–3 and 6–9 h of the dark period for 21 days produces a sustained reduction in daily caloric intake of 11–32% and prevents body weight gain and fat deposition. Together, these results indicate that chronic, intermittent infusion of PYY(3-36) can produce a sustained reduction in daily food intake and prevent weight gain and fat deposition in lean and obese rats. These studies helped to resolve the intense debate regarding the inhibitory effects of PYY(3-36) on food intake and body weight (18, 20, 27). The goal in treating obese humans, however, is reducing body weight and adiposity, not just preventing or attenuating weight gain and fat deposition. Thus, our aim here was to determine whether intermittent intraperitoneal administration of PYY(3-36) can produce a similar sustained reduction in daily food intake in diet-induced obese rats when body weight and adiposity are decreasing in response to PYY(3-36) administration.
MATERIALS AND METHODS
Synthesis and Purification of PYY(3-36)
Rat PYY(3-36) was synthesized by fluorenylmethoxycarbonyl solid-phase methodology (3) and purified by reverse-phase high-performance liquid chromatography. Proof of structure was provided by coelution with a known sample and by electrospray mass spectrometry. PYY(3-36) stock was prepared by dissolving the purified peptide in 0.15 M NaCl, 0.1% BSA. Single-use aliquots were stored at −70°C.
Animals
Male Sprague-Dawley rats (Sasco; Charles River, Portage, MI; initially weighing 225–350 g) were housed in a room with controlled temperature (19–21°C) and a 12:12-h light-dark cycle (lights off at 1700). Rats were provided pelleted rat chow (Labdiet, 5001 Rodent diet; PMI Nutrition International) and water ad libitum for about a week before being subjected to induction of obesity. The Animal Studies Subcommittee of the Omaha Veterans Affairs Medical Center approved the experimental protocol.
Dietary Induction of Obesity
Obesity was induced in the rats as previously described (8). Briefly, animals were provided both a high-fat pelleted food (45% calories from fat, 4.73 kcal/g, D12451; Research Diets, New Brunswick, NJ) and vanilla Ensure Plus liquid food (29% calories from fat, 1.5 kcal/ml; Ross Nutrition, Abbott Laboratories, Columbus, OH). An EchoMRI-700 quantitative nuclear magnetic resonance (QMR) analyzer (Echo Medical Systems, Houston, TX) was used to measure total fat mass and lean mass (not including skeletal mass) in the rats at monthly intervals.
Surgical and Postsurgical Adaptation Procedures
Diet-induced obese rats (n = 92, 696 ± 8 g body weight; 199 ± 4 g body fat; 279 ± 11 days of obesity induction) were surgically implanted with intraperitoneal catheters under isoflurane anesthesia using procedures that were described previously (8). The intraperitoneal catheters, which exited the skin in the dorsal cervical region, were plugged with stainless steel wire and kept patent by flushing weekly with 1 ml of normal saline. After surgery, rats were transferred to a room with a 12:12-h light-dark cycle with lights off at 1100. During the postsurgical recovery period, the animals had continued access to both the Ensure and pelleted high-fat food. Rats were allowed 3 wk to regain lost body weight. The animals were then fitted with light-weight harnesses (IITC Life Science Inc., Woodlands, CA) used for tethering to infusion swivels and were allowed an additional 3 wk to adapt to the harnesses.
Experimental Design
Rationale.
In our previous study of the effects of chronic PYY(3-36) administration in diet-induced obese rats (9), both Ensure and high-fat powdered food were provided during the test period, vehicle-treated rats gained weight and adiposity, and PYY(3-36) administration in a second group of matched obese rats produced a sustained reduction in daily food intake, which only prevented weight gain and fat deposition. In a subsequent 7-wk study of the effects of salmon calcitonin administration in diet-induced obese rats (8), only the high-fat powdered food was provided during the test period; vehicle-treated obese rats maintained a relatively stable body weight and adiposity during the test period, and none of 10 salmon calcitonin dosing strategies administered to a second group of matched obese rats produced a sustained reduction in daily food intake for more than 5 days, although body weight and adiposity in these rats were reduced across the period by 9 and 22%, respectively. Here we used the latter experimental approach to determine whether intermittent intraperitoneal administration of PYY(3-36) can produce a similar sustained ∼20% reduction in daily food intake in diet-induced obese rats when body weight and adiposity are decreasing in response to PYY(3-36) administration. Our goal was to define the lowest dose and frequency of PYY(3-36) administration that would induce a sustained 15–25% reduction in average daily food intake for at least 2 wk. A dosing regimen was usually changed after observing two or more consecutive days of daily food intake reductions either below or above this criterion. We and others have provided evidence that PYY(3-36) produces dose-dependent malaise in rodents (7, 19) and humans (12). It is also well known that continuous or frequent administration of high doses of agonists can produce receptor downregulation and tolerance. This is why our goal was to define the lowest dose and frequency of PYY(3-36) administration that can produce a sustained decrease in daily food intake.
Experimental procedures.
Of the 92 obese rats implanted with peritoneal catheters, the 45 most weight-stable rats (708 ± 10 g body wt; 243 ± 7 g body fat; 291 ± 15 days of obesity induction) were selected for experimentation. Each was housed individually in a metabolism cage modified to include a stainless steel side compartment with a 3-cm diameter hole in the base. Below the hole was a food cup for powdered food. For 32 of these rats, the food bowl was fixed to a digital balance, which was connected to a computer through a code-activated switch (CAS-161; Western Telematic, Irvine, CA). Output from each balance was monitored at ∼20-s intervals, and changes in food container weight were recorded. Data were processed daily to determine the amount of food ingested each hour, and total food intake cumulated hourly. For the other 13 animals, daily food intake was determined by manually weighing the food container at the start and end of each day. Thus, daily food intakes were measured in all 45 rats and cumulative hourly intakes were determined each day in 32 of the 45 rats.
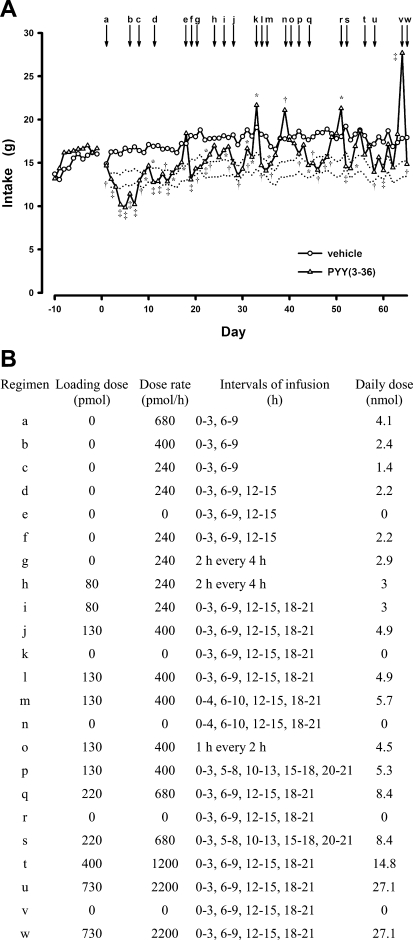
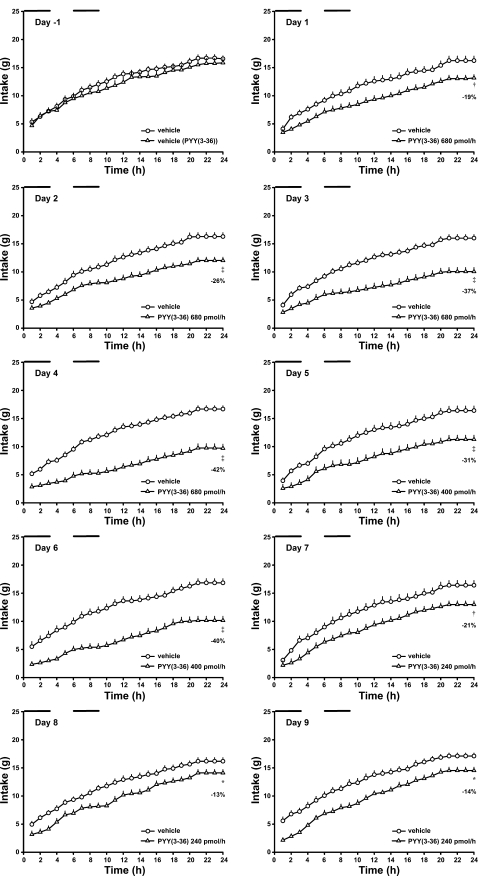
Each rat had its intraperitoneal catheter connected to a 40-cm length of tubing passed through a protective spring coil connected between the light-weight harness worn by the rat and a single-channel infusion swivel (Instech Laboratories, Plymouth Meeting, PA), which allowed free movement of each rat in its individual cage. Rats were provided powdered high-fat solid food (45% calories from fat, 4.73 kcal/g, D12451M; Research Diets) and water each day from 1100 to 0800 the next morning (dark period was from 1100–2300). Experimental setup and routine maintenance were performed each day between 0800 and 1100. Animals were allowed an additional 2 wk to adapt to tethering and experimental conditions. During an initial 10-day baseline period, all rats received an intraperitoneal infusion of vehicle (0.15 M NaCl, 0.1% BSA; 0.9 ml/h) during intervals 0–3 and 6–9 h of the dark period (1100–1400 and 1700–2000, respectively). Rats were weighed at the beginning and end of the baseline period, and their total body fat was determined by QMR at the end of the baseline period. Animals were then divided into two groups, one to receive vehicle and the other, PYY(3-36). Groups were matched for average daily caloric intake during the last 3 days of the baseline period, weight gain during the baseline period, and body weight and fat mass at the end of the baseline period. Groups to receive vehicle (n = 23) and PYY(3-36) (n = 22) had distributions of body weight (661 ± 17 vs. 670 ± 11 g), fat mass (161 ± 12 vs. 170 ± 7 g), lean mass (415 ± 6 vs. 414 ± 7 g), weight gain during the baseline period (−9 ± 4 vs. −8 ± 3 g), and average daily food intake (16.0 ± 0.6 vs. 16.4 ± 0.6 g) that were not statistically different (P > 0.05, Fig. 1A). Cumulative hourly food intakes in these groups on the last day of the baseline period (day −1) were also not different (Fig. 2).
Fig. 1.
Effects of intermittent intraperitoneal infusions of peptide YY(3-36) [PYY(3-36)] on daily food intake in diet-induced obese rats that had ad libitum access to a high-fat solid food. A: during a 10-day baseline period (days −10 to −1) rats (n = 45) received intraperitoneal infusions of vehicle during intervals 0–3 and 6–9 h of the dark period. During the 9-wk treatment period, separate groups of rats received intraperitoneal infusions during the same periods of either vehicle (n = 19–23) or PYY(3-36) (n = 13–20) at 15 different dosing regimens. Dotted lines border a range in food intake that is 15–25% less than that observed in vehicle-treated control rats. B: dosing regimens. Some dosing regimens employed a loading dose of PYY(3-36) administered just before food presentation at the onset of the dark period (time 0). Values are means ± SE. *P < 0.05, †P < 0.01, ‡P < 0.001 vs. vehicle.
Fig. 2.
Effects of intermittent intraperitoneal infusions of PYY(3-36) on cumulative hourly food intake in diet-induced obese rats during the last day of the baseline period (day −1) and first 9 days of PYY(3-36) treatments. Data are from the experiment described in Fig. 1. Time 0, start of 12-h dark period. Horizontal bars indicate periods of infusion. Values are means ± SE. *P < 0.05, †P < 0.01, ‡P < 0.001 vs. vehicle.
On the 1st day of treatment, PYY(3-36) was infused intraperitoneally at 17 pmol·kg−1·min−1 (680 pmol/h) during intervals 0–3 and 6–9 h of the dark period. We previously determined that this dosing regimen produced a sustained reduction in daily caloric intake and prevented weight gain in diet-induced obese rats consuming both the high-fat solid diet and Ensure (9). On subsequent days, dosing level and/or pattern of PYY(3-36) administration was adjusted, as necessary, in an attempt to define the lowest dose and frequency of PYY(3-36) administration that would induce a sustained 15–25% reduction in average daily caloric intake for ≥ 2 wk, compared with average daily food intake in rats administered vehicle at the same infusion rate during the same intervals. We chose this 15–25% criterion because we previously observed that intermittent PYY(3-36) administration produced a sustained ∼20% inhibition of daily food intake and prevented weight gain and fat deposition in growing lean and obese rats. Our aim here was to determine whether intermittent intraperitoneal administration of PYY(3-36) can still produce a sustained reduction in daily food intake in diet-induced obese rats when body weight and adiposity are decreasing in response to PYY(3-36) administration. A dosing regimen was usually changed after observing two or more consecutive days of daily food intake reductions either below or above this 15–25% criterion for reduction in daily food intake. We tested the effects of 15 different PYY(3-36) dosing regimens during the 9-wk period (Fig. 1). On five different occasions, PYY(3-36) treatment was discontinued for 1 day to assess whether loss in efficacy of a treatment might be due to receptor downregulation and tolerance to PYY(3-36) administration or activation of a homeostatic response to counteract the inhibitory effect of PYY(3-36) on food intake and energy reserves. Rats were weighed weekly and their body fat was measured by QMR at the end of the 9-wk period. During the experiment, three vehicle-treated rats and seven PYY(3-36)-treated rats were removed due to catheter malfunction (6 rats) and illness or distress (4 rats).
Statistical Analyses
Values are presented as group means ± SE. Data were analyzed by ANOVA. Planned comparisons of treatment means were evaluated by Student's t-tests and paired t-tests. Differences were considered significant if P < 0.05.
RESULTS
Effects of Intermittent Intraperitoneal Infusion of PYY(3-36) on Food Intake, Body Weight, and Adiposity in Diet-Induced Obese Rats
In vehicle-treated rats, daily food intake remained relatively constant across the 9-wk period (Fig. 1A). In the 20 vehicle-treated rats completing the study, body weight, fat mass, and lean mass did not change from the beginning to the end of this period (body wt: 643 ± 17 vs. 656 ± 19 g; fat: 154 ± 10 vs. 148 ± 13 g; lean: 416 ± 7 vs. 418 ± 7 g).
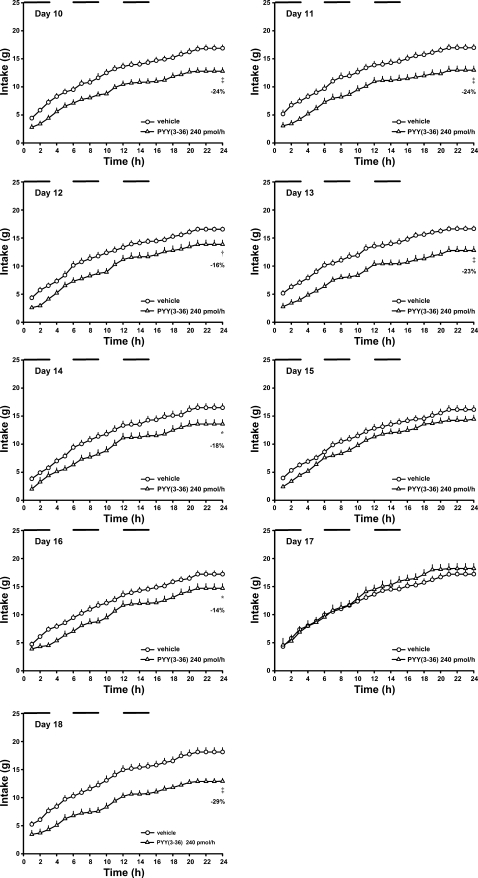
Our goal was to define the lowest dose and frequency of PYY(3-36) administration that would induce a sustained 15–25% reduction in average daily food intake for at least 2 wk. A dosing regimen was usually changed after observing two or more consecutive days of daily food intake reductions either below or above this criterion. Responses to the various dosing regimens were as follows. On days 1–4, PYY(3-36) was administered by infusion at 680 pmol/h during intervals 0–3 and 6–9 h of the dark period (Fig. 1B, regimen a); food intakes were reduced by 19, 26, 37 and 42%, respectively, compared with those of vehicle-treated rats (Figs. 1A and 2; Table 1). The second interval of PYY(3-36) infusion inhibited food intake at least as well as the first interval of infusion. On day 5, PYY(3-36) dose was reduced to 400 pmol/h during intervals 0–3 and 6–9 h of the dark period to determine whether a lower dose would produce a sustained 15–25% reduction in daily food intake (Fig. 1B, regimen b). Food intakes on days 5 and 6 were reduced by 31 and 40%, respectively (Figs. 1A and 2). On day 7, the PYY(3-36) dose was reduced to 240 pmol/h during intervals 0–3 and 6–9 h of the dark period to determine whether a lower dose would produce a sustained 15–25% reduction in daily food intake (Fig. 1B, regimen c). Food intakes on days 7–9 were reduced by 21, 13, and 14%, respectively (Figs. 1A and 2). PYY(3-36) infusion similarly inhibited food intake during infusion intervals on each day. In contrast, food intake between infusion intervals gradually increased during the 3-day period in the PYY(3-36)-treated vs. control rats (Table 1). On day 10, an additional 3-h infusion of PYY(3-36) at 240 pmol/h was introduced during interval 12–15 h in an attempt to attenuate this increase in food intake (Fig. 1B, regimen d). Food intakes on days 10–14 were reduced by 24, 24, 16, 23, and 18%, respectively; intake on day 15 was not different from that observed in control rats, and intake on day 16 was reduced by 14% (Figs. 1A and 3). During the 7-day period, food intake during and between infusion intervals appeared to gradually increase relative to that in control rats (Table 1).
Table 1.
Effects of PYY(3-36) dosing regimens on grams of food consumed in specified periods
| Day | Dose Regimen |
24 h |
1st 3 h
|
Infusion Periods
|
Noninfusion Periods
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Veh | PYY | Veh | PYY | Veh | PYY | Veh | PYY | ||
| −1 | 15.9 | 16.6 | 7.2 | 7.3 | 8.5 | 9.4 | 7.3 | 7.3 | |
| 1 | a | 16.2 | 13.0‡ | 6.9 | 4.9 | 8.6 | 5.9† | 7.7 | 6.9 |
| 2 | a | 16.3 | 12.3‡ | 6.5 | 4.5† | 7.9 | 5.7† | 8.4 | 6.5* |
| 3 | a | 16.0 | 10.3‡ | 7.1 | 4.1‡ | 9.2 | 4.6‡ | 6.8 | 5.7 |
| 4 | a | 16.7 | 10.0‡ | 7.3 | 3.6‡ | 9.5 | 4.0‡ | 7.1 | 6.0 |
| 5 | b | 16.4 | 11.5‡ | 6.7 | 3.5‡ | 8.3 | 4.3‡ | 8.1 | 7.2 |
| 6 | b | 16.8 | 10.5‡ | 7.4 | 3.0‡ | 9.3 | 3.4‡ | 7.5 | 7.1 |
| 7 | c | 16.4 | 13.0† | 6.6 | 3.5† | 8.9 | 5.1† | 7.5 | 7.9 |
| 8 | c | 16.2 | 14.0* | 7.0 | 4.1‡ | 9.0 | 5.4‡ | 7.2 | 8.6 |
| 9 | c | 17.1 | 14.9† | 7.2 | 3.7‡ | 9.3 | 5.0‡ | 7.8 | 9.9† |
| 10 | d | 16.9 | 12.9‡ | 7.3 | 4.3‡ | 10.1 | 6.2‡ | 6.8 | 6.6 |
| 11 | d | 17.0 | 13.0‡ | 7.4 | 4.3‡ | 10.4 | 6.2‡ | 6.6 | 6.8 |
| 12 | d | 16.6 | 13.9† | 6.5 | 4.2‡ | 9.2 | 6.2‡ | 7.3 | 7.7 |
| 13 | d | 16.7 | 13.0‡ | 7.1 | 4.0‡ | 9.3 | 5.6‡ | 7.4 | 7.5 |
| 14 | d | 16.5 | 13.9* | 5.8 | 4.4* | 8.7 | 6.6* | 7.8 | 7.3 |
| 15 | d | 16.2 | 14.7 | 6.3 | 4.7† | 9.6 | 6.8† | 6.5 | 7.8 |
| 16 | d | 17.2 | 15.1* | 7.4 | 4.7‡ | 10.6 | 7.0‡ | 6.6 | 8.1 |
| 17 | e | 17.2 | 18.7 | 7.4 | 6.9 | 10.0 | 10.4 | 7.2 | 8.3 |
| 18 | f | 18.1 | 13.2‡ | 7.6 | 4.5‡ | 10.2 | 5.9‡ | 7.9 | 7.3 |
| 19 | g | 18.0 | 14.6† | 7.2 | 5.2* | 10.9 | 7.3‡ | 7.2 | 7.3 |
| 20 | g | 18.8 | 14.7‡ | 7.6 | 5.5* | 10.0 | 7.2* | 8.8 | 7.5 |
| 21 | g | 17.6 | 14.8† | 6.0 | 5.8 | 10.6 | 6.6‡ | 7.0 | 8.3 |
| 22 | g | 17.8 | 16.0* | 7.3 | 5.7 | 11.8 | 9.9 | 6.0 | 6.0 |
| 23 | h | 17.8 | 17.0 | 7.1 | 6.6 | 11.2 | 9.4 | 6.6 | 7.6 |
| 24 | h | 17.8 | 15.8* | 7.4 | 5.4* | 11.3 | 7.5‡ | 6.6 | 8.2 |
| 25 | i | 18.1 | 16.2* | 6.8 | 5.5 | 11.3 | 9.7 | 6.9 | 6.5 |
| 26 | i | 17.9 | 16.8 | 7.5 | 6.2 | 11.8 | 9.6 | 6.1 | 7.2 |
| 27 | j | 18.2 | 15.0† | 7.6 | 6.3 | 12.1 | 9.2† | 6.1 | 5.8 |
| 28 | j | 17.1 | 13.5‡ | 6.9 | 4.2† | 11.5 | 7.0‡ | 5.6 | 6.5 |
| 29 | j | 18.0 | 14.4‡ | 7.6 | 5.2‡ | 13.1 | 8.3‡ | 5.0 | 6.0 |
| 30 | j | 18.8 | 16.5* | 7.0 | 6.3 | 12.7 | 9.2† | 6.1 | 7.3 |
| 31 | j | 18.1 | 15.7* | 7.2 | 5.8 | 11.6 | 9.7* | 6.5 | 6.0 |
| 32 | k | 19.1 | 21.4* | 7.0 | 9.5* | 11.9 | 14.2* | 7.1 | 7.2 |
| 33 | l | 18.3 | 14.8‡ | 6.8 | 5.6* | 11.1 | 8.0† | 7.2 | 6.8 |
Values are means. Data presented are from the first 33 days of the 9-week experiment. Dosing regimens are as described in Fig. 1. Veh, vehicle: PYY(3-36), peptide YY (3-36).
P < 0.05,
P < 0.01,
P < 0.001 vs. vehicle.
Fig. 3.
Effects of intermittent intraperitoneal infusions of PYY(3-36) on cumulative hourly food intake in diet-induced obese rats during days 10–18 of PYY(3-36) treatments. Data are from the experiment described in Fig. 1. Time 0, start of 12-h dark period. Horizontal bars indicate periods of infusion. Values are means ± SE. *P < 0.05, †P < 0.01, ‡P < 0.001 vs. vehicle.
On day 17, PYY(3-36) treatment was replaced with vehicle (Fig. 1B, regimen e). Food intake in rats that had been receiving PYY(3-36) was not different from that in control rats, yet intake was 24% greater than that observed in the same animals on the previous day when PYY(3-36) was administered (Figs. 1A and 3). This rebound in food intake, which occurred primarily during intervals of infusion (Table 1), indicates that PYY(3-36) had not lost its efficacy during the previous dosing regimen, but that an orexigenic mechanism had been activated to counteract the inhibitory effect of PYY(3-36) on food intake. On day 18, PYY(3-36) was administered using the same dosing regimen as employed on days 10–16 (Fig. 1B, regimen f). PYY(3-36) reduced 24-h food intake by 29% (Figs. 1A and 3) by decreasing food intake during infusion intervals (Table 1). Thus, an intervening day of no treatment restored the inhibitory potency of the dosing regimen on food intake.
On day 19, PYY(3-36) infusion rate remained the same (240 pmol/h) and infusion intervals were changed to six 2-h intervals of infusion each separated by 2 h of no infusion in an attempt to attenuate the increase in food intake that had developed between infusion intervals when the interinfusion interval was 3 h (Fig. 1B, regimen g). [Because of the sheer volume of daily food intake data produced during the 9-wk study, cumulative hourly intakes are not shown for days 19 to 64.] Food intakes on days 19–22 were reduced by 21, 24, 16, and 11%, respectively (Fig. 1A). On days 21 and 22 food intakes during the first 3 h of the dark period were reduced only slightly by PYY(3-36) infusion, and on day 22 food intakes during infusion intervals were not reduced (Table 1). Thus, on day 23, an 80-pmol loading dose of PYY(3-36) was administered just prior to onset of the dark period to elevate tissue levels of PYY(3-36) more quickly at dark onset, when feeding is most active (Fig. 1B, regimen h). Food intake on day 23 was not significantly reduced, while that on day 24 was reduced by 15% (Fig. 1A). These results suggested that frequent PYY(3-36) administration may have produced receptor downregulation and tolerance. On day 25, infusion intervals were changed to four 3-h infusions each separated by 3 h (Fig. 1B, regimen i). Food intake was reduced by 13% on day 25 and not significantly reduced on day 26 (Fig. 1A). These results suggested that this dosing paradigm caused either receptor downregulation and tolerance or further activation of an orexigenic mechanism to counteract the anorexic response to PYY(3-36).
On day 27, the PYY(3-36) loading dose was increased to 130 pmol, the PYY(3-36) infusion rate was increased to 400 pmol/h, and infusion intervals were kept the same (four 3-h infusions separated by 3 h; Fig. 1B, regimen j). Food intakes on days 27–31 were reduced by 19, 24, 23, 14, and 14%, respectively (Fig. 1A). The gradual loss in PYY(3-36) efficacy appeared to be due to a gradual loss in efficacy during infusions (Table 1). On day 32, PYY(3-36) treatment was again replaced with vehicle (Fig. 1B, regimen k). Food intake in rats that had been receiving PYY(3-36) was 12% larger than in control rats, and 40% greater than that observed in the same animals on the previous day when PYY(3-36) was administered (Fig. 1A). This rebound in food intake during the 1-day washout, which occurred primarily during infusion intervals (Table 1), indicates that PYY(3-36) had not lost its efficacy during the previous dosing regimen but that an orexigenic mechanism had been activated to counteract the inhibitory effect of PYY(3-36) on food intake. On day 33, PYY(3-36) was administered using the same strategy as employed on days 27–31 (Fig. 1B, regimen l). PYY(3-36) reduced 24-h food intake by 21% (Figs. 1A) by decreasing food intake during infusion intervals (Table 1). Thus, an intervening day of no treatment was sufficient to restore the anorexic potency of the dosing regimen.
On day 34, loading dose and PYY(3-36) infusion rate (130 pmol and 400 pmol/h) remained the same, while infusion intervals were changed to 0–4, 6–10, 12–15, and 18–21 h (Fig. 1B, regimen m). The longer infusion intervals and shorter interinfusion interval during the dark period were chosen to minimize possible compensatory hyperphagia between infusions. Food intakes on days 34 and 35 were reduced by 21 and 11%, respectively, while intakes on days 36 and 37 were not different from controls (Fig. 1A). On day 38, PYY(3-36) treatment was again replaced with vehicle (Fig. 1B, regimen n). Food intake in rats that had been receiving PYY(3-36) was 17% larger than in control rats and 17% greater than that observed in the same animals on the previous day when PYY(3-36) was administered (Fig. 1A). This rebound in food intake during the 1-day washout indicates that PYY(3-36) had not lost its efficacy during the previous dosing regimen but that an orexigenic mechanism had been activated to counteract the inhibitory effect of PYY(3-36) on food intake.
On day 39, loading dose and PYY(3-36) infusion rate (130 pmol and 400 pmol/h) remained the same, while infusion intervals were changed to eleven 1-h intervals, each separated by 1 h of no infusion (Fig. 1B, regimen o). This dosing regimen was tested because we previously showed that a similar regimen in lean rats produced a sustained suppression in daily food intake for 10 days (5). Food intakes on days 39 and 40 were not different from controls (Fig. 1A). On day 41, infusion intervals were changed to 0–3, 5–8, 10–13, 15–18, and 20–21 h (Fig. 1B, regimen p). Food intake on day 41 was reduced by 13%, while intake on day 42 was not different from controls (Fig. 1A). These results suggested that the frequent PYY(3-36) infusions may have produced receptor downregulation and tolerance or further activation of an orexigenic mechanism to counteract the anorexic response to PY(3-36).
On day 43, the PYY(3-36) loading dose was increased to 220 pmol, the PYY(3-36) infusion rate was increased to 680 pmol/h, and infusion intervals were changed to four 3-h intervals each separated by 3 h of no infusion (Fig. 1B, regimen q). Food intakes on days 43–47 were reduced by 17, 19, 23, 15, and 17%, respectively, while intakes on days 48 and 49 were not different from controls (Fig. 1A). The loss in PYY(3-36) efficacy across days appeared to be due to a loss in efficacy during infusions (data not shown). On day 50, PYY(3-36) treatment was again replaced with vehicle (Fig. 1B, regimen r). Food intake in rats that had been receiving PYY(3-36) was 16% larger than in control rats, and 17% greater than that observed in the same animals on the previous day when PYY(3-36) was administered (Fig. 1A). This rebound in food intake during the 1-day washout, which occurred primarily during infusion intervals (data not shown), indicates that PYY(3-36) had not lost its efficacy during the previous dosing regimen, but that an orexigenic mechanism had been activated to counteract the inhibitory effect of PYY(3-36) on food intake.
On day 51, PYY(3-36) was administered at a loading dose of 220 pmol and at an infusion rate of 680 pmol/h during intervals of 0–3, 5–8, 10–13, 15–18, and 20–21 h (Fig. 1B, regimen s). Food intakes on days 51 and 52 were reduced by 25 and 21%, respectively, while intakes on days 53 and 54 were not different from controls (Fig. 1A).
On day 55, the PYY(3-36) loading dose was increased to 400 pmol, the PYY(3-36) infusion rate was increased to 1,200 pmol/h, and infusion intervals were changed to four 3-h intervals each separated by 3 h of no infusion (Fig. 1B, regimen t). Food intakes on days 55 and 56 were not different from controls (Fig. 1A). On day 57, the PYY(3-36) loading dose was increased to 730 pmol and the PYY(3-36) infusion rate was increased to 2,200 pmol/h, while infusion intervals were kept the same (Fig. 1B, regimen u). Food intakes on days 57 and 59 were reduced by 21 and 22%, respectively, while intakes on days 58, 60, 61, and 62 were not different from controls (Fig. 1A). On day 63, PYY(3-36) treatment was again replaced with vehicle (Fig. 1B, regimen v). Food intake in rats that had been receiving PYY(3-36) was 55% larger than in control rats, and 51% greater than that observed in the same animals on the previous day when PYY(3-36) was administered (Fig. 1A). This large rebound in food intake during the 1-day washout, which occurred primarily during infusion intervals (data not shown), indicates that PYY(3-36) had not lost its efficacy during the previous dosing regimen but that a potent orexigenic mechanism had been activated to counteract the inhibitory effect of PYY(3-36) on food intake. On day 64, PYY(3-36) was administered using the same strategy as employed on days 57–62 (Fig. 1B, regimen w), and food intake was reduced by 21% (Fig. 1A). Thus, an intervening day of no treatment was sufficient to restore the anorexic potency of the dosing regimen.
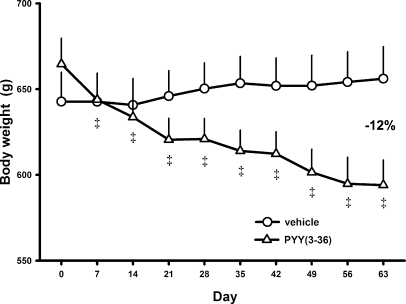
At the end of the 9-wk experiment, body weight, fat mass, and lean mass in the 20 vehicle-treated rats completing the study was unchanged from the beginning to the end of the experiment (body wt: 643 ± 17 vs. 656 ± 19 g; fat mass: 154 ± 10 vs. 148 ± 13 g; lean mass: 416 ± 7 vs. 418 ± 7 g). In contrast, in the 15 PYY(3-36)-treated rats completing the study, body weight was reduced by 12% from 672 ± 15 to 594 ± 15 g (P < 0.001; Fig. 4), fat mass was reduced by 43% from 169 ± 9 to 96 ± 7 g (P < 0.001), and lean mass was unchanged (407 ± 8 vs. 419 ± 7 g, P > 0.05).
Fig. 4.
Effects of intermittent intraperitoneal infusions of PYY(3-36) on body weight in diet-induced obese rats. Data are from the experiment described in Fig. 1. Values are means ± SE for the vehicle-treated rats (n = 20) and the PYY(3-36)-treated rats (n = 15) completing the study. ‡P < 0.001 vs. day 0 value within same treatment group.
DISCUSSION
We previously reported that in lean rats intermittent intravenous infusion of PYY(3-36) for 10 days produced a sustained reduction in daily caloric intake of ∼20%, and prevented weight gain (5). We subsequently reported that in diet-induced obese rats consuming two palatable foods intermittent intraperitoneal infusion of PYY(3-36) for 21 days produced a similar sustained reduction in daily food intake and prevented weight gain and fat deposition (9). The goal in treating obese humans, however, is reducing body weight and adiposity, not just preventing weight gain and fat deposition. Our aim here was to determine whether intermittent intraperitoneal administration of PYY(3-36) can produce a similar sustained reduction in daily food intake in diet-induced obese rats when body weight and adiposity are decreasing in response to administration of the substance.
Our results demonstrate several important properties of the effects of intermittent intraperitoneal administration of PYY(3-36) on food intake, body weight, and adiposity in the diet-induced obese rats. First, vehicle-treated obese rats had relatively stable food intake, body weight, and adiposity during the 9-wk test period. Second, none of the 15 PYY(3-36) dosing regimens administered in succession during the 9-wk period produced a sustained 15–25% reduction in daily food intake for more than 5 days, although body weight and total fat mass were reduced across the period by 12 and 43%, respectively. Third, the declining inhibitory effect of PYY(3-36) on daily food intake when the interinfusion interval was ≥ 3 h appeared to be due in part to an increase in food intake between infusions. Fourth, the declining inhibitory effect of PYY(3-36) on daily food intake when interinfusion interval was < 3 h suggested possible receptor downregulation and tolerance to frequent PYY(3-36) administration; however, food intake significantly increased when PYY(3-36) treatments were discontinued for 1 day following apparent loss in treatment efficacies. These results demonstrate the development of a potent homeostatic response to increase food intake when PYY(3-36) reduces food intake and energy reserves in diet-induced obese rats. They further suggest that reducing body weight and adiposity is more difficult to accomplish than attenuating weight gain and fat deposition.
The inability of intermittent infusion of PYY(3-36) to produce a sustained reduction in daily food intake in the present study, similar to that observed in our previous studies in lean (5) and diet-induced obese rats (9), is not likely due to differences in dosing regimens tested. In our previous work using lean rats, a daily PYY(3-36) dose of 20 nmol/kg was given as eleven 1-h infusions (1.8 nmol·kg−1·h−1) each separated by 1 h. In our previous study using obese rats, daily doses of PYY(3-36) from 4 to 11 nmol/kg were given during the dark period as two 3-h infusions (0.6 to 1.8 nmol·kg−1·h−1) separated by 3 h. In the present study in weight-stable obese rats, daily doses of PYY(3-36) from 2 to 40 nmol/kg were administered at rates of 0.36 to 3.3 nmol·kg−1·h−1 at various frequencies (Fig. 1B), which included those used in our previous studies.
No previous study has demonstrated that chronic administration of PYY(3-36) can produce a sustained reduction in food intake, body weight, and adiposity in animals or humans. Several studies have shown that continuous administration of PYY(3-36) to rodents by osmotic minipump produces a transient reduction in daily food intake and weight gain (1, 29, 34, 36, 37). Continuous infusion of other anorexigenic substances has also been reported to produce transient reductions in daily food intake (10, 13, 22–24, 26, 28, 32). It is unclear from these studies whether transient responses were due to receptor downregulation and tolerance or redundancy and plasticity in the energy regulatory system. Our earlier studies employing intermittent delivery of PYY(3-36) to lean and obese rats (5) suggest that transient feeding responses to continuous infusion of PYY(3-36) were due in part to receptor downregulation and tolerance. Here we provide evidence that transient feeding responses to intermittent PYY(3-36) administration in mature, weight-stable obese rats were likely due to redundancy and plasticity in the energy regulatory system, rather than to downregulation of PYY(3-36) receptors. This is supported by our finding that food intake significantly increased on each of five occasions when PYY(3-36) treatments were discontinued for 1 day following apparent loss in treatment efficacies. If loss of PYY(3-36) receptors was primarily responsible for loss in PYY(3-36) efficacy, then discontinuing PYY(3-36) treatment should have had little, if any, effect on food intake. Rather, our results suggest that a potent homeostatic mechanism was activated to increase food intake when PYY(3-36) treatments reduced food intake and energy reserves. A similar mechanism may have been activated to offset salmon calcitonin's potent inhibitory effect on food intake in our recent study (8). The nature of this mechanism remains to be determined. One possibility is that early PYY(3-36)-induced reductions in daily food intake and adiposity elicit a delayed compensatory response to restore energy balance mediated by a reduction in leptin signaling to the brain (2, 15). In lean rats, coinfusion of leptin has been reported to extend the anorexic response to continuous infusion of PYY(3-36) (35). It remains to be determined whether intermittent coadministration of leptin with either PYY(3-36) or salmon calcitonin can produce a sustained reduction in daily food intake and adiposity in diet-induced obese rats.
There is a growing consensus that multidrug therapy aimed at different components of the food intake regulatory system will be required to produce significant weight loss in obese individuals (4, 11, 14, 16, 21, 33). Roth et al. (30) recently reported that in rats prone to diet-induced obesity, continuous coadministration of gastrointestinal peptides amylin and PYY(3-36) for 2 wk synergistically reduced food intake and additively reduced body weight across the treatment period. However, data were reported only as vehicle-corrected percentage changes in food intake and body weight. Measurements of absolute food intake, body weight, and adiposity before and after treatments were not provided. Daily food intakes also were not reported. So it is unclear whether subjects were obese or whether treatments produced real weight and fat loss, which would be important conditions and goals in human trials. Rather, the data they do report suggest that 1) the obesity-prone rats were preobese at start of treatments (rats were ∼500 g), 2) vehicle-treated rats gained significant weight during the treatment period (rats were provided free access to an obesity inducing diet before and throughout drug treatment), and 3) coadministration of amylin and PYY(3-36) likely only attenuated weight gain. As discussed above, our work suggests that it is easier to attenuate weight gain in lean and obese individuals than to produce significant weight loss. Thus, we believe that the experimental design and method of data reporting employed by Roth et al. (30) significantly overestimated the ability of their test substances to inhibit food intake and promote weight loss.
Perspectives and Significance
There is an extensive body of evidence indicating that a sustained reduction in caloric intake in obese individuals will produce steady weight loss. Thus, an important early step in discovery of antiobesity drugs is defining methods of administration of anorexigenic agents that can produce a sustained reduction in daily food intake and body weight in obese experimental animals. We previously showed that infusion of PYY(3-36) rapidly and potently suppresses food intake in rats. In contrast, several days of PYY(3-36) administration would likely be required to produce a measurable reduction in body weight. Thus, monitoring the effects of specific PYY(3-36) dosing strategies on daily food intake in diet-induced obese rats enabled us to quickly rule out potentially ineffective strategies for producing significant weight loss. Because we were able to measure the instantaneous effects of dosing strategy on pattern of food intake each day, we were able to adjust PYY(3-36) dosing daily in the same animals in an attempt to optimize reduction in daily food intake. This approach is not the only way to test the effects of chronic administration of PYY(3-36) on food intake and body weight, but it certainly is a rapid and novel way to screen many possible dosing paradigms in the same animals. Such an approach is commonly used in clinical settings, in which dose and schedule of drug administration are adjusted across time in individual patients in an attempt to produce a desired response. Here we showed that none of the 15 dosing strategies tested in the obese rats during a 9-wk period produced a sustained 15–25% reduction in daily caloric intake for > 5 days, although body weight and adiposity were reduced across the test period by 12 and 43%, respectively. Our results further suggest that PYY(3-36)'s inability to sustain a reduction in food intake is due to activation of a potent homeostatic response to counteract PYY(3-36)'s inhibitory effect on food intake, rather than to downregulation of receptors.
GRANTS
The research was supported by the Medical Research Service of the Department of Veterans Affairs, National Institutes of Health grants DK-55830, DK-73152, DK-70851, and P20-RR-16469, and the Canadian Institutes of Health Research.
Acknowledgments
We thank Dean Heimann, Rani Padanilam, and Amanda Ross for their technical assistance.
Present address of P. K. Chelikani: Dept. of Production Animal Health, Faculty of Veterinary Medicine, University of Calgary, HRIC 2C66, 3330 Hospital Drive NW, Calgary, AB T2N 4N1, Canada.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams SH, Lei C, Jodka CM, Nikoulina SE, Hoyt JA, Gedulin B, Mack CM, Kendall ES. PYY[3-36] administration decreases the respiratory quotient and reduces adiposity in diet-induced obese mice. J Nutr 136: 195–201, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Al Barazanji KA, Arch JR, Buckingham RE, Tadayyon M. Central exendin-4 infusion reduces body weight without altering plasma leptin in (fa/fa) Zucker rats. Obes Res 8: 317–323, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase peptide synthesis. Mol Biotechnol 33: 239–254, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature 404: 672–677, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Chelikani PK, Haver AC, Reeve JR Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol 290: R298–R305, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3-36) potently inhibits food intake in rats. Endocrinology 146: 879–888, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3-36) on conditioned taste aversion in rats. Peptides 27: 3193–3201, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Chelikani PK, Haver AC, Reidelberger RD. Effects of intermittent intraperitoneal infusion of salmon calcitonin on food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol 293: R1798–R1808, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Chelikani PK, Haver AC, Reidelberger RD. Intermittent intraperitoneal infusion of peptide YY(3-36) reduces daily food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol 293: R39–R46, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Crawley JN, Beinfeld MC. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature 302: 703–706, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology 129: 1430–1436, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res 779: 75–83, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Finer N Pharmacotherapy of obesity. Best Pract Res Clin Endocrinol Metab 16: 717–742, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Friedman JM The function of leptin in nutrition, weight, and physiology. Nutr Rev 60: S1–S14, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides 33: 360–368, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Grady EF, Bohm SK, Bunnett NW. Turning off the signal: mechanisms that attenuate signaling by G protein-coupled receptors. Am J Physiol Gastrointest Liver Physiol 273: G586–G601, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Gura T Obesity research: new data on appetite-suppressing peptide challenge critics. Science 306: 1453–1454, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Ladenheim EE Peptide YY(3-36) and food intake: a peptide waiting for a paradigm? Am J Physiol Regul Integr Comp Physiol 293: R37–R38, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Levin BE Factors promoting and ameliorating the development of obesity. Physiol Behav 86: 633–639, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lukaszewski L, Praissman M. Effect of continuous infusions of CCK-8 on food intake and body and pancreatic weights in rats. Am J Physiol Regul Integr Comp Physiol 254: R17–R22, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol 293: R1855–R1863, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 30: 1332–1340, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Morley JE, Flood JF. An investigation of tolerance to the actions of leptogenic and anorexigenic drugs in mice. Life Sci 41: 2157–2165, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Olsson M, Herrington MK, Reidelberger RD, Permert J, Arnelo U. Comparison of the effects of chronic central administration and chronic peripheral administration of islet amyloid polypeptide on food intake and meal pattern in the rat. Peptides 28: 1416–1423, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Persson P Highlights from the literature: What underlies the inability of several labs to reproduce the appetite suppressing effects of peptide YY(3-36) [PYY(3-36)]? Physiology 21: 4–5, 2006. [Google Scholar]
- 28.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51: 1337–1345, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Pittner RA, Moore CX, Bhavsar SP, Gedulin BR, Smith PA, Jodka CM, Parkes DG, Paterniti JR, Srivastava VP, Young AA. Effects of PYY[3-36] in rodent models of diabetes and obesity. Int J Obes Relat Metab Disord 28: 963–971, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, Weyer C, Parkes DG. Combination therapy with amylin and peptide YY[3-36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology 148: 6054–6061, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Salva LP, Garcia Vicente JA, Costa PJ, Lucio MP. Causes and problems of nonresponse or poor response to drugs. Drugs 51: 552–570, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Seeley RJ, Burklow ML, Wilmer KA, Matthews CC, Reizes O, McOsker CC, Trokhan DP, Gross MC, Sheldon RJ. The effect of the melanocortin agonist, MT-II, on the defended level of body adiposity. Endocrinology 146: 3732–3738, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev 85: 1131–1158, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-Sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature 430: 1, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Unniappan S, Kieffer TJ. Leptin extends the anorectic effects of chronic PYY(3-36) administration in ad lib fed rats. Am J Physiol Regul Integr Comp Physiol 295: R51–R58, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unniappan S, McIntosh CH, Demuth HU, Heiser U, Wolf R, Kieffer TJ. Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia 49: 1915–1923, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Vrang N, Madsen AN, Tang-Christensen M, Hansen G, Larsen PJ. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 291: R367–R375, 2006. [DOI] [PubMed] [Google Scholar]