Abstract
Recent awareness of cardiovascular diseases as a number one killer of the middle-aged women has prompted interest in sex differences leading to heart failure (HF). Therefore, we evaluated cardiac function in female and male mice following myocardial infarction (MI) using the Millar pressure-volume (P-V) conductance system in vivo, at time points corresponding to early (2 wk), late compensatory hypertrophy (4 wk), and decompensation (10 wk) to HF. A significant deterioration of the load dependent and independent hemodynamic measurements occurred in both female and male mice during the early phase of hypertrophy. Later, compensatory hypertrophy was marked by a normalization of volumes to control levels in females compared with males. The most notable differences between sexes occurred in the measurements of cardiac contractility during the decompensation to HF. In females, there was a significant improvement in contractility compared with males, which was apparent in the load-independent measurements of preload recruitable stroke work (10 wk post-MI, female = 48.7 ± 8.0 vs. male = 25.2 ± 1.8 mmHg, P < 0.05) and maximum dP/dt vs. maximum end-diastolic volume (10 wk post-MI, female=359 ± 58 vs. male=149 ± 28 mmHg·s−1·μl−1, P < 0.05). Despite these differences, there were no differences in the heart weight to body weight ratio and infarct size between the sexes. These data demonstrate that compensatory hypertrophy is associated with an improvement in contractility and a delayed decompensation to HF in females. However, compensatory hypertrophy in males appears to be undermined by a steady decline in contractility associated with decompensation to HF.
Keywords: contractility, pressure-volume loops
damage to the myocardium following myocardial infarction (MI) is associated with compensatory responses such as left ventricular (LV) hypertrophy and activation of the sympathetic nervous system to maintain cardiac output. Eventually, these changes are inadequate and contractile dysfunction along with subsequent chamber dilation underlie the transition to heart failure (HF) (11, 32).
There are known sex-related responses to ischemia following myocardial infarction (MI) ranging from symptoms, diagnoses, preventions, courses of disease and outcomes of existing therapies. Women have more diverse symptoms than men, which are the major causes for delayed diagnoses and treatments (18, 29, 37). Also, women have poorer outcomes for a number of clinical complications such as a non-Q wave MI and invasive percutaneous transluminal coronary angioplasty (16, 25). Despite these sex differences in incidence, manifestation and outcome, clinical data on cardiac function changes following MI are obscured by numerous baseline differences such as age, risk factors, symptoms, and the presence of coexisting diseases (18, 24, 37). Therefore, the sex differences in cardiac function have not been well established.
On the other hand, women are known to benefit from the cardioprotective actions of sex hormones which discontinue with the onset of menopause (21, 24). Estrogen indirectly affects cardiac function by increasing elastin and decreasing collagen accumulation in blood vessels. In addition, estrogen increases the production of nitric oxide synthases, which catalyzes the oxidation of l-arginine to nitric oxide to increase vasodilation (24). Estradiol inhibits LV hypertrophy, whereas testosterone facilitates it. Even later in life, the prevalence of HF is lower in women than in men.
Animal studies have demonstrated cardioprotective effect in females with a better survival following MI and a slower transition to HF (2, 3, 5, 9, 23). The incidence of cardiac rupture is lower in females than males due to reduced muscle tensile strength during infarct expansion (12). There is a sex-related dimorphic response to hypoxia with differences in the translocation of the transcription factor, hypoxia inducible factor-1α, to the nucleus in females compared with males following MI (41). In addition, the pattern of LV remodeling in female rats differs with a smaller increase in hypertrophy of the viable portions of the LV, but comparable LV cavity enlargement and systolic dysfunction. Despite similar infarct size, females have less abnormal LV diastolic filling compared with males (21). However, there are no reports to date of sex-related deterioration of cardiac function in post-MI mice.
Cardiac function is thought to be a stronger predictor of long-term prognosis rather than the extent of coronary artery disease (24). Thus, our goal was to undertake a comprehensive assessment of sex-related changes in global cardiac function during the adaptive and maladaptive responses in young adult mice following MI, using the Millar pressure-volume (P-V) conductance system in vivo (1, 4, 10, 22, 40). Thus, a better understanding of physiological and pathophysiological function during the transition to HF following MI in mice will provide the necessary groundwork for further mechanistic studies aimed at dissecting sex differences.
METHODS
Experiments were conducted in accordance with the Institutional Animal Care and Use Committee and National Institutes of Health guidelines.
Animals.
Fifty eight 3–4 mo old, sexually mature female and male B6SJL outbred mice weighing 20–25 g were used: 26 females and 32 males. Six females were designated as control without coronary artery ligation and 20 females underwent coronary artery ligation. There were 2 acute deaths, but 18 (90%) of female mice survived to their respective end points. Seven males were designated as control and 25 males underwent coronary artery ligation. In the males, there were 3 acute deaths, 1 mouse died 1 wk post-MI, but 21 (∼88%) mice survived to their respective end points. On the basis of post-procedural inspection, 2 (∼8%) males did not develop MI, and an additional two mice (one control and one 4 wk post-MI) did not yield PV loops with an end-systolic pressure higher than 60 mmHg in the open chest configuration, so they were excluded from final analysis. There were eight experimental groups of female and male mice (n = 6): control, 2 wk, 4 wk, and 10 wk post-MI. Mice were kept in a temperature- and humidity-controlled environment with standard chow and water given ad libitum and on a 12:12-h light-dark cycle.
Surgical preparation.
Anesthesia was induced with 3% isoflurane inhaled in a closed chamber and an intraperitoneal injection of etomidate (10 mg/kg). After intubation, mice were connected to a rodent ventilator with a stroke volume 0.2–0.4 ml/min and respiration rate 135 breaths/min. A plane of anesthesia for surgery was maintained by delivery of 1.5% isoflurane through a vaporizer with 100% oxygen. Both coronary artery ligations and pressure volume loop measurements were performed under the same anesthesia, and a Zeiss dissecting microscope maintaining aseptic conditions on a heated surgical pad at 40 C.
Myocardial infarction.
A left thoracotomy was performed and the left main coronary artery ligated with 8-0 prolene suture 1–2 mm below the ostium as previously described (31, 34). Myocardial blanching indicated a lack of perfusion. The chest cavity was closed in three layers (intercostal muscles, pectoral muscles, and skin) with 7-0 silk sutures. Mice were gradually weaned off the respirator, and once spontaneous respiration resumed, the endotracheal tube was removed and were placed in a cage on a heating pad until fully conscious.
Pressure-volume loop analyses.
A standard 1.4 French pressure-conductance catheter with 4.5-mm signal electrode spacing (SPR-839; Millar Instruments, Houston, TX) was volume calibrated by submerging it into heparin-treated murine blood in a series of cylindrical holes with known diameters (1.5 to 4.0 mm) and 1 cm deep. This catheter was inserted into the right carotid artery to measure baseline arterial pressure, then advanced retrograde into the LV to record closed chest hemodynamics with the ARIA Pressure Volume Conductance System (Millar Instruments). Then, a small incision in the diaphragm was made to record baseline open chest hemodynamics and to ensure stability of the preparation prior to recording hemodynamics during thoracic vena cava (TVC) occlusion (14, 17, 31). Subsequently, parallel conductance was determined by a 10-μl injection of 15% saline into the right femoral vein to establish the offset due to the conductivity of structures external to the blood pool (13, 38). Afterward, mice were euthanized with an overdose of 5% isoflurane, their whole hearts were weighed and used for infarct quantification.
Pressure-volume (P-V) loops were analyzed with the PVAN 3.4 software package (Millar Instruments) with the conversion of relative volume units (RVU) to absolute volume by applying the formula (slope of 3.213·RVU − intercept of 8.867) based on initial catheter calibration. The major hemodynamic parameters collected were heart rate (HR), end-systolic pressure (ESP), end-diastolic pressure (EDP), end systolic volume (ESV), end diastolic volume (EDV), stroke volume (SV), stroke work (SW), cardiac output (CO). ejection fraction (EF), maximum first derivative of change in systolic pressure rise with respect to time (dP/dtmax), maximum first derivative of change in diastolic pressure fall with respect to time (dP/dtmin), time constant of the fall in ventricular pressure by the Glantz method, as the regression of dP/dt vs. pressure (tau-G). Preload adjusted maximal power (PAMP) is the maximum value of power during cardiac cycle over EDV2 (33). Arterial elastance (Ea) is an index of afterload (ESP/SV) (30, 40). Cardiac index (CI) was calculated by CO/body weight and total peripheral resistance (TPR) calculated as mean arterial pressure/CO·1,000. On the basis of the loops collected during the transient occlusions of TVC in the open chest, the following parameters were derived: end systolic pressure volume relationship (ESPVR), preload recruitable stroke work (PRSW), maximum dP/dt vs. maximum EDV (dP/dtmax vs. EDVmax). The volume intercept (Vint) represented the x-axis intercept of the slope ESPVR and cardiac contractile efficiency (CCE), is the ratio of external work over the pressure volume area (potential energy+external work).
Infarct quantification.
Duplicate 1 mm mid-LV sections were cut and incubated with 1% triphenyltetrazolium chloride for 20 min at 37°C, then fixed in 10% buffered formalin. The infarct size was determined by planimetry of the infarct zone (white) and expressed as a percentage of the total LV area on digital images at ×10 magnification (Advanced SPOT Diagnostic Instruments) (31, 36).
Statistical analysis.
The differences in the means were tested using ANOVA. Three-way ANOVA was used to test influence of three independent variables: sex, infarct, and baseline hemodynamic data collected in the closed vs. open chest at each time point post-MI. Two-way ANOVA was used to test influence of two independent variables: sex and infarct at each time point post-MI. The Holm-Sidak method was used for post hoc analysis and to test for statistical significance (P < 0.05).
RESULTS
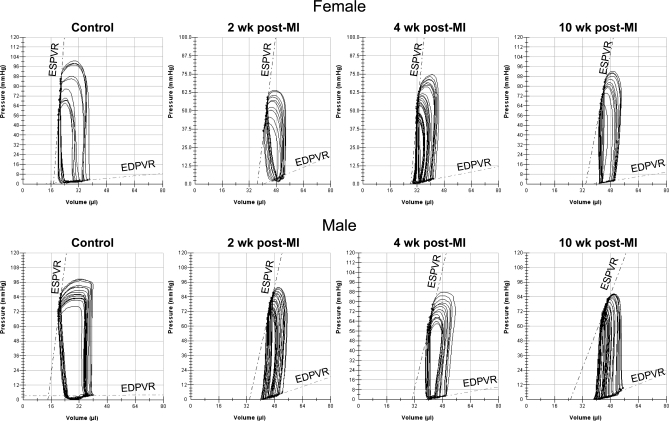
Overall cardiac function declined in post-MI mice compared with control mice, which was demonstrated in the series of P-V loops obtained during transient TVC occlusions (Fig. 1). A characteristic right shift in the loops and a decline in the amplitude of the pressure signal indicated a greater LV operating volume due to dilation of the chamber and a decrease in contractility following MI. During early compensation (2 wk post-MI), the response was similar in females and males with a notable right shift of the P-V loops relative to controls. During late compensation (4 wk post-MI), there was a substantial left shift of the P-V loops in females compared with males. At decompensation (10 wk post-MI), the right shift of the P-V loops was more prominent in males compared with females. Furthermore, additional signs of HF; rapid breathing, cyanosis of the extremities, and enlargement of the spleen and ascites were noted in 10 wk post-MI mice.
Fig. 1.
Representative left ventricular pressure-volume (P-V) loops from control, 2, 4, and 10 wk post-myocardial infarction (MI) mice following thoracic vena cava (TVC) occlusion in the open chest configuration. ESPVR, end-systolic pressure volume relationship; EDPVR, end-diastolic pressure volume relationship.
On the basis of measurements in the right carotid artery, the mean arterial pressure declined post-MI in both females (control, 79 ± 14; 2 wk MI, 64 ± 12; 4 wk MI, 53 ± 10; 10 wk MI = 60 ± 15 mmHg, P < 0.05 vs. control) and males (control, 77 ± 11; 2 wk MI, 60 ± 8; 4 wk MI, 71 ± 21; 10 wk MI, 53 ± 13 mmHg, P < 0.05 vs. control).
The load-dependent hemodynamic parameters derived in the closed chest showed that at 2 wk post-MI, there was a statistically significant decline in cardiac function in both female and male mice compared with their respective controls (Table 1). HR fluctuated but was not statistically different among the groups.
Table 1.
Deterioration of cardiac function in mice at 2, 4, and 10 wk following MI
|
Female |
Male
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group/Parameter | Control | 2 wk MI | 4 wk MI | 10 wk MI | Control | 2 wk MI | 4 wk MI | 10 wk MI |
| HR, bpm | 547±22 | 586±23 | 449±19 | 540±25 | 581±23 | 535±21 | 583±18 | 515±31 |
| ESP, mmHg | 94±3 | 92±10 | 70±3 | 88±7 | 93±2 | 81±6 | 82±5 | 82±4 |
| EDP, mmHg | 5.7±0.8 | 10.3±2.0 | 6.2±0.8 | 11.0±2.0 | 7.2±1.0 | 7.5±1.9 | 6.2±1.5 | 10.7±2.4 |
| ESV, μl | 21±2 | 44±4* | 27±2 | 39±4* | 20±2 | 42±3* | 30±5 | 40±5* |
| EDV, μl | 37±2 | 52±4* | 38±2 | 50±5* | 38±3 | 51±4* | 40±4 | 50±4* |
| SV, μl | 16.4±1.6 | 8.4±0.8*‡ | 9.9±1.2* | 10.8±1.5* | 18.2±1.7 | 9.5±0.4† | 10.5±1.0† | 9.7±1.5† |
| SW, mmHg/μl | 1178±119 | 533±62† | 497±85†‡ | 653±125*‡ | 1349±78 | 574±42† | 711±62† | 510±87† |
| CO, ml/min | 8923±784 | 5367±692*‡ | 4263±793† | 5779±768* | 10330±719 | 5107±297† | 6074±570† | 4835±574† |
| CI, μl·min−1·kg−1 | 324±33 | 175±19* | 202±31 | 206±30 | 366±30 | 205±19* | 235±25† | 187±26† |
| EF, % | 43.9±3.2 | 16.3±0.8†‡ | 27.1±5.1 | 21.8±2.4* | 47.5±2.9 | 19.0±1.2† | 27.2±3.4* | 20.3±3.3† |
| dP/dtmax, mmHg/s | 9450±765 | 7304±473* | 4357±284†‡ | 7179±769 | 9681±624 | 5927±556† | 6333±422† | 6248±546† |
| dP/dtmin, mmHg/s | −8975±407 | −7006±539* | 4802±509†‡ | −7282±855 | −8633±353 | −6112±744 | 6147±310* | 6000±367* |
| Tau-G, ms | 8.6±0.4 | 10.2±1.2 | 11.8±1.3 | 10.5±1.4 | 8.2±0.7 | 11.3±2.3 | 12.1±2.3 | 10.2±0.7 |
| PAMP, mWatts/ml2 | 32.9±5.2 | 11.7±1.8† | 16.7±4.1* | 15.1±4.8* | 40.4±5.8 | 10.1±2.0* | 20.4±4.3 | 10.5±1.8* |
| Ea, mmHg/μl | 6.6±0.7 | 11.7±2.0†‡ | 8.3±1.1 | 9.0±1.2 | 5.3±0.6 | 8.6±0.8* | 8.4±1.2* | 9.7±1.6* |
| TPR, mmHg·ml−1·min−1 | 8.4±0.8 | 12.9±2.0 | 14.6±3.1 | 11.3±1.7 | 7.7±0.9 | 11.8±0.5* | 12.7±2.7 | 11.9±2.0* |
Data presented are based on pressure-volume loop measurements collected in the closed chest and are presented as means ± SE. HR, heart rate; ESP, end-systolic pressure; EDP, end-diastolic pressure; ESV, end systolic volume; EDV, end diastolic volume; SV, stroke volume; SW, stroke work; CO, cardiac output; CI, cardiac index; EF, ejection fraction; dP/dtmax, maximum first derivative of change in systolic pressure rise with respect to time; dP/dtmin, maximum first derivative of change in diastolic pressure fall with respect to time; tau-G, time constant of fall in ventricular pressure by Glantz method; PAMP, preload adjusted maximal power; Ea, arterial elastance; TPR, total peripheral resistance changes due to infarct.
P < 0.05,
P < 0.01 and changes due to gender
P < 0.05.
ESP declined by 4 wk and rebounded at 10 wk post-MI in females, while in males, it dropped at 2 wk and remained low. EDP fluctuated but increased in both females and males by 10 wk post-MI. Both ESV and EDV showed similar directional changes in females and males, with increases occurring at 2 wk, normalization at 4 wk, before increasing again at 10 wk post-MI. The increase in ESV was comparable in females and males at 2 wk post-MI relative to control, but it was substantially less by 4 wk (females 28% vs. males 50%), before being similar by 10 wk post-MI (females 85% vs. males 100%). However, the change in EDV was similar between females and males at all time points relative to their respective controls (Table 1).
The load-dependent parameters of systolic function such as SV, SW, and CO showed signs of improvement in females 10 wk post-MI, whereas in males they continued to decline after a transient rebound at 4 wk post-MI. In particular, SW was significantly better in 10 wk post-MI females, after being significantly worse than in 4 wk post-MI males. Similarly, CI significantly decreased initially at 2 wk and remained low in females, but in males, CI continually declined following MI. The decrease in EF was greater in females, but by 10 wk-post MI, it did not differ between females and males (Table 1).
Both dP/dtmax and dP/dtmin demonstrated a significant decline in 2 wk post-MI mice. Both parameters deteriorated to a greater extent in 4-wk post-MI females and were significantly lower than in males. This was followed by a notable rebound in 10-wk post-MI females, and were no longer statistically different compared with controls. Conversely, there was a significant decline in dP/dtmax and dP/dtmin in post-MI males, which persisted without evidence of improvement at 10 wk post-MI.
Tau-G was elevated in both female and male post-MI mice, reaching a peak at 4 wk post-MI. PAMP showed a greater drop in LV contractility in males than in females at 2 wk post-MI, but then it significantly declined in both male and female mice.
Arterial elastance (Ea) was elevated and significantly greater in 2 wk post-MI females compared with males. Ea then declined in females, suggesting an initial vascular response, whereas in males, Ea remained elevated. TPR increased in post-MI females, but it was not statistically significant. In post-MI males, TPR increased significantly, suggesting that a different vascular response exists between sexes that may contribute to impairment of cardiac function during the transition to HF.
The baseline load-dependent hemodynamics obtained in the open chest prior to TVC occlusion were slightly lower than those collected in the closed chest but showed similar directional changes (data not shown). However, the pressures recorded in the ventricles at 10-wk post-MI in female mice were considerably lower, which may be explained by the absence of negative thoracic pressure compared with the closed chest. Only SW differed significantly in 10 wk post-MI female mice between the closed and open chest baseline data. Conversely, the males demonstrated a greater degree of consistency between the closed and open chest baseline data, as previously reported (31).
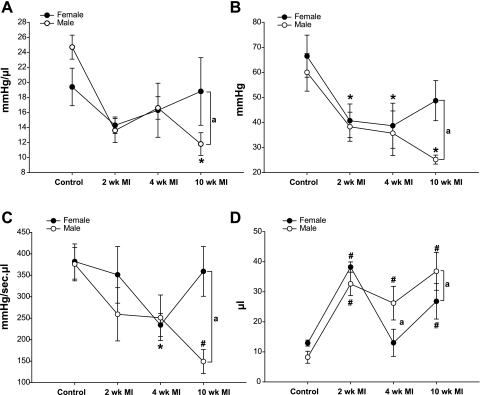
Hemodynamic measurements obtained while varying preload during transient TVC occlusion, revealed significant differences in LV contractility between post-MI females and males (Fig. 2). In females, the slope of the end-systolic pressure volume relationship recovered to control levels in 10 wk post-MI mice. Conversely, in males ESPVR declined and was significantly lower relative to control and females at 10 wk post-MI (Fig. 2A). Likewise, similar differences were also noted between female and males at 10 wk post-MI in other parameters of contractility. Both PRSW and dP/dtmax vs. EDVmax showed improvement in females by 10 wk post-MI relative to control and a significant difference relative to males at 10 wk post-MI (Fig. 2, B and C). Likewise, time-varying elastance (Emax) also showed similar differences between females and males (data not shown).
Fig. 2.
Measurements of left ventricular contractility obtained during transient occlusion of TVC and volume intercept at 2, 4, and 10 wk post-MI mice. A: slope of the end-systolic pressure volume relationship. B: preload recruitable stroke work. C: maximum dP/dt vs. maximum EDV. D: volume intercept, representing the x-axis intercept of the slope of the ESPVR. Data are means ± SE; changes are due to infarct. *P < 0.05, #P < 0.01 vs. respective controls and changes due to sex. aP < 0.05.
The volume intercept showed evidence of a different degree of compensation between females compared with males. Volumes increased similarly at 2 wk but normalized to control levels in 4 wk post-MI females, which was significantly different than the normalization noted in males. This difference persisted in females and males at 10 wk post-MI (Fig. 2D).
Finally, CCE representing heart work was obtained during TVC occlusions and corrected for parallel conductance indicated a similar decline in both females (control, 82 ± 2; 2 wk MI, 71 ± 6; 4 wk MI, 72 ± 6; 10 wk MI =73 ± 5%) and males (control, 88 ± 1; 2 wk MI, 71 ± 3; 4 wk MI, 75 ± 3; 10 wk MI, 69 ± 4%, P < 0.05 vs. control) following MI, but only declined significantly in males by 10 wk post-MI.
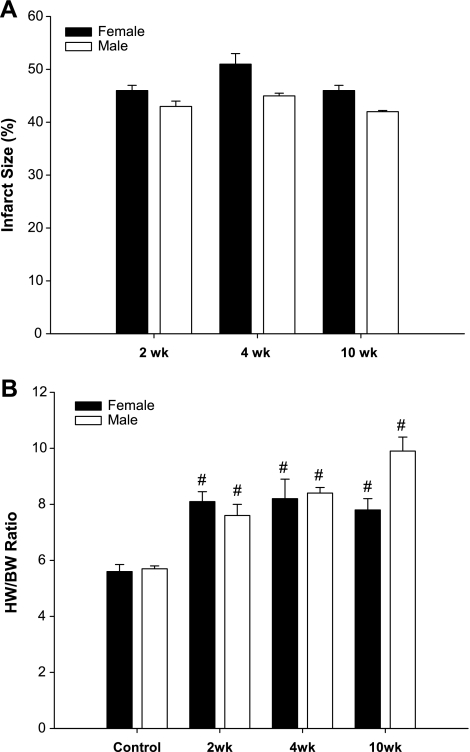
The infarcted area expressed as a percent of the total LV, showed that infarcts consistently greater than 40% were created at all time points, although slightly higher in females than males (Fig. 3A). To relate the global cardiac function to LV hypertrophy, the heart weight-to-body weight ratio (HW/BW) was calculated as an index of cardiac hypertrophy, which was similar in both females and males mice by 2 wk post-MI. However, the increase in the HW/BW ratio remained steady in the females, whereas males showed a trend to continually increase at 10 wk post-MI compared with their respective controls (Fig. 3B).
Fig. 3.
Quantification of infarct size and hypertrophic response in female and male post-MI mice. A: infarct size. B: heart-weight-to-body weight (HW/BW) ratios. Data are presented as means ± SE. #P < 0.01 vs. respective controls.
DISCUSSION
To our knowledge, this is the first assessment of the sex-related changes in cardiac function during the compensatory hypertrophic response, and decompensation to HF following MI in mice using the Millar P-V loop conductance system in vivo. Our hemodynamic data obtained in the closed chest compared functional changes in more a physiologically relevant context with normal intrathoracic pressures, but transient occlusions of TVC in the open chest were necessary to evaluate LV contractility. The baseline hemodynamic data obtained in the closed and open chest were not significantly different, but they did show an interesting dichotomy in females at 10 wk post-MI, which suggested a delayed decompensation to HF. This may be due to a relatively smaller chest cavity in females and that surrounding structures may aid load-dependent function of the dilated heart. Although a similar early compensatory response occurred in females and males, the late compensatory response differed with normalized ESV in females compared with males. Although some parameters did not reach statistical significance among the groups, their variability could be attributed to the individual responses to large infarcts of more than 40%, heterogeneous changes in LV geometry, nonlinear electrical properties of infarcted myocardium, and the potentially confounding effects of anesthetics necessary for invasive surgical procedures. Nevertheless, despite these potential limitations, we observed no differences in heart rate between sexes, and we believe our data highlight relevant physiological differences in cardiovascular function between females and males following MI.
Overall, the major P-V loop-derived parameters indicated deterioration in LV function in both female and male mice following MI. The overall decline in LV function appeared to result from an initial significant decline in systolic function at 2 wk post-MI, before evidence of diastolic dysfunction, which was more evident in females at 4 wk post-MI. There were notable rebounds in the ESV and EDV, which occurred at the 4 wk post-MI, before they declined further by 10 wk post-MI, which were also reflected in PAMP, representing the LV contractility derived from load-dependent parameters (33). This apparent improvement may be due to beneficial effects of compensatory hypertrophy, as well as scar formation and stabilization, which have been shown to occur in rodents at 2–3 wk post-MI (20). However, the compensatory responses associated with LV hypertrophy appear to differ between females and males. Thickening of the wall with a normal LV chamber diameter occurs during compensated hypertrophy, which is followed by an increase in the ratio of the chamber diameter to wall thickness during decompensated hypertrophy (20, 24, 32). Gender-related differences have been described in the extent and patterns of LV remodeling post-MI. Generally, there is attenuated hypertrophy in females with a lower incidence of rupture, lower rate of infarct expansion, and reduced thickening of the noninfarcted wall, which contribute to better LV systolic function and a better outcome during the development of HF (5, 24, 39). This attenuated remodeling has been credited, in part, to the cardioprotective effects of female sex hormones. Ovariectomized rats develop comparable remodeling and LV dysfunction, as males with volume overload, and while estrogen replacement reduced infarct size, it increases ventricular remodeling and mortality post-MI in mice (3, 28). We found that infarct size measurements did not differ between females and males and that HW/BW ratios were similar. Thus, these data suggest that despite similarly sized infarcts, chronic compensated contractile function is associated with adaptive remodeling in females, whereas a continual decline in contractile function is associated with maladaptive remodeling in males.
The P-V loop data derived in the closed chest are dependent, to some extent, on the level of arterial pressure, which influences intrinsic cardiac function and are also influenced by the peripheral vascular and neurohormonal mechanisms. Alterations in both cardiac and vascular properties occur to maintain blood pressure and flow in disease states, making it difficult to assess the relative changes in each system independently. To evaluate the effects of MI on cardiac contractility, TVC occlusions were preformed to alter the loading conditions and assess whether there was a sex-related response in the regulation of intrinsic contractility (14, 30, 40).
The work performed by the heart is confined within the boundaries defined by end-diastolic pressure volume relationship and ESPVR (26, 27). The slope of the ESPVR significantly declined by 2 wk post-MI and then nearly recovered in females, whereas ESPVR continued to decline in males. PRSW represents the slope of the relationship between SW and EDV and has been described as being independent of chamber geometry and is sensitive to chamber contractile function (10, 26, 27). Likewise, dP/dtmax vs. EDVmax permits analysis of the change in pressure rise over time at a given EDV, thereby allowing examining of contractility independent of preload. Both of these indexes of cardiac muscle contractility (PRSW and dP/dtmax vs. EDVmax), demonstrated a sex-related difference in 10 wk post-MI mice during the decompensation to failure. Despite the activation of neurohormonal compensatory mechanisms, myocyte contractility is depressed in the infarcted heart, partly due to dysregulation of excitation-contraction (E-C) coupling (8, 15, 19). The expression of key proteins and the signaling components involved in E-C coupling regulation differ between females and males and are associated with slight functional differences (6). A greater abundance of L-type Ca2+ channel protein and dihydropyridine binding sites in concert with larger Ca2+ current density (Ica) have been reported in female myocytes (6, 35). Expression of several calcium-handling proteins appears to be selectively regulated by estrogen, but the contribution to basal and pathologic contractile function is unclear (7). However, deletion of the estrogen receptor (ER), mainly the β-receptor isomer, induces alterations in the ECG parameters post-MI. Prolongation of ventricular repolarization (the Q-T interval) occurred in the β-ER-deficient female mice, suggesting that estrogen plays a role in maintaining the autorhythmaticity of the female heart post-MI (23).
Our study provided an examination of cardiovascular function changes during the transition to HF following MI, in female and male mice. P-V loop analysis was used to quantitatively assess global function and revealed a decrease in systolic function despite late compensatory hypertrophy post-MI. Interestingly female mice, demonstrated an improvement in systolic function and contractility by 10 wk post-MI compared with males, indicating that a sex-related basis underlies the physiological and pathophysiological regulation of cardiovascular function during the transition to heart failure.
Perspectives and Significance
A great deal of our understanding of sex differences in HF is based on postmenopausal women with HF, without the cardioprotective actions of estrogen. While our study used young adult female mice, the prevalence of HF in young women due to cigarette smoking, physical inactivity, inappropriate diet, diabetes, hypertension, dyslipidemia, and metabolic disorder is increasing (24, 37). As such, appreciating the sex differences in physiological function in small animal models that allow for specific genetic alterations in either the heart or vascular system, could contribute to earlier diagnosis, treatment, and/or prevention of HF in women.
GRANTS
K. M. Shioura was supported by National Institutes of Health T32-HL-07692. This work was also supported by P01-HL-62426.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baan J, van der Velde ET, de Bruin HG, Smeek GJ, Koops J, van Dijk AD, Temmerman D, Senden J, Buis B. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation 70: 812–23, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Bridgman P, Aronovitz MA, Kakkar R, Oliverio MI, Coffman TM, Rand WM, Konstam MA, Mendelsohn ME, Patten RD. Gender-specific patterns of left ventricular and myocyte remodeling following myocardial infarction in mice deficient in the angiotensin II type 1a receptor. Am J Physiol Heart Circ Physiol 289: H586–H592, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Brower GL, Gardner JD, Janicki JS. Gender-mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem 251: 89–95, 2003. [PubMed] [Google Scholar]
- 4.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci 75: 2181–2182, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium handling proteins, β-adrenergic receptors, and function in rat heart. Life Sci 79: 1257–1267, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chu SH, Sutherland K, Beck J, Kowalski J, Goldspink P, Schwertz D. Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci 76: 2735–2749, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Daniels MC, Naya T, Rundell VL, de Tombe PP. Development of contractile dysfunction in rat heart failure: hierarchy of cellular events. Am J Physiol Regul Integr Comp Physiol 293: R284–R292, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Douglas P, Katz S, Weinberg M, Chen M, Bishop S, Lorell B. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol 32: 1118–1125, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher PJ, Pfeffer JM, Pfeffer MA, Braunwald E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction. Effects on systolic function. Circ Res 49: 618–626, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Francis GS, McDonald KM, Cohn JN. Neurohumoral activation in preclinical heart failure. Remodeling and the potential for intervention. Circulation 87: IV90–IV96, 1993. [PubMed] [Google Scholar]
- 12.Gao XM, Xu Q, Kiriaziz H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course. Strain- and gender dependency, tensile strength, and histopathology. Cardiovasc Res 65: 469–477, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Georgakopoulos D, Kass DA. Estimation of parallel conductance by dual-frequency conductance catheter in mice. Am J Physiol Heart Circ Physiol 279: H443–H450, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol Heart Circ Physiol 274: H1416–H1422, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation 104: 688–693, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Gowda MS, Vacek JL, Hallas D. Gender related risk factors and outcomes for non-Q wave myocardial infarction patients receiving in-hospital PTCA. J Invasive Cardiol 11: 121–126, 1999. [PubMed] [Google Scholar]
- 17.Hasnat K, van der Velde ET, Hon JK, Yacoub MH. Reproducible model of post-infarction left ventricular dysfunction: haemodynamic characterization by conductance catheter. Eur J Cardiothorac Surg 24: 98–104, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hayes SN Preventing cardiovascular disease in women. Am Fam Physician 74: 1331–1340, 2006. [PubMed] [Google Scholar]
- 19.Holt E, Tonnessen T, Lunde PK, Semb SO, Wasserstrom JA, Sejersted OM, Christensen G. Mechanisms of cardiomyocyte dysfunction in heart failure following myocardial infarction in rats. J Mol Cell Cardiol 8: 1581–1593, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Jugdutt BI, Joliart MJ, Khan MI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation 94: 94–101, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of β1-adenoreceptor upon ischemic insult in the rat heart. J Exp Pharmacol Exp Therapeutics 309: 8–15, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kass DA, Yamazaki T, Burkhoff D, Maughan WL, Sagawa K. Determination of left ventricular end-systolic pressure-volume relationships by the conductance (volume) catheter technique. Circulation 73: 586–93, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Korte T, Fuchs M, Arkudas A, Geertz S, Meyer R, Gardiwal A, Klein G, Niehaus M, Krust A, Chambon P, Drexler H, Fink K, Grohé C. Female mice lacking estrogen receptor β display prolonged ventricular repolarization and reduced ventricular automaticity after myocardial infarction. Circulation 111: 2282–2290, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Litwin SE, Katz SE, Litwin CM, Morgan JP, Douglas PS. Gender differences in postinfarction left ventricular remodeling. Cardiology 91: 173–183, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen OS, Bjorner JB, Newman B, Oldenburg B, Groenvold M, Madsen JK, Andersen HR. Gender differences in health-related quality of life following ST-elevation myocardial infarction: women and men do not benefit from primary percutaneous coronary intervention to the same degree. Eur J Cardiovasc Prev Rehabil 14: 37–43, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Liu J, Harvey-White J, Brassai A, Járai Z, Cravatt BJ, Kunos G. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 289: H533–H541, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacher P, Liaudet L, Mabley JG, Cziráki A, Haskó G, Szabó C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Intern J Mol Med 17: 369–375, 2006. [PMC free article] [PubMed] [Google Scholar]
- 28.Patten RD, Karas RH. Estrogen replacement and cardiomyocyte protection. Trends Cardiovasc Med 16: 69–75, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Schenck-Gustafsson K Are the symptoms of myocardial infarction different in men and women, if so, will there be any consequences? Scand Cardiovasc J 6: 325–326, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Segers P, Georgakopoulos D, Afanasyeva M, Champion HC, Judge DP, Millar HD, Verdonck P, Kass DA, Stergiopulos N, Westerhof N. Conductance catheter-based assessment of arterial input impedance, arterial function and ventricular-vascular interaction in mice. Am J Physiol Heart Circ Physiol 288: H1157–H1164, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Shioura KM, Geenen DL, Goldspink PH. Assessment of cardiac function with the pressure-volume conductance system following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 293: H2870–H2877, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Solomon SD, Greaves SC, Rayan M, Finn P, Pfeffer MA, Pfeffer JM. Temporal dissociation of left ventricular function and remodeling following experimental myocardial infarction in rats. J Card Fail 5: 213–223, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Takagaki M, McCarthy PM, Chung M, Connor J, Dessoffy R, Ochiai Y, Howard M, Doi K, Kopcak M, Mazgalev TN, Fukamachi K. Pre-load adjusted maximal power: a novel index of left ventricular contractility in atrial fibrillation. Heart 88: 170–176, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW. Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol 282: H256–H263, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Wang GY, Wu S, Pei JM, Yu XC, Wong TM. κ- and δ-opioid receptors mediate effects of ischemic preconditioning on both infarct and arrhythmia in rats. Am J Physiol Heart Circ Physiol 280: H384–H391, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wenger NK Coronary heart disease in women: highlights of the past 2 years-stepping stones, milestones, and obstructing boulders. Nat Clin Pract Cardiovasc Med 3: 194–202, 2006. [DOI] [PubMed] [Google Scholar]
- 38.White PA, Brookes CIO, Ravn H, Hjortdal V, Chaturvedi RR, Redington AN. Validation and utility of novel volume reduction technique for determination of parallel conductance. Am J Physiol Heart Circ Physiol 280: H475–H482, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Wu JC, Nasseri BA, Bloch KD, Picard MH, Scherrer-Crosbie M. Influence of sex on ventricular remodeling after myocardial infarction in mice. J Am Soc Echocardiogr 16: 1158–62, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol Heart Circ Physiol 277: H1906–H1913, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Zampino M, Yuzhakova M, Hansen J, McKinney RD, Goldspink PH, Geenen DL, Buttrick PM. Sex-related dimorphic response of HIF-1α expression in myocardial ischemia. Am J Physiol Heart Circ Physiol 291: H957–H964, 2006. [DOI] [PubMed] [Google Scholar]