Abstract
The role(s) of central Gα-proteins in the regulation of cardiovascular and renal function is unknown. We examined how inhibition/downregulation of central Gαi/Gαo, Gαz or Gαq proteins altered the characteristic cardiovascular (depressor), renal excretory (diuretic), and plasma AVP (inhibitory) responses to intracerebroventricular injection of nociceptin/orphanin FQ (N/OFQ) in rats. Before investigation, rats were pretreated intracerebroventricularly with saline vehicle (5 μl, 48 h, n = 6), pertussis toxin (PTX; 48-h, 1 μg, n = 6), or Gαz, Gαq, or scrambled oligodeoxynucleotide (ODN) (25 μg, 24 h, n = 6 per group). On the study day, intracerebroventricular N/OFQ (5.5 nmol) or vehicle (5 μl) was injected into pretreated conscious rats. Mean arterial pressure (MAP) and heart rate (HR) were recorded, and urine was collected for 90 min. In vehicle or scrambled ODN groups, intracerebroventricular N/OFQ decreased MAP and HR and produced water diuresis (sensitive to UFP-101, N/OFQ receptor antagonist). The hypotension and bradycardia, but not diuresis, to N/OFQ were abolished in PTX-pretreated rats. In contrast, intracerebroventricular ODN pretreatment markedly blunted (Gαz) or augmented (Gαq) the diuresis to intracerebroventricular N/OFQ. In separate studies, the action of central N/OFQ to decrease plasma AVP levels in naïve water-restricted rats was differentially altered by intracerebroventricular Gαz ODN (blunted) and Gαq ODN (augmented) pretreatment. These studies demonstrate central Gαi/Gαo activity mediates intracerebroventricular N/OFQ's cardiovascular depressor function. Alternatively, central Gαz (inhibitory) and Gαq (stimulatory) activity differentially modulates AVP release to control the pattern of diuresis to intracerebroventricular N/OFQ. These findings highlight the novel selective central Gα-subunit protein-mediated control of cardiovascular vs. renal excretory function.
Keywords: central Gα-subunit proteins, central nervous system, cardiovascular and renal excretory function, vasopressin/antidiuretic hormone, nociceptin/orphanin FQ
it is well established that G protein-coupled receptor (GPCR) ligands act in the central nervous system (CNS) to alter cardiovascular and renal excretory function through diverse intracellular signaling pathways. However, the role(s) that specific central Gα-subunit proteins play in controlling cardiovascular and renal function in vivo has not been investigated. To initially investigate this question, we examined the cardiovascular and renal excretory responses produced by intracerebroventricular injection of nociceptin/orphanin FQ (N/OFQ) in conscious rats following the selective inhibition/downregulation of Gα-protein signaling pathways. N/OFQ, an opioid-like peptide, which binds to the G protein-coupled N/OFQ peptide (NOP) receptor (15, 18), was used for these studies since intracerebroventricular administration of this ligand to conscious rats evokes concurrent changes in cardiovascular (bradycardia, hypotension) and renal excretory (water diuresis) function, the latter of which is mediated via a central action of the peptide to inhibit AVP secretion (9, 11, 12). The NOP receptor is widely distributed throughout the CNS, particularly in sites that control cardiovascular and renal function and AVP synthesis/release (16).
Although the cardiovascular and renal responses to exogenous central N/OFQ are well characterized, the endogenous Gα-protein-signaling pathways involved in mediating these responses in vivo have yet to be elucidated. NOP receptor activation leads to multiple cellular responses, including activation of potassium channels and inhibition of calcium channels, adenylyl cyclase, and MAPK phosphorylation, all of which can modulate neuronal activity and neurotransmitter release (18). At present, there are essentially no data correlating intracellular Gα-protein-gated pathways to the physiological responses elicited by central N/OFQ in vivo. Following NOP receptor stimulation, initial signal transduction occurs via NOP receptor coupling to GTP-binding regulatory proteins. On the basis of studies performed in vitro, it is possible that central NOP receptors can signal through pathways that involve the following Gα subunit proteins, Gαi1,2,3 (5), GαoA,B (25), Gαz (2, 7), and potentially through Gαq (26) in the manner of traditional opioid receptors (8).
The aim of this study was to identify the central NOP Gα protein-coupled pathways, which mediate the cardiovascular and renal excretory effects produced by intracerebroventricular N/OFQ in conscious Sprague-Dawley rats. Initial studies used University of Ferrara Peptide 101 (UFP-101), a selective NOP receptor antagonist (1), to establish that the effects of N/OFQ were mediated via central NOP receptors. In other studies, groups of rats were pretreated with selective inhibitors of specific Gα-protein pathways via intracerebroventricular pertussis toxin (PTX) or oligodeoxynucleotide (ODN) administration to selectively inhibit Gαi/Gαo and reduce Gαz/Gαq protein levels in the CNS (4), respectively. After PTX (48 h) or ODN (24 h) treatment, we examined the cardiovascular and renal excretory responses to central N/OFQ in conscious chronically instrumented rats. Finally, separate studies were performed in rats, in which basal plasma AVP levels were elevated by water restriction to determine whether intracerebroventricular Gαz/Gαq ODN pretreatment altered the ability of central N/OFQ to suppress circulating levels of this hormone. These studies demonstrate that N/OFQ acts in the CNS to activate NOP receptor signaling pathways mediated through Gα-proteins, which selectively control cardiovascular function (Gαi/Gαo) vs. the renal excretion of water (Gαz or Gαq), the latter of which involves opposing Gαz (inhibitory) and Gαq (stimulatory) influences on AVP secretion.
METHODS
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN), 275–300 g, were housed individually under a 12:12-h light-dark cycle. Rats were fed standard rodent diet and allowed tap water ad libitum and were randomly assigned to experimental treatment groups. All procedures were conducted in accordance with National Institutes of Health and Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee guidelines for the Care and Use of Animals.
Measurement of BP and HR and Renal Excretory Function
A stainless-steel cannula was stereotaxically implanted into the right lateral cerebral ventricle of rats anesthetized with ketamine in combination with xylazine 5–7 days before experimentation, as previously described (11, 12). On the day of study, rats were anesthetized with sodium methohexital and instrumented with catheters in the left femoral artery, left femoral vein, and bladder, as described previously (11, 12, 14). Following surgical preparation, rats were placed in a rat holder, and an intravenous infusion of isotonic saline (55 μl/min) was started and continued for the duration of the experiment. The experimental protocol commenced after the animal regained full consciousness and cardiovascular, renal, and excretory functions had stabilized (4–6 h). Mean arterial pressure (MAP) and heart rate (HR) were continuously recorded using computer-driven BIOPAC data acquisition software (MP100 and AcqKnowledge 3.8.2) connected to a pressure transducer (P23XL; Viggo Spectramed, Oxnard, CA). Urine was collected during a 20-min control period. Following this, N/OFQ (5.5 nmol) (10) or saline vehicle was injected intracerebroventricularly (n = 6 per group), urine was collected during consecutive 10-min experimental periods for 90 min. Urine volume was determined gravimetrically. Urine sodium concentration was measured by flame photometry (model 943; Instrumentation Laboratories, Lexington, MA) and expressed as urinary sodium (UNaV) excretion.
Experimental Protocols
UFP-101 antagonist studies.
In UFP-101 pretreatment studies, animals were continuously infused with intracerebroventricular UFP-101 (18 nmol·5μl−1·h−1). Following 60 min of UFP-101 infusion pretreatment, N/OFQ (5.5 nmol/5 μl) (10) was injected intracerebroventricularly (n = 6 per group), and UFP-101 infusion was continued for the duration of the experiment (90 min).
Gα-protein studies.
In Gα-protein studies, rats (n = 6 per group) were pretreated with a single intracerebroventricular injection of either saline vehicle (5 μl, 48 h), PTX (1 μg/5 μl, 48 h) (3, 13), Gαz ODN (25 μg/5 μl, 24 h; 5′-GGGCCAGTAGCCCAATGGG-3′), Gαq ODN (25 μg/5 μl, 24-h; 5′-GCTTGAGCTCCCGGCGGGCG-3′) or a scrambled ODN (25 μg/5 μl, 24 h; 5′-GGGGGAAGTAGGTCTTGG-3′) (4, 19, 24). Following an NCBI Basic Local Alignment Search Tool (Blast) search of the Rattus norvegicus RefSeq protein database, it was confirmed that there is no similarity between the scrambled ODN sequence and any rat protein gene sequence. On the day of the experiment, all animals received a single intracerebroventricular injection of N/OFQ (5.5 nmol/5 μl).
AVP studies and measurement.
Using 48-h water restriction as a means to elevate basal AVP plasma levels, Kakiya et al. (9) have shown that intracerebroventricular N/OFQ inhibits circulating levels of this hormone in conscious rats. As noted below, we modified this protocol to determine the role(s) of central Gαz and Gαq in the action of N/OFQ to suppress plasma AVP. Rats chronically implanted with an intracerebroventricular cannula were water deprived for a total period of 48 h. After the first 24 h of water deprivation, animals received a single intracerebroventricular injection of saline vehicle (5 μl) or ODN sequence (25 μg/5 μl) (n = 5 per group). Then, 24 h after this pretreatment (i.e., 48 h total water deprivation), the following experimental protocol was conducted.
Saline vehicle-pretreated animals were administered either intracerebroventricular saline vehicle (5 μl) or N/OFQ (5.5 nmol/5 μl). All animals pretreated with an ODN sequence received intracerebroventricular N/OFQ (5.5 nmol/5 μl). Ten minutes postinjection, animals were decapitated, and plasma AVP was determined using an AVP ELISA kit, according to the manufacturer's instruction (Assay Designs, Ann Arbor, MI).
G protein immunoblotting.
Twenty-four hours following a single intracerebroventricular administration of saline vehicle (5 μl) or an ODN sequence (25 μg/5 μl), the CNS sites of interest (brain cortex, hypothalamus, medulla) were identified by visual landmarks (17) and were dissected on ice (n = 6 per group), tissue lysates were prepared, and protein levels were quantified. Lysates were resolved on SDS-PAGE gels and transferred to nitrocellulose membrane (GE Healthcare, Piscataway, NJ). Gαz and Gαq levels were determined using anti-Gαz antibody (Santa Cruz, CA) (1:3,000) and anti-Gαq antibody (Calbiochem, San Diego, CA) (1:2,000); protein levels were normalized to GAPDH (anti-GAPDH 1:1,000, Abcam, Cambridge, MA). Chemiluminescent immunoreactive bands were detected by horseradish peroxidase-conjugated secondary antibody; data were imaged and quantified using Bio-Rad Quantity One image analysis software.
Statistical Analysis
All data are expressed as means ± SE. The magnitude of the changes in cardiovascular and renal excretory parameters at different time points after intracerebroventricular injection of N/OFQ were compared with respective group control values by a one-way repeated-measures ANOVA with subsequent Dunnett's test. Differences occurring between treatment groups (e.g., intracerebroventricular saline vehicle and scrambled ODN) were assessed by a two-way repeated-measures ANOVA with pretreatment group (e.g., saline vehicle) being one fixed effect and time the other, with the interaction included. The time (min) was then the repeated factor. Post hoc analysis was performed using Bonferroni's test. Where appropriate, a Student's t-test was also used to compare means between two groups. In each case, statistical significance was defined as P < 0.05.
RESULTS
UFP-101 Blocks the Cardiovascular and Renal Responses to Intracerebroventricular N/OFQ
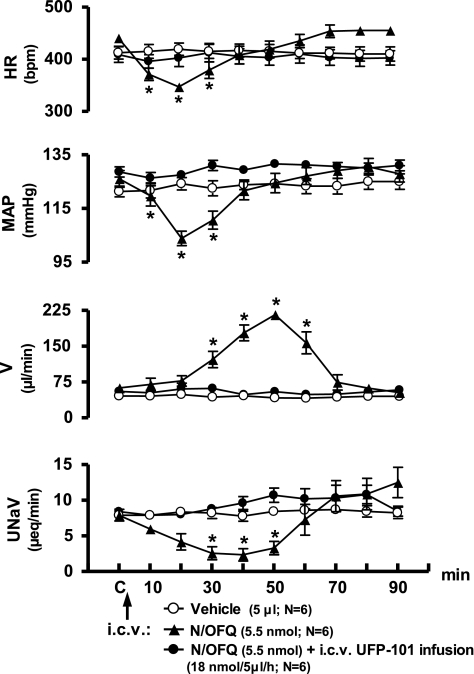
In conscious Sprague-Dawley rats pretreated with isotonic saline vehicle (5 μl, 48 h), intracerebroventricular N/OFQ (5.5 nmol), but not intracerebroventricular isotonic saline vehicle, produced characteristic reductions in HR, MAP, and urinary sodium excretion, and an increase in urine flow rate (Fig. 1). Peak bradycardia and hypotension were observed 20 min after intracerebroventricular N/OFQ, with responses returning to predrug control levels by 40 min. Maximal delayed onset diuresis was observed 50 min post-N/OFQ with urine flow rate returning to predrug levels by 70 min. Central infusion of the NOP receptor antagonist, UFP-101 (18 nmol·5 μl−1·h−1), did not alter the measured physiological parameters before (60 min) or after (120 min) intracerebroventricular vehicle injection (data not shown). However, central UFP-101 infusion (60 min intracerebroventricular pretreatment and subsequent infusion; 18 nmol·5μl−1·h−1) completely blocked the cardiovascular and renal excretory responses to intracerebroventricular N/OFQ (Fig. 1).
Fig. 1.
Effect of intracerebroventricular UFP-101 pretreatment on cardiovascular and renal excretory responses to central nociceptin/orphanin FQ (N/OFQ) in conscious male Sprague-Dawley rats. The values are means ± SE and illustrate the cardiovascular and renal effects of intracerebroventricular N/OFQ (5.5 nmol) or isotonic saline vehicle (5 μl) in six conscious rats/group pretreated intracerebroventricularly with isotonic saline vehicle (5 μl, 48 h). In a separate group (n = 6), University of Ferrara Peptide 101 (UFP-101) (18 nmol·5μl−1·h−1) was infused intracerebroventrcularly in rats for 60 min prior to N/OFQ injection, and then the infusate was maintained for the duration of the experimental protocol. HR, heart rate; MAP, mean arterial pressure; V, urine flow rate; UNaV, urinary sodium excretion. *P < 0.05 compared with respective group control value (designated C).
Central Gαi/Gαo Activity Is Required to Mediate Central N/OFQ-Evoked Cardiovascular Depressor Function
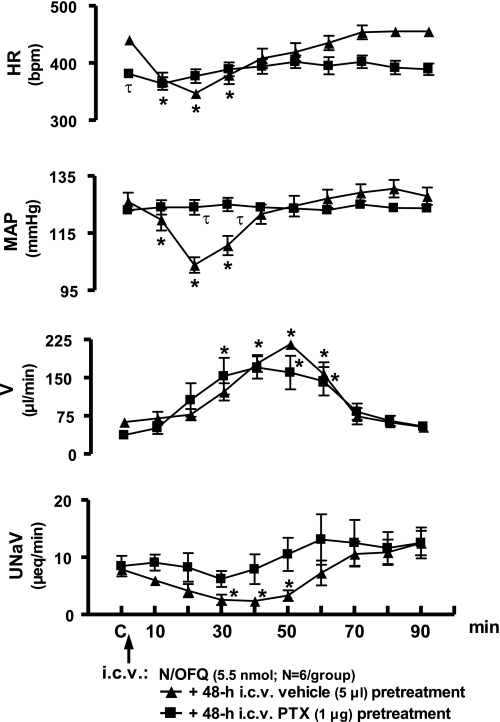
Intracerebroventricular PTX pretreatment (1 μg, 48 h) produced a slight, but not statistically significant, reduction in body weight in rats (Δ −21 ± 3 g). Further, compared with rats pretreated with vehicle, PTX pretreatment did not alter baseline control (C) levels for MAP or renal excretory function, although HR was slightly reduced (Fig. 2). In vehicle-treated animals (data transposed from Fig. 1), the peak bradycardia and hypotension were observed 20 min after central N/OFQ injection [Δ 20 min; HR, −98 ± 12 beats/min (bpm), MAP −20 ± 2 mmHg]. In contrast, intracerebroventricular N/OFQ did not alter either cardiovascular parameter in PTX-treated animals (Δ 20 min; HR, +8 ± 5 bpm, MAP +1 ± 1 mmHg). In both saline vehicle and PTX-pretreated groups, intracerebroventricular N/OFQ produced a delayed onset diuresis of comparable magnitude (cumulative urine output; saline vehicle pretreatment, 5,635 ± 619 μl; PTX pretreatment, 5,346 ± 683 μl). However, intracerebroventricular N/OFQ did not produce a decrease in urinary sodium excretion in PTX-pretreated animals (40 min UNaV; saline vehicle pretreatment, 2.3 ± 0.8 μeq/min; PTX pretreatment, 7.9 ± 2.6 μeq/min).
Fig. 2.
Effect of intracerebroventrcular pertussis toxin (PTX) pretreatment on cardiovascular and renal excretory responses to central N/OFQ in conscious male Sprague-Dawley rats. Values are presented as means ± SE and illustrate the cardiovascular and renal effects of intracerebroventricular N/OFQ (5.5 nmol) in 6 conscious rats that were pretreated (48 h) with intracerebroventricular PTX (1 μg). Data for intracerebroventricular N/OFQ are transposed from Fig. 1 for comparison. Abbreviations as in Fig. 1. *P < 0.05 compared with respective group control value (designated C). τP < 0.05 significantly different compared with intracerebroventricular saline vehicle pretreatment group at respective time points.
Central Gαq and Gαz Proteins Participate in the Diuretic Response to Central N/OFQ
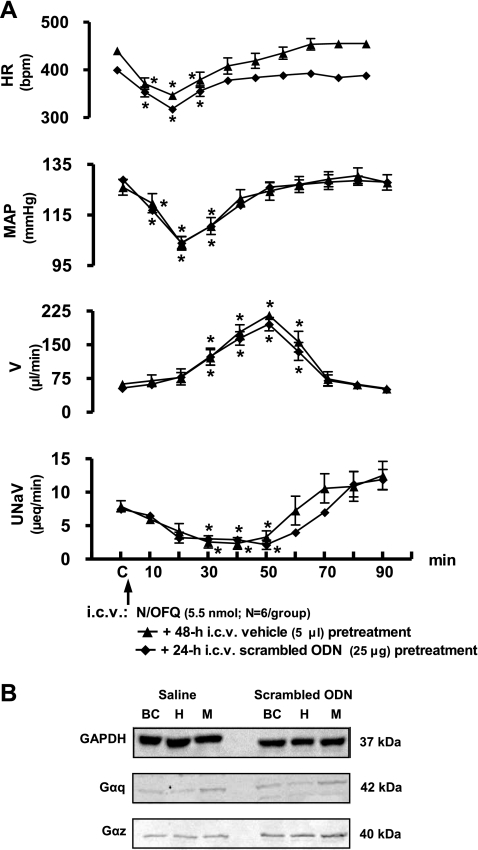
Scrambled ODN administration did not alter baseline control levels for cardiovascular/renal excretory function (Fig. 3A), the characteristic physiological responses to intracerebroventricular N/OFQ (Fig. 3A) or brain Gα protein expression (Fig. 3B). In animals pretreated with scrambled ODN, intracerebroventricular N/OFQ produced characteristic reductions in HR, MAP, and urinary sodium excretion and an increase in urine flow rate, with the pattern and duration of physiological responses mirroring those responses to central N/OFQ observed in saline vehicle-pretreated animals (Fig. 3A). Peak bradycardia and hypotension were observed 20 min after intracerebroventricular N/OFQ with responses returning to predrug control levels by 40 min. Maximal diuresis was observed 50 min post-N/OFQ with urine flow rate returning to predrug levels by 70 min.
Fig. 3.
A: effect of intracerebroventricular scrambled oligodeoxynucleotide (ODN) pretreatment on cardiovascular and renal excretory responses to central N/OFQ in conscious Sprague-Dawley rats. The values are expressed as means ± SE and illustrate the cardiovascular and renal effects of intracerebroventricular N/OFQ (5.5 nmol) in six conscious rats that were pretreated (24 h) with intracerebroventricular scrambled ODN (25 μg). Data for intracerebroventricular N/OFQ are transposed from Fig. 1 for comparison. Abbreviations are the same as in Fig. 1. *P < 0.05 compared with respective group control value (designated C). B: representative immunoblot showing GAPDH, Gαq and Gαz subunit protein levels in brain cortex (BC), hypothalamus (H), and medulla (M) sample from a male Sprague-Dawley rat that was pretreated (24 h) with intracerebroventricular scrambled ODN (25 μg) or saline vehicle (5 μl).
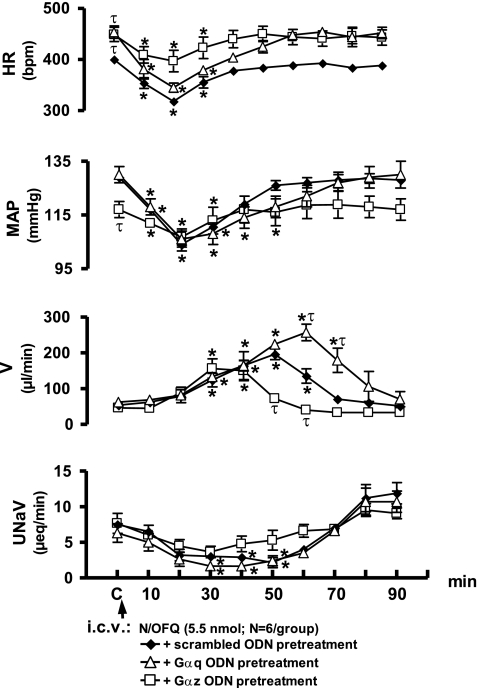
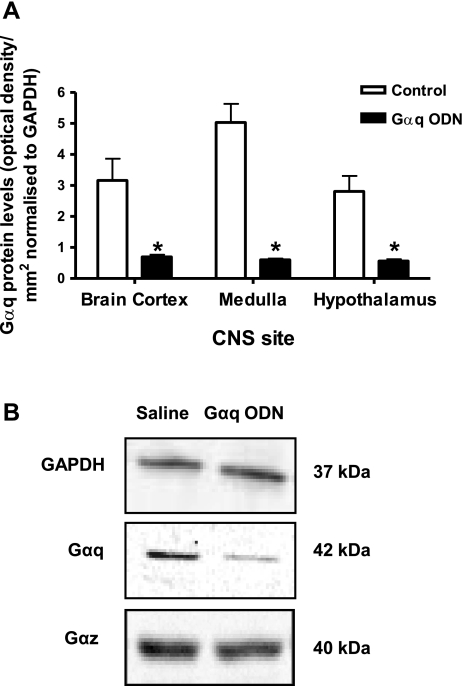
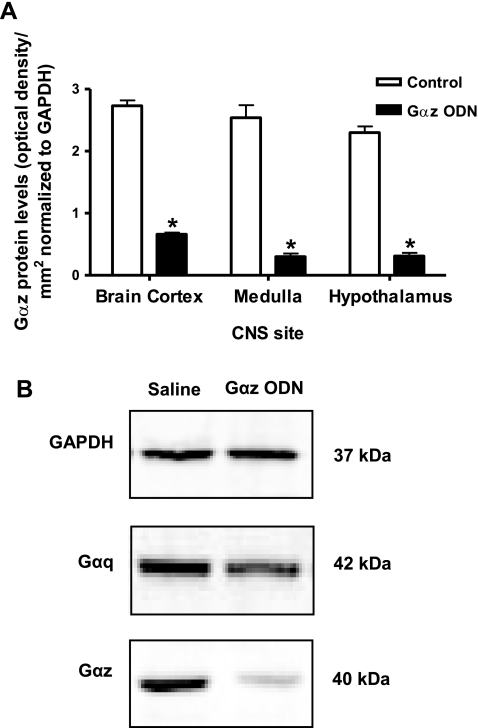
Intracerebroventricular Gαq or Gαz ODN pretreatment (25 μg each/24 h) did not significantly alter animal body weight (Gαq ODN, Δ +2 ± 1 g; Gαz ODN, Δ −14 ± 4 g) or baseline control levels (Fig. 4) for renal excretory function. It is of interest to note that 24 h intracerebroventricular pretreatment with either a Gαq or Gαz ODN resulted in a small elevation in basal HR, of ∼40 bpm, compared with scrambled ODN pretreatment (Fig. 4). Additionally, pretreatment with a Gαz, but not a Gαq ODN, resulted in a slight reduction in basal MAP of ∼12 mmHg (Fig. 4). Following 24-h intracerebroventricular pretreatment, both Gαq and Gαz ODN sequences reduced Gα protein expression in the brain cortex, hypothalamus, and medulla, respectively (Figs. 5, A and B, 6, A and B). With both sequences, protein levels were reduced significantly, in an ODN sequence-specific manner, by at least 85% in the medulla and hypothalamus, indicating ODN dispersal and efficacy throughout the CNS, following intracerebroventricular administration.
Fig. 4.
Effect of intracerebroventricular Gαq or Gαz ODN pretreatment on cardiovascular and renal excretory responses to central N/OFQ in conscious male Sprague-Dawley rats. The values are expressed as means ± SE and illustrate the cardiovascular and renal effects of intracerebroventricular N/OFQ (5.5 nmol) in six conscious rats that were pretreated (24 h) with either intracerebroventricular Gαq ODN (25 μg) or intracerebroventricular Gαq ODN (25 μg). Data for intracerebroventricular N/OFQ in intracerebroventricular scrambled ODN pretreated animals transposed from Fig. 3 for comparison. Abbreviations are the same as in Fig. 1. *P < 0.05 compared with respective group control value (designated C). τP < 0.05 significantly different compared with intracerebroventricular scrambled ODN pretreatment group at respective time points.
Fig. 5.
A: Gαq protein levels normalized to GAPDH expressed as optical density units/mm2 in CNS sites in Sprague-Dawley rats pretreated (24 h) with intracerebroventricular Gαq ODN (25 μg, n = 6) or isotonic saline vehicle (5 μl, n = 6). *P < 0.05 compared with control. B: representative immunoblots for GAPDH, Gαq, and Gαz taken from a hypothalamic sample from a male Sprague-Dawley rat pretreated (24 h) with intracerebroventricular Gαq ODN (25 μg) or saline vehicle (5 μl).
Fig. 6.
A. Gαz protein levels normalized to GAPDH expressed as optical density units/mm2 in central nervous system (CNS) sites in Sprague-Dawley rats pretreated (24 h) with intracerebroventricular Gαz ODN (25 μg, n = 6) or isotonic saline vehicle (5 μl, n = 6). *P < 0.05 compared with control. B: representative immunoblots for GAPDH, Gαz, and Gαq taken from a hypothalamic sample from a male Sprague-Dawley rat pretreated (24 h) with intracerebroventrcular Gαz ODN (25 μg) or saline vehicle (5 μl).
In Gαq ODN-pretreated rats, intracerebroventricular N/OFQ produced bradycardia, hypotension, and antinatriuresis, which followed the same pattern and magnitude as that produced by central N/OFQ in scrambled ODN-pretreated animals (Fig. 4). However, the hypotensive response to intracerebroventricular N/OFQ persisted for a longer duration than in scrambled ODN-treated animals. The magnitude and duration of the diuretic response to intracerebroventricular N/OFQ were increased in Gαq ODN-pretreated animals. Peak diuresis was observed later and was of a significantly greater magnitude (scrambled ODN pretreatment, 50 min, 196 ± 15 μl/min; Gαq ODN pretreatment, 60 min, 256 ± 20 μl/min). In addition, the cumulative urine output was significantly increased in Gαq ODN-treated animals (scrambled ODN pretreatment, 4,930 ± 561 μl; Gαq ODN pretreatment, 8,286 ± 783 μl, P < 0.05, Student's t-test).
Following Gαz ODN pretreatment, intracerebroventricular N/OFQ evoked hypotension and a decrease in urinary sodium excretion of the same magnitude and time course as that observed in scrambled ODN-pretreated animals (Fig. 4). However, the peak bradycardia was blunted in Gαz ODN-pretreated animals (scrambled ODN pretreatment, Δ 20 min, HR, −86 ± 9 bpm; Gαz ODN pretreatment, Δ 20 min, HR, −52 ± 14 bpm). Intracerebroventricular Gαz ODN pretreatment reduced both the magnitude and duration of the central N/OFQ-evoked diuresis. This is demonstrated by the earlier time, and reduced magnitude, of the peak diuresis (scrambled ODN pretreatment, 50 min, 196 ± 15 μl/min; Gαz pretreatment, 30 min, 156 ± 27 μl/min). In addition, cumulative urine output to intracerebroventricular N/OFQ was significantly decreased in Gαz ODN-pretreated animals (scrambled ODN pretreatment, 4,930 ± 561 μl; Gαz pretreatment, 3,154 ± 564 μl, P < 0.05, Students t-test). Thus, intracerebroventricular Gαz and Gαq pretreatment produced opposing effects on the diuretic responses to central N/OFQ (Fig. 4).
Central Gαq and Gαz Proteins Participate in the Effects of Intracerebroventricular N/OFQ on AVP Release
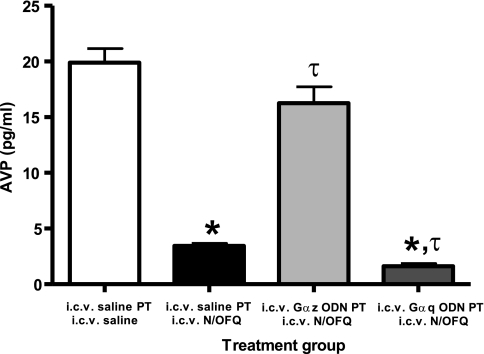
Rats were water deprived (48 h) to increase basal plasma levels of AVP (9) and then pretreated (after 24-h water deprivation) with isotonic saline vehicle or Gα-subunit ODN sequences. On the experimental day (i.e., following 48-h water deprivation and 24-h vehicle/Gα ODN pretreatment), in vehicle-pretreated rats, the levels for plasma AVP measured 10 min following intracerebroventricular vehicle injection were 19.9 ± 1.3 pg/ml (Fig. 7). In contrast, in vehicle-pretreated rats, plasma AVP levels were significantly suppressed by intracerebroventricular N/OFQ (5.5 nmol; AVP, 3.4 ± 0.2 pg/ml) (Fig. 7). In animals pretreated with Gαz ODN, central N/OFQ suppression of plasma AVP levels was significantly blunted (plasma AVP; Gαz ODN pretreatment plus intracerebroventricular N/OFQ, 16.3 ± 1.5 pg/ml). Alternatively, in Gαq ODN-pretreated animals, plasma AVP levels were suppressed by intracerebroventricular N/OFQ to a significantly greater level than that observed in vehicle-pretreated animals (plasma AVP; Gαq ODN pretreatment plus intracerebroventricular N/OFQ, 1.6 ± 0.2 pg/ml) (Fig. 7).
Fig. 7.
Effect of intracerebroventricular N/OFQ on plasma AVP levels in water-deprived rats pretreated with Gα ODNs. Values are expressed as means ± SE and illustrate plasma vasopressin levels measured in water restricted (48 h) rats 10-min post intracerebroventricular N/OFQ injection (5.5 nmol, n = 5/group). Rats were pretreated (24 h) intracerebroventricularly with either isotonic saline vehicle (5 μl), Gαq, or Gαz ODN (25 μg each). PT, pretreatment. *P < 0.05 compared with intracerebroventricular saline vehicle pretreatment and intracerebroventricular saline vehicle treatment group. τP < 0.05 compared with intracerebroventricular saline vehicle pretreatment and intracerebroventricular N/OFQ treatment group.
DISCUSSION
The role(s) of central Gα-protein signaling pathways in the regulation of cardiovascular and renal function in vivo remains elusive. In this study, we demonstrate that in conscious Sprague-Dawley rats, the GPCR ligand N/OFQ acts within the brain via the NOP receptor to activate separate Gα-protein signaling pathways that control cardiovascular (Gαi/Gαo) vs. renal excretory (Gαz or Gαq) function. In addition, these data highlight a novel role for Gαz (inhibitory influence) and Gαq (stimulatory influence) proteins as a CNS gating system through which GPCR ligands affect AVP secretion and consequently the renal handling of water.
Our initial study examined whether intracerebroventricular N/OFQ elicits cardiovascular and renal excretory responses through activation of central NOP receptors in conscious rats. N/OFQ was selected as a pharmacological tool to explore the role of central Gα-protein mechanisms in the regulation of cardiovascular and renal excretory function because of the multiple well characterized centrally evoked cardiovascular (bradycardia, hypotension) and renal excretory (diuresis and antinatriuresis) effects of this peptide (10, 11, 12). We have previously demonstrated that microinjection of UFP-101, a peptide antagonist selective for the NOP receptor (1), into the hypothalamic PVN of conscious rats prevents the cardiovascular and renal responses to N/OFQ microinjected into the same site (14). The present results extend this finding and demonstrate that intracerebroventricular UFP-101 infusion completely prevented the changes in cardiovascular and renal function to intracerebroventricular N/OFQ, thus further indicating the participation of central NOP receptors in mediating these responses.
Intracerebroventricular pretreatment with PTX, a bacterial toxin that inactivates Gαi and Gαo proteins by ADP ribosylation of their α-subunit (3, 13), completely prevented the cardiovascular depressor effects of intracerebroventricular N/OFQ. In contrast, following central administration of Gαq or Gαz ODN sequences, the pattern of the N/OFQ-induced bradycardia and hypotension was maintained. However, in Gαz ODN-pretreated animals, the magnitude of the decrease in HR was blunted, and in Gαq ODN-pretreated animals, the duration (but not magnitude) of the hypotension to intracerebroventricular N/OFQ was prolonged. These findings suggest that N/OFQ acts centrally to evoke its cardiovascular depressor responses primarily through a PTX-sensitive Gαi/Gαo protein-gated signal transduction pathways. However, it appears that following central NOP receptor stimulation, PTX-insensitive Gαz and Gαq protein pathways have some influence on the final pattern of the cardiovascular depressor responses to N/OFQ, possibly by interacting in a yet to be determined manner with the predominant Gαi/o pathway. It is also of interest that in central PTX-pretreated animals, the antinatriuresis to N/OFQ was also abolished. This finding suggests that the antinatriuresis produced by intracerebroventricular N/OFQ might occur secondary to a reduction in mean arterial pressure and subsequent changes in intrarenal (e.g., cortical vs. medullary) hemodynamics. This possibility, however, remains to be further investigated since in previous studies we have shown that intracerebroventricular N/OFQ failed to alter glomerular filtration rate or effective renal plasma flow in conscious rats (12).
The present study does not discern which specific Gαi/Gαo subunit(s) is responsible for mediating the cardiovascular depressor responses to central N/OFQ; this will require further investigation using specific ODN sequences directed against each subtype of Gαi/Gαo protein. Within the CNS, the likely sites of action in which N/OFQ elicits cardiovascular depressor effects are regions that regulate sympathetic control of cardiovascular function, including the PVN (14) and the rostral ventrolateral medulla (20). The mechanism(s) through which Gαi/o (and potentially Gαz) pathways mediate decreases in HR and MAP to central N/OFQ remains unknown. It is reasonable to postulate that central Gαi/o proteins (and to some extent Gαz) mediate bradycardia and hypotension through alteration of neuronal firing patterns and subsequent changes in central sympathetic outflow. However, further CNS microinjection and electrophysiological recording (whole animal and brain slices) studies are required to establish the central sites and underlying cellular mechanisms involved in these responses. Of merit, it is likely that central Gαi/o proteins have a more global physiological role to modulate the cardiovascular responses to other GPCR ligands. This is suggested because in pilot studies, we showed that the bradycardia and hypotension, but not diuresis, to intracerebroventricular clonidine (alpha-2 adrenoceptor/imidazoline agonist) is also abolished in conscious rats pretreated intracerebroventricularly with PTX (personal observation).
Studies were performed to investigate the role(s) that central Gαq and Gαz proteins play in mediating the cardiovascular and renal excretory responses to central N/OFQ. For these investigations, we used ODN sequences to selectively reduce brain Gαq and Gαz protein expression (4, 19, 22, 24). The ODN sequences used in these studies are specific for their respective Gα subunit protein sequences, as verified by a GenBank search and have been used extensively in vivo for selective targeting of Gα-subunit proteins (4, 19, 22, 24). In agreement with previous studies (4, 19, 24), the current data show a sequence-specific reduction in Gα-subunit protein levels 24 h following intracerebroventricular administration of each respective ODN (25 μg). We further demonstrated the specific and selective nature of the ODN sequences using a miss-sense ODN (25 μg), which did not alter CNS Gα-subunit protein levels or the cardiovascular and renal responses to intracerebroventricular N/OFQ. Our findings demonstrate that the pattern of central N/OFQ-evoked diuresis is mediated largely through Gαz (inhibitory) and Gαq (stimulatory) influences on AVP release. Thus, pretreatment of rats with a Gαz ODN blunted the magnitude and duration of the diuresis to central N/OFQ. In an opposite manner, Gαq ODN pretreatment significantly enhanced the magnitude and duration of the N/OFQ-evoked diuresis. These findings are of key physiological significance, since this is the first report that Gα-subunit signaling pathways can influence AVP release into the systemic circulation. Further, these data suggest that the pattern of urine output evoked by intracerebroventricular N/OFQ reflects, in part, the balance of CNS activity of these two counteracting Gα-subunits on respective downstream signaling pathways that affect AVP secretion.
The diuresis to central N/OFQ in rats pretreated with Gαz ODN was markedly blunted but not abolished. This observation suggests that other non-Gαz/αq-mediated pathways, possibly involving βγ, β-arrestins, or receptor kinases, may also contribute to the diuretic response elicited by central N/OFQ. Although these pathways have yet to be identified, it is clear that Gαi/o signaling pathways are not involved since PTX inhibition of central Gαi/o proteins did not alter the N/OFQ-evoked diuresis.
Following circulation in the brain, it is likely that N/OFQ may activate central Gαz and Gαq protein pathways within the hypothalamus to influence AVP secretion/release from magnocellular neurons of the PVN and SON. This premise is supported by evidence that magnocellular neurons in these nuclei possess both NOP receptors and AVP peptide (6, 23) and that N/OFQ inhibits activity of hypothalamic PVN and SON AVP-containing neurons in vitro (21, 23). Furthermore, central N/OFQ inhibits AVP release (9), and the microinjection of N/OFQ into the hypothalamic PVN of conscious rats produces a water diuresis (14). However, the findings reported in this manuscript do not establish whether the Gαz and Gαq protein interactions downstream of the NOP receptor, which influence AVP secretion occur on the same or different cells/neurons. Further studies are required, however, to elucidate the physiological importance and cellular/neuronal sites and mechanisms by which Gαz/Gαq pathways in the PVN (or potentially other brain sites) control AVP secretion and subsequently urine output.
Perspectives and Significance
This study demonstrates that separate central Gα-subunit protein signaling pathways downstream of the NOP receptor selectively mediate the cardiovascular depressor (Gαi/o) vs. diuretic responses to central N/OFQ. The diuretic response to central N/OFQ occurs through a novel interaction of this GPCR ligand with opposing Gαz, and Gαq, pathways that provide an inhibitory and stimulatory influence on AVP release, respectively. On the basis of these studies with N/OFQ, it appears that central Gα-protein signaling pathways may have an important physiological and potentially pathological role in the regulation of cardiovascular function and the renal excretion of water. On the basis of these findings, it is likely that the exogenous administration or endogenous release of other GPCR ligands (opioids, α-adrenoceptor agonists, ANG II), which are recognized to signal, at least in part, through Gαi/o, Gαz, or Gαq, may also influence systemic cardiovascular function, AVP secretion, and/or urine output through similar central Gα-protein pathways downstream of GPCRs for each respective ligand. Although this possibility has yet to be fully explored, the concept that separate central Gα-protein subunit pathways can be targeted to selectively modify the effects of GPCR ligands on cardiovascular function vs. AVP secretion and the renal handling of water in vivo is novel and opens a new approach to study the contribution of each physiological pathway to complex pathological states (e.g., salt-sensitive hypertension and congestive heart failure).
GRANTS
The research presented in this manuscript was funded to D. R. Kapusta by the National Institute of Health grants, National Institute of Diabetes and Digestive and Kidney Diseases DK43337, National Heart, Lung, and Blood Institute HL 071212, and the National Center for Research Resources COBRE P20 RR018766.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Calo G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug Rev 11: 97–112, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JSC, Yung LY, Lee JWM, Wi YL, Pei G, Wong YH. Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. J Neurochem 71: 2203–2210, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Gullapalli S, Ramarao P. Role of L-type Ca2+ channels in pertussis toxin induced antagonism of U50, 488H analgesia and hypothermia. Brain Res 946: 191–191, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Hadjimarkou MM, Silva RM, Rossi GC, Pasternak GW, Bodnar RJ. Feeding induced by food depravation is differentially reduced by G-protein α-subunit antisense probes in rats. Brain Res 955: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Hawes BE, Fried S, Yao X, Weig B, Graziano MP. Nociceptin (ORL-1) and μ-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: Evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem 71: 1024–1033, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Isgor C, Shieh KR, Akil H, Watson J. Colocalization of estrogen beta-receptor messenger RNA with orphanin FQ, vasopressin and oxytocin in the rat hypothalamic paraventricular and supraoptic nuclei. Anat Embryol 206:461–469, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Jeong SW, Ikeda SRG. Protein α subunit Gαz couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron 21: 1201–1212, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Joshi S, Lee JWM, Wong YH. Stimulation of phospholipase C by the cloned μ, δ, and κ opioid receptors via chimeric Gαq mutants. Eur J Neurosci 11: 383–388, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Kakiya S, Murase T, Aimra H, Yokoi H, Iwasaki Y, Miura Y, Oiso Y. Role of endogenous nociceptin in the regulation of arginine vasopressin release in conscious rats. Endocrinology 141: 4466–4471, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinaturetic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci 60: PL15–PL21, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Kapusta DR, Chang JK, Kenigs VA. Central administration of [Phe1Ψ (CH2-NH)Gly2]nociceptin(1–13)-NH2 and orphanin FQ/nociceptin (OFQ/N) produce similar cardiovascular and renal responses in conscious rats. J Pharmacol Exp Therap 289: 173–180, 1999. [PubMed] [Google Scholar]
- 12.Kapusta DR, Kenigs VA. Cardiovascular and renal responses produced by central orphanin FQ/nociceptin occur independent of renal nerves. Am J Physiol 77: R987–R995, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Katada T, Oinuma M, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci USA 79: 3129–3133, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krowicki ZK, Kapusta DR. Tonic nociceptinergic inputs to neurons in the hypothalamic paraventricular nucleus contribute to sympathetic vasomotor tone and water and electrolyte homeostasis in conscious rats. J Pharmacol Exp Ther 317: 446–453, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid-like ORL1 receptor. Nature 377: 532–535, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Mollereau C, Modledous L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Nature 377: 532–535, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th ed. New York: Academic, 1998.
- 18.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioid G protein-coupled receptor. Science 270: 792–794, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Rossi GC, Standifer KM, Pasternak GW. Differential blockade of morphine, and morphine-6 beta-glucuronide analgesia by antisense oligodeoxynucleotides directed against M.OR-1 and G-protein alpha subunits in rats. Neurosci Lett 198: 99–102, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Sapru HN, Chitravanshi VC. Responses to microinjections of endomorphin and nociceptin into the medullary cardiovascular areas. Clin Exp Pharmacol Physiol 29: 243–247, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Shirasaka T, Miyahara S, Takasaki M, Kannan H. Nociceptin/orphanin FQ and [Phe(1)psi(CH2-NH)Gly2]nociceptin(1–13)NH2 modulates the activity of hypothalamic paraventricular nucleus neurons in vitro. Brain Res 890: 147–153, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Silva RM, Rossi GC, Mathis JP, Standifer KM, Pasternak GW, Bodnar RJ. Morphine and morphinw-6-beta-glucaronide-induced feeding are differentially reduced by G-protein alpha-subunit antisense in rats. Brain Res 876: 62–75, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Slugg RM, Rønnekleiv OK, Grandy DK, Kelly MJ. Activation of inwardly rectifying K+ conductance by orphanin-FQ/nociceptin in vasopressin-containing neurons. Neuroendocrinology 385–396, 1999. [DOI] [PubMed]
- 24.Standifer KM, Rossi GC, Pasternak GW. Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G protein alpha subunits. Mol Pharmacol 50: 293–298, 1996. [PubMed] [Google Scholar]
- 25.Tso W, Wong YH. Opioid receptor-like (ORL1) receptor utilizes both GoA and GoB for signal transduction. Prot Pep Lett 13: 437–441, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Yung LY, Joshi SA, Chan RY, Chan JS, Pei G, Wong YH. G alphaL1 (Galpha14) couples the opioid receptor-like 1 receptor to stimulation of phospholipase C. J Pharma Exp Ther 288: 232–238, 1999. [PubMed] [Google Scholar]