Abstract
Many obese elderly persons have impaired physical function associated with an increased chronic inflammatory response. We evaluated 12 wk of exercise (aerobic and resistance) or 12 wk of weight loss (∼7% reduction) on skeletal muscle mRNAs for toll-like receptor-4 (TLR-4), mechanogrowth factor (MGF), TNF-α, and IL-6 in 16 obese (body mass index 38 ± 2 kg/m2) older (69 ± 1 yr) physically frail individuals. Vastus lateralis muscle biopsies were obtained at 0 and 12 wk and analyzed by real-time RT-PCR. Body composition was assessed by dual-energy x-ray absorptiometry. Body weight decreased (−7.5 ± 1.2 kg, P = 0.001) in the weight loss group but not in the exercise group (−0.3 ± 0.8 kg, P = 0.74). Fat-free mass (FFM) decreased (−2.9 ± 0.6 kg, P = 0.010) in the weight loss group and increased (1.6 ± 0.6 kg, P = 0.03) in the exercise group. Exercise resulted in a 37% decrease in TLR-4 mRNA (P < 0.05) while weight loss had no significant effect. Additionally, exercise led to a significant (50%) decrease in IL-6 and TNF-α mRNA (P < 0.05) while weight loss had no effect. Exercise increased MGF mRNA (∼2 fold, P < 0.05), but weight loss had no effect. In conclusion, exercise but not weight loss had a beneficial effect on markers of muscle inflammation and anabolism in frail obese elderly individuals.
Keywords: exercise, weight loss, cytokines, growth factors
aging is associated with an increase in the chronic systemic inflammation (17). Obesity also induces chronic inflammation (37) and, thus, may further contribute to the age-related increase in the production of inflammatory cytokines. Indeed, sarcopenic obesity is associated with increased levels of circulating inflammatory markers (7, 34). Inflammatory cytokines have direct catabolic effects on skeletal muscle: TNF-α impairs muscle protein synthesis (22, 23, 44) and increases muscle protein degradation (8, 26, 36) while IL-6 increases muscle protein degradation (13). High plasma concentrations of TNF-α and IL-6 are associated with lower muscle mass or strength and mobility disability (10, 11, 41), and high IL-6 and low IGF-1 levels contribute synergistically to impaired mobility (6). Elevated levels of C-reactive protein and serum IL-6 strongly predict mortality and functional decline in older persons (31). Not only does adipose tissue release cytokines, but also skeletal muscles express cytokines that have direct autocrine and paracrine effects (33). Further, obese persons have increased levels of intramuscular cytokines (33).
Diet-induced weight loss appears to lower levels of circulating cytokines, and the effect is greater with larger amounts of weight loss (2, 9, 21). The results regarding the effect of exercise training is much less convincing: some studies have shown exercise-induced reductions (24, 25) or unchanged levels (29, 43) of plasma/serum inflammatory cytokines. Nevertheless, exercise training may induce local anti-inflammatory effects in skeletal muscle that may not be reflected in the systemic circulation, as evidenced in the study of Gielen et al. (14). These investigators found that aerobic training reduced TNF-α, IL-6, and IL-1 gene expression in skeletal muscles but had no effect on levels of these cytokines in the systemic circulation. Further evidence for an important role of local cytokines in skeletal muscle is provided by Greiwe et al. (16). These investigators found that TNF-α is transcribed by human myocytes, elevated in the muscles of frail elderly, and decreased by resistance training. Moreover, the levels of TNF-α correlated inversely with muscle protein synthesis rate. Singh et al. (35) reported that resistance training increased IGF-1 staining in muscles of frail older patients. In addition, Kim et al. (20) reported that an acute bout of resistance training increased the expression of mechanogrowth factor (MGF) in older persons. Thus these findings suggest that the anabolic and functional benefits of regular exercise may be mediated, in part, through direct actions within skeletal muscle (14, 16, 20, 35). However, no previous study has directly compared the independent effects of weight loss to regular exercise on muscle cytokines and growth factors, particularly in obese older adults who constitute a growing segment of the elderly population (1). These individuals represent a unique population because of increased frailty (3, 40), which putatively derives from the additive effects of obesity and aging on chronic inflammation (17, 32, 34).
Thus the purpose of this investigation was to compare the effects of weight loss vs. exercise (combined resistance and aerobic) on the expression of inflammatory and anabolic genes in the skeletal muscle of frail-obese elderly volunteers. Specifically, we quantified the expression of mRNAs for IL-6, TNF-α, MGF, and toll-like receptor-4 (TLR-4).
MATERIALS AND METHODS
Subjects.
Subjects were sixteen obese (body mass index = 38 ± 6 kg/m2) older (age = 69 ± 1 yr) men and women who had evidence of frailty based on two of three criteria (4, 40): physical performance test (PPT) score of 18–32, peak oxygen consumption (V̇o2peak) of 11–18 ml·min−1·kg body wt−1, and self-reported difficulty or need for assistance in two instrumental activities of daily living (ADL) or one basic ADL. All subjects were sedentary (i.e., did not participate in regular exercise more than 2 times/wk) and reported having had a stable body weight (±2 kg) and had no change in medications for ≥6 mo before the study. All subjects provided written informed consent before participation in the study, which was approved by the Human Studies Committee. They were randomized to receive either ∼12 wk of weight loss or ∼12 wk of regular physical exercise.
Weight loss.
Participants in the weight loss group received a combination of an energy-deficit diet and behavior therapy. They were prescribed a balanced diet to provide an energy deficit of 500–750 kcal/day with a goal of 1–2% loss of body weight per week. They met weekly as a group with a study dietitian, who was experienced in group behavioral therapy for obesity. Standard behavioral strategies were used to modify eating habits (21).
Exercise.
Participants in the exercise group participated in exercise-training sessions (3 days/wk) led by a physical therapist. The exercise program consisted of physical therapy, endurance, and resistance exercise designed to improve physical function. Each session lasted ∼90 min: 15 min of flexibility exercises, 20–30 min of aerobic exercise, 30–40 min of progressive resistance training (PRT), and 15 min of balance exercises. Aerobic exercises included walking on a treadmill, step-ups, stair climbing, stationary cycling, and Stairmaster exercise. Initially, subjects exercised at moderate intensity (∼75% of peak heart rate), and the intensity of exercise was gradually increased to between 80% and 90% of peak heart rate. The PRT consisted of nine exercises (squats, leg press, knee extension, knee flexion, seated row, upright row, seated chest press, biceps curl, and triceps extensions) performed using a squat rack and weight-lifting machines (Hoist Fitness Systems, San Diego, Ca). The one-repetition maximum (1RM) was used to adjust exercise intensity. Initially, weightlifting sessions consisted of one to two sets performed at ∼65% of 1RM, which allowed the completion of 8–12 repetitions. The volume of exercise was gradually increased to two to three sets at a resistance of ∼80% of 1RM, which allowed the completion of 6–8 repetitions. Participants performed make-up sessions if they missed one, so each participant performed the goal of 36 sessions. Missed/made-up sessions were minimal and did not extend the average training duration (12 wk).
Quantification of mRNA expression.
Total RNA was isolated from biopsies of vastus lateralis muscle taken before and after the 12 wk of weight loss or exercise therapy. RNAzol B (Tel-test, Frienswood, TX) was used according to the manufacturer's instructions. RNA concentration was determined by spectrophotometric absorbancy at two dilutions. First-strand cDNA was generated by reverse transcription using 500 ng total RNA and the Applied Biosystems (Foster City, CA) reverse transcription (RT) kit. Real-time RT-PCR was performed using the ABI PRISM 7500 sequence detection system and PowerSYBR mix (Applied Biosystems, Foster City, CA). Arbitrary units of TLR-4, MGF, IL-6, and TNF-α mRNA were corrected to 36B4 RNA content to control for loading. The oligonucleotide primer pairs used for the mRNA determination are listed in Table 1.
Table 1.
Oligonucleotide primer pairs used for the mRNA determination
| Sense Primer | Antisense Primer | |
|---|---|---|
| TLR-4 | 5′-ATGCTGCCGTTTTATCACGGA-3′ | 5′-CTAAACTCTGGATGGGGTTTCC-3′ |
| IL-6 | 5′-GGGACTGTTGGGGATAGTAA-3′ | 5′-AGCCATCTTTGGAAGGTTCAGG-3′ |
| TNF-α | 5′-CCCAGGCAGTCAGATCATCTTC-3′ | 5′-AGCTGCCCCTCAGCTTGA-3′ |
| MGF | 5′-ACCAACAAGAACACGAAGTC-3′ | 5′-CAAGGTGCAAATCACTCCTA-3′ |
| 36B4 | 5′-GCAGACAACGTGGGCTCCAAGCAGAT-3′ | 5′-GGTCCTCCTTGGTGAACACGAAGCCC-3′ |
TLR, toll-like receptor; MGF, mechanogrowth factor.
Serum markers of inflammation.
Serum high sensitive C-reactive protein (HsCRP) was measured by using a particle-enhanced immunoturbidimetric assay on a Hitachi 917 analyzer (Roche Diagnostics, Mannheim, Germany). Serum IL-6 and TNF-α were measured by using enzyme-linked immunoabsorbent assay (Quantikine High Sensitivity kit: R&D Systems, Minneapolis, MN).
Body weight and body composition.
Body weight was measured in duplicate in the morning following a 12-h fast. Fat mass (FM) and fat-free mass (FFM) were measured by using dual-energy X-ray absorptiometry (DXA; Delphi 4500-W; Hologic, Waltham, MA). Phantom calibration was performed before each scan. The coefficient of variation for DXA measurements for both FFM and FM in our laboratory is <2% (39).
Statistical analysis.
Baseline characteristics were compared by using independent t-tests or chi-square tests. Analysis of covariance was used to determine whether the changes in the outcomes were significantly different between groups while using the baseline values as the covariates. Student's t-tests for paired samples were performed to determine whether there were significant within-group changes in the outcomes. Pearson's correlation was performed to assess associations between changes in gene expression and FFM. Values are presented as means ± SE. Data were considered significant at P < 0.05.
RESULTS
Baseline characteristics such as age, body weight, sex, and body composition did not differ between the weight loss and exercise groups (Table 2). Moreover, indexes of frailty, such as PPT score (28.6 ± 1.0 vs. 26.1 ± 1.0) and V̇o2peak (18.2 ± 0.9 vs. 17.5 ± 1.1 ml·kg−1·min−1) were similar between the weight loss and exercise groups, respectively (both P > 0.05). Because all participants in the exercise group were required to complete the 36 exercise sessions, compliance with the exercise program was 100%. In response to the exercise program, improvements in 1RM, were detected for both upper body exercises (biceps curl, 14 ± 6%; bench press, 25 ± 9%; seated row, 16 ± 5%;) and lower body exercises (knee flexion, 24 ± 5%; knee extension, 18 ± 7%; leg press, 26 ± 7%; all P < 0.05).
Table 2.
Characteristics of the study population before and after weight loss and exercise therapy
| Diet (n = 8) |
Exercise (n = 8) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age, yr | 69.6±1.4 | 68.5±1.4 | ||
| Women, n (%) | 4 (50) | 4 (50) | ||
| BMI, kg/m2 | 38.5±1.7 | 35.6±1.5* | 37.1±2.8 | 37.1±2.6 |
| Weight, kg | 108.1±5.2 | 100.6±5.3* | 104.6±6.4 | 104.4±6.0 |
| FFM, kg | 62.3±4.4 | 59.4±4.4* | 58.0±2.9 | 59.5±3.1* |
| FM, kg | 43.2±2.6 | 39.3±2.7* | 46.7±5.9 | 44.8±5.4* |
BMI, body mass index; FFM, far-free mass; FM, fat mass.
Pre/post differences are statistically significant (P < .05) based on t-tests for paired samples.
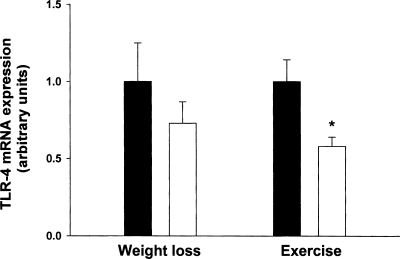
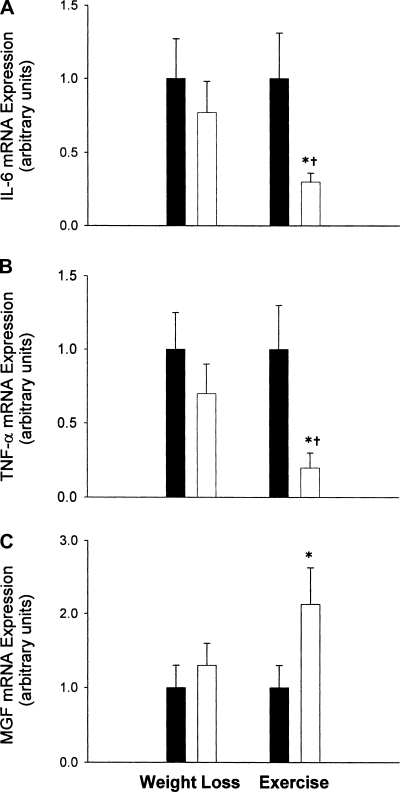
Body weight decreased in the weight loss group by 7.1% but not in the exercise group (−7.5 ± 1.2 vs. −0.3 ± 0.8 kg, P < 0.001) (Table 2). FFM decreased by 4.8% as a result of weight loss and increased as a result of exercise (+2.7%) (−2.9 ± 0.6 vs. 1.6 ± 0.6 kg, P < 0.001). TLR-4 mRNA was reduced by 37% (P < 0.05) as a result of exercise, and no significant change was observed in the weight loss group (the sample size required for a significant effect is equal to 103 subjects) (Fig. 1). Exercise led to a significant 50% decrease (P < 0.05) in the expression of IL-6 and TNF-α, whereas weight loss had no significant effect on TNF-α (the sample size required for a significant effect is equal to 337 subjects) or IL-6 expression (the sample size required for a significant effect is equal to 210 subjects) (−0.70 ± 0.30 vs. −0.23 ± 0.19 and −0.71 ± 0.30 vs. −0.30 ± 0.35, respectively; both P <.05) (Fig. 2, A and B). Exercise increased MGF expression 223% (P < 0.05) whereas weight loss therapy had no effect (the sample size required for a significant effect is equal to 25 subjects) (1.2 ± 0.38 vs. 0.30 ± 0.18; P = 0.07) (Fig. 2C).
Fig. 1.
Expression of toll-like receptor-4 (TLR-4) in skeletal muscle of frail obese elderly patients before (filled bars) and after (open bars) randomization to weight loss therapy (n = 8) or exercise therapy (n = 8). Values are means ± SE. Values are also normalized (=1.0) to baseline for each subject and corrected for the expression of a housekeeping gene (36B4). *Value significantly different from before treatment value, P < 0.05.
Fig. 2.
Expression of IL-6 (A), TNF-α (B), and mechanogrowth factor (MGF; C) in skeletal muscle of frail obese elderly patients before (filled bars) and after (open bars) randomization to weight loss therapy (n = 8) or exercise therapy (n = 8). Values are means ± SE. Values are also normalized (=1.0) to baseline for each subject and corrected for the expression of a housekeeping gene (36B4). *Value significantly different from before treatment value, P < 0.05. †Change from baseline was significantly different between the treatment and control group, P < 0.05.
Data for serum HsCRP, IL-6, and TNF-α are presented in Table 3. Serum HsCRP was significantly lower (P < 0.05) after weight loss compared with before weight loss. No significant effect of weight loss or exercise on serum levels of IL-6 or TNF-α was observed.
Table 3.
Serum concentration of inflammatory markers before and after weight loss and exercise therapy
| Diet |
Exercise | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| HsCRP, mg/l | 2.6±0.6 | 1.8±0.4* | 3.0±0.6 | 3.1±1.1 |
| IL-6, pg/ml | 2.7±0.4 | 2.6±0.4 | 2.7±0.6 | 2.7±0.6 |
| TNF-α, pg/ml | 1.8±0.6 | 1.7±0.1 | 1.6±0.1 | 1.6±0.1 |
HsCRP, high sensitive C-reactive protein.
Pre/post differences are statistically significant (P < .05) based on t-tests for paired sample.
The improvements in 1RM in the exercise group were accompanied by positive changes in functional status, as indicated by improvements in time to 1) lift a book (from 2.9 ± 0.4 to 2.1 ± 0.5 s; P = 0.04); 2) put on a coat (from 12.3 ± 4.1 to 9.2 ± 1.8 s; P = 0.01; 3) pick up a coin (from 4.6 ± 1.4 to 3.4 ± 1.1 s; P = < 0.001), and 4) walk 25 ft back and forth (from 14.9 ± 1.9 to 13.1 ± 12.3 s; P = 0.02).
DISCUSSION
The major finding of this investigation was that mRNAs for proteins involved in inflammation were significantly reduced by exercise but not by weight loss in obese elderly individuals with functional impairment. Further, TLR-4 mRNA was significantly reduced by exercise but not by weight loss. To our knowledge, this is the first investigation to report TLR-4 mRNA changes in human skeletal muscle as a result of exercise training or weight loss.
Cytokines are inflammatory mediators produced primarily by peripheral blood mononuclear cells, adipocytes, hepatocytes, and skeletal muscle. Two cytokines, TNF-α and IL-6, are linked to higher rates of skeletal muscle catabolism. TNF-α impairs muscle protein synthesis (22, 23, 44) and increases muscle protein degradation (8, 26, 36) while IL-6 increases muscle protein degradation (13). These two cytokines are thought to play a major role in the age-related decline in muscle mass and physical function. For example, Visser et al. (41) reported that circulating TNF-α and IL-6 were inversely related to muscle mass in 3,075 elderly men and women. The exact source of the circulating TNF-α and IL-6 is unclear, but these circulating cytokines are likely derived from the adipose tissue depot and/or peripheral blood mononuclear cells (PBMC). It would appear plausible that on a large scale, such as in the Visser et al. (41) study, the increase in adipose tissue as one ages or the increase in secretion of cytokines from PBMC could cause the loss of muscle mass. However, the practical importance of this elevation in circulating cytokines on skeletal muscle in large population-based studies with thousands of subjects remains to be determined as sample size has a large influence on statistical significance while the practical significance of the elevation of circulating cytokines on skeletal muscle for a given individual may be small. From our data, based on few subjects, it appears that cytokine gene expression in muscle is derived from muscle, as serum concentrations of TNF-α and IL-6 did not change with exercise but the mRNAs for these cytokines were reduced in muscle as a result of exercise. Indeed, from our study, it appears that muscle contraction through combined aerobic and resistance exercise for 12 wk acts to decrease muscle inflammatory cytokine expression, whereas weight loss has no effect.
One mechanism by which IL-6 and TNF-α are secreted is through stimulation of the toll-like receptors (TLRs) (12). The TLRs are highly conserved proteins that detect infection in humans (38). The most potent ligand for TLR-4 is lipopolysaccharide, which on binding stimulates a cascade of cellular events that culminate in the release of inflammatory cytokines (38). We found that TLR-4 mRNA is reduced as a result of combined aerobic and resistance exercise; however, ∼7% weight loss did not significantly reduce skeletal muscle TLR-4 mRNA. Data that support our data on the effects of exercise training on skeletal muscle TLR-4 mRNA expression are data from McFarlin et al. (27a), in which CD14+ cells from blood of physically active individuals had a 32% lower TLR-4 protein expression compared with physically inactive individuals. Thus it appears at least at the mRNA level that exercise training decreases TLR-4 in both monocytes and skeletal muscle.
The downregulation of TLR-4 mRNA provides a potential mechanism by which exercise training decreases the mRNA for IL-6 and TNF-α as TLR-4 stimulation leads to the production of IL-6 and TNF-α. Reduced TLR-4 stimulation decreases signaling through the IκB kinase complex, decreasing NF-κB and reducing IL-6 and TNF-α production by reduced binding of NF-κB to the promoters for IL-6 and TNF-α (22).
The mechanisms for the reduction in TLR-4 mRNA as a result of exercise training are unknown and speculative at this time. Many potential mechanisms have been summarized by Flynn et al. (12). One putative mechanism is cross-tolerance or tolerance to heat shock proteins. Heat shock proteins are known endogenous ligands for TLR and may tolerize the cell, which results in a receptor downregulation or reduced intracellular signaling. Evidence suggests that intense exercise training acts to substantially increase heat shock proteins in human skeletal muscle (27). Further, the exposure of monocytes with HSP60 resulted in a reduced signaling in response to lipopolysaccharide as well as downregulation of cell surface expression of TLR-4 (19). Other endogenous ligands that become elevated during exercise could also be involved in the tolerance or cross-tolerance associated with exercise training.
Bruun et al. (5) reported that a combined caloric restriction and exercise regimen resulted in weight loss and a reduction in skeletal muscle IL-6 mRNA. However, the contribution of weight loss, energy restriction, and/or exercise to the observed reduction in IL-6 mRNA was unclear in that investigation. Gielen et al. (14) reported that aerobic exercise training (heart rate equivalent to ∼70% V̇o2max) for 20 min/day and one group training session of 60 min each week lowered IL-6 mRNA in skeletal muscle by ∼42% in chronic heart failure patients. Importantly, we show that combined resistance and aerobic exercise training but not diet-induced weight loss, in humans, results in reduced skeletal muscle IL-6 mRNA expression by ∼50% in frail obese elderly individuals.
Another important finding of this investigation is that TNF-α gene expression was reduced by ∼50% in response to exercise training but not due to weight loss. The exercise in this investigation was primarily resistance exercise with some aerobic exercise. This is consistent with the work of Greiwe et al. (16), indicating that resistance exercise reduces TNF-α gene expression in the skeletal muscle of frail elderly although their subjects were not obese. Likewise, Gielen et al. (14) reported that aerobic exercise training lowered skeletal muscle TNF-α mRNA by ∼38% in chronic heart failure patients. Thus both resistance and aerobic exercise training decrease TNF-α mRNA in skeletal muscle.
The lack of a significant effect of ∼7% weight loss on TNF-α mRNA in skeletal muscle is similar to the results of Mingrone et al. (28), who reported that a 4.6% decrease in body weight did not significantly decrease TNF-α mRNA. Further, Bruun et al.(5) reported that a 12.7% decrease in body weight was insufficient to decrease TNF-α mRNA in skeletal muscle. It is possible that there is a threshold of weight loss that will result in a significant decrease in TNF-α mRNA, as Mingrone et al. (28) also reported that a 23.6% decrease in body weight via gastric bypass resulted in an 82% decrease in muscle TNF-α mRNA. Thus it appears that only severe weight loss is sufficient to decrease TNF-α mRNA in skeletal muscle.
The lack of significant effect of weight loss on inflammatory markers (IL-6, and TNF-α mRNA) in skeletal muscle may be related to the fact that there appears to be a low abundance of macrophages (CD68+ cells) in skeletal muscle (5). It has been suggested that CD68+ cells are the source of cytokines in adipose tissue (8). Bruun et al. (5) found that IL-6 mRNA and TNF-α mRNA and CD68+ cells were reduced by weight loss in adipose tissue where their abundance is high but there was no effect of weight loss on skeletal muscle IL-6 mRNA, TNF-α mRNA, and CD68+ cells where the abundance of CD68+ cells is low. Thus difference in the abundance of CD68+ cells in skeletal muscle and adipose tissue may explain the difference between these two tissues with regard to the effects of weight loss. We hypothesize that the effects of exercise on TLR-4, IL-6, and TNF-α mRNA is direct effect on skeletal muscle cells. This contention is supported by the work of Greiwe et al. (16), who found that TNF-α protein expression, localized to skeletal muscle via in situ hybridization, was reduced as a result of chronic exercise in frail elders.
MGF is a circulating muscle growth factor produced by alternative splicing of the IGF-I transcript and is involved in muscle tissue remodeling (15). Previous work has shown that MGF expression increases in response to one bout of resistance training in young but not old subjects (18). Similar results have been observed in an animal model of intense muscle contraction (30). Another important finding of this investigation was that exercise training markedly increased the skeletal muscle mRNA for MGF in the elderly. This is supported by the work of Hameed et al. (18), who reported a substantial increase in the mRNA for MGF as a result of 5 and 12 wk of exercise training. Interestingly, despite a ∼7% decrease in body weight in the weight loss group and therefore presumably a ∼7% decrease in the load on the muscles, there was no significant decrease in MGF expression.
A potential criticism of the present study is that the lack of effect of weight loss on inflammatory cytokine/growth factor expression may be due to a low sample size. We have, however, performed sample size calculations based on the observed difference between pre and post values for the weight loss group and the SD of the difference. We found that the number of subjects required to reach a significant effect were 210, 337, 103, and 25 for IL-6, TNF-α, TLR-4, and MGF, respectively. Therefore, when considering the feasibility of performing such interventions, it does not appear that a moderate increase in our sample size would have significantly influenced the results. A potential limitation of this study is that changes in mRNA expression in response to exercise training still need to be confirmed by changes in protein expression. The typical yield of muscle biopsy from obese elderly subjects was 50–100 mg of tissue sample. Therefore, although our findings of a discordant effect of weight loss vs. exercise on changes in cytokine/growth factor gene expression in local skeletal muscles are novel and interesting, they should be considered preliminary.
To our knowledge, this is the first study to directly compare the effects of weight loss alone vs. exercise alone on local regulators of skeletal mass, particularly in frail obese elderly. We conclude that exercise but not weight loss downregulates mRNA expression of TLR-4 and of the inflammatory factors TNF-α and IL-6, linked to increased muscle catabolism while upregulating mRNA expression of an anabolic factor, MGF, in skeletal muscles. Thus exercise but not weight loss is anti-inflammatory and anabolic to skeletal muscle in the frail obese. The anti-inflammatory and anabolic effects of exercise on gene expression were accompanied by increases in FFM, which was presumably muscle mass. These data suggest that, with regard to skeletal muscle anabolic/inflammatory gene expression, exercise is more efficacious than weight loss. Future research should evaluate the effectiveness of combining resistance exercise and weight loss together on skeletal muscle mass and the intramuscular expression of cytokines and growth factors. Exercise appears to be an important intervention for maintaining and/or increasing muscle mass during weight loss. Indeed, data from our laboratory, in younger individuals, suggest that lower-body aerobic exercise-induced weight loss resulted in a large decrease in body fat but maintenance of lower body muscle mass while caloric restriction-induced weight loss resulted in a significant decrease in lower body muscle mass (42).
GRANTS
This research was supported by the following National Institutes of Health grants: R01-AG-025501, R21-AG-025721, P30-DK-56341 (Clinical Nutrition Research Unit Pilot and Feasibility), and M01-RR-000036 (General Clinical Research Center).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc 52: 1907–1912, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 85: 3338–3342, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the women's health and aging studies. J Am Geriatr Soc 53: 927–934, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci 55: M350–M355, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290: E961–E967, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab 88: 2019–2025, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr 82: 428–434, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Coletti D, Moresi V, Adamo S, Molinaro M, Sassoon D. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43: 120–128, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab 83: 2907–2910, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons (see comments). J Am Geriatr Soc 47: 639–646, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 50: 1947–1954, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev 34: 176–181, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Fujita J, Tsujinaka T, Ebisui C, Yano M, Shiozaki H, Katsume A, Ohsugi Y, Monden M. Role of interleukin-6 in skeletal muscle protein breakdown and cathepsin activity in vivo. Eur Surg Res 28: 361–366, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42: 861–868, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Goldspink G, Williams P, Simpson H. Gene expression in response to muscle stretch. Clin Orthop Relat Res S146–S152, 2002. [DOI] [PubMed]
- 16.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 15: 475–482, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Grimble RF Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care 6: 21–29, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547: 247–254, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilmartin B, Reen DJ. HSP60 induces self-tolerance to repeated HSP60 stimulation and cross-tolerance to other pro-inflammatory stimuli. Eur J Immunol 34: 2041–2051, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288: E1110–E1119, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kopp HP, Krzyzanowska K, Mohlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes 29: 766–771, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism 56: 49–57, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab 282: E336–E347, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Larsen AI, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol 88: 805–808, 2001. [DOI] [PubMed] [Google Scholar]
- 25.LeMaitre JP, Harris S, Fox KA, Denvir M. Change in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart J 147: 100–105, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Lormes W, Wang L, Reissnecker S, Steinacker JM. Different skeletal muscle HSP70 responses to high-intensity strength training and low-intensity endurance training. Eur J Appl Physiol 91: 330–335, 2004. [DOI] [PubMed] [Google Scholar]
- 27a.McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, Timmerman KL, Coen PM. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci 61: 388–393, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Mingrone G, Rosa G, Di RP, Manco M, Capristo E, Castagneto M, Vettor R, Gasbarrini G, Greco AV. Skeletal muscle triglycerides lowering is associated with net improvement of insulin sensitivity, TNF-alpha reduction and GLUT4 expression enhancement. Int J Obes Relat Metab Disord 26: 1165–1172, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr 79: 544–551, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett 505: 259–263, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 50: 638–644, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Roubenoff R Sarcopenic obesity: the confluence of two epidemics. Obes Res 12: 887–888, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest 97: 1111–1116, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 2006. [DOI] [PMC free article] [PubMed]
- 35.Singh MA, Ding W, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab 277: E135–E143, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y, Peng X, Wang F, You Z, Dong Y, Wang S. Effects of tumor necrosis factor-alpha on the 26S proteasome and 19S regulator in skeletal muscle of severely scalded mice. J Burn Care Res 27: 226–233, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- 38.van DD, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc 55: 1438–1444, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 166: 860–866, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res 12: 913–920, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57: M326–MM332, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 102: 634–640, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White LJ, Castellano V, McCoy SC. Cytokine responses to resistance training in people with multiple sclerosis. J Sports Sci 24: 911–914, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Williamson DL, Kimball SR, Jefferson LS. Acute treatment with TNF-alpha attenuates insulin-stimulated protein synthesis in cultures of C2C12 myotubes through a MEK1-sensitive mechanism. Am J Physiol Endocrinol Metab 289: E95–E104, 2005. [DOI] [PubMed] [Google Scholar]