Abstract
Buspirone, a partial agonist of the serotonergic 5-HT1A receptor, improves breathing irregularities in humans with Rett syndrome or brain stem injury. The purpose of this study was to examine whether buspirone alters posthypoxic ventilatory behavior in C57BL/6J (B6) and A/J mouse strains. Measurements of ventilatory behavior were collected from unanesthetized adult male mice (n = 6 for each strain) using the plethysmographic method. Mice were given intraperitoneal injections of vehicle or several doses of buspirone and exposed to 2 min of hypoxia (10% O2) followed by rapid reoxygenation (100% O2). Twenty minutes later, mice were tested for hypercapnic response (8% CO2-92% O2). On a separate day, mice were injected with the 5-HT1A receptor antagonist 4-iodo-N-{2-[4-(methoxyphenyl)-1-piperazinyl] ethyl}-N-2-pyridinylbenzamide (p-MPPI) before the injection of buspirone, and measurements were repeated. In separate studies, arterial blood-gas analysis was performed for each strain (n = 12 in B6 and 10 in A/J) with buspirone or vehicle. In both strains, buspirone stimulated ventilation at rest. In the B6 mice, the hypoxic response was unchanged, but the response to hypercapnia was reduced with buspirone (5 mg/kg; P < 0.05). With reoxygenation, vehicle-treated B6 exhibited periodic breathing and greater variation in ventilation compared with A/J (P < 0.01). In B6 animals, ≥3 mg/kg of buspirone reduced variation and prevented the occurrence of posthypoxic periodic breathing. Both effects were reversed by p-MPPI. Treatment effect of buspirone was not explained by a difference in resting arterial blood gases. We conclude that buspirone improves posthypoxic ventilatory irregularities in the B6 mouse through its agonist effects on the 5-HT1A receptor.
Keywords: ventilation, periodic breathing, 5-HT1A receptor, apnea
serotonin (5-hydroxytryptamine or 5-HT)-containing neurons of the central nervous system are involved in respiratory control. Although the pharmacology of serotonin in mammals is complicated by the presence of many serotonin receptor subtypes (3), one subtype in particular, the 5-HT1A receptor, when activated by an agonist has been shown to counteract respiratory disturbances produced by hypoxia, pentobarbital, and antagonists of the N-methyl-d-aspartate receptor complex in anesthetized cats (13, 28) and to reverse morphine-induced respiratory depression in anesthetized rats (24). In addition, buspirone was reported to reverse apneustic breathing in a pediatric patient after an operation to remove an astrocytoma located in the pons and medulla (28), and to improve respiratory dysfunction in a patient with Rett syndrome (2) and in another patient after brain stem infarction (7). Overall, according to these former studies, an agonist action of buspirone on the 5-HT1A receptor seems to have a beneficial effect on breathing irregularity.
One mechanism proposed to protect against periodic breathing is short-term potentiation (STP; a phenomenon in which ventilation remains elevated above baseline values after hypoxic exposure). The contrasting response, posthypoxic frequency decline (PHFD; an observed phenomenon in which frequency falls below baseline values after hypoxic exposure) is associated with periodic breathing in patients with heart failure (1). Thus one might consider modification of posthypoxic ventilatory behavior as an important element to modify in the treatment of recurrent central apneas. In our laboratory's previous study, inbred C57BL/6J (B6) mice show PHFD and inbred A/J mouse show STP. Furthermore the B6 mouse exhibited posthypoxic breathing instability including periodic breathing, while the A/J mouse did not (10, 11, 23). Another mechanism identified as responsible for initiation and propagation of periodic breathing is an increased chemosensitivity, especially hypercapnic responsiveness (15).
The hypothesis for this study was that buspirone would modify posthypoxic ventilatory behavior and improve posthypoxic unstable breathing, including periodic breathing, present in the B6 mouse (11, 23). Results in the B6 would be compared with those in the A/J to determine buspirone effects in a strain without posthypoxic periodic breathing. Mechanisms examined for the effects of buspirone included production of STP and/or a change in hypoxic or hypercapnic sensitivity.
METHODS
Animals.
Experiments were performed using male B6 and A/J mice (n = 18 for B6 and 16 for A/J; Jackson Laboratory, Bar Harbor, ME). Animals were housed in the Louis Stokes Department of Veterans Affairs Medical Center (LSDVAMC) Animal Research Facility for at least 3 wk before investigation (food and water ad libitum; with a 7 AM to 7 PM and 7 PM to 7 AM light-dark cycle). Age was matched at 3–4 mo in both strains and all experiments in a given animal were completed within 3 wk. The experimental protocols were approved by the LSDVAMC Animal Care and Use Committee and were in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental protocols for ventilatory behavior.
Six animals from each strain were used for measurement of ventilatory behavior. Measurements were made between 10:00 AM and 2:00 PM to limit the effect of circadian rhythm. All experiments were carried out when the animals appeared awake, as determined by behavioral observation. The day before testing, mice were put into the chambers from 10:00 AM to 1:00 PM, but they did not undergo testing. After testing hours, they were returned to the Animal Resource Facility. The next day animals were weighed and then placed in the test apparatus at ∼10:00 AM for a 60-min acclimatization period. At 11:00 AM, the mice received an intraperitoneal injection of vehicle (saline), buspirone, or buspirone and 4-iodo-N-{2-[4-(methoxyphenyl)-1-piperazinyl]ethyl}-N-2-pyridinylbenzamide (p-MPPI). To investigate the dose-related effect of buspirone, several doses (1.5, 3, 5, 10 mg/kg) were injected. The order for injection was random. The volume of the injection was body weight (g)/60 ml, or usually 0.4–0.5 ml. Approximately 25 min later, the animals underwent testing. Animals were given a rest day between testing days, before the protocol was repeated with another dose. On a separate day, mice were given an intraperitoneal injection of p-MPPI (5 mg/kg), a selective 5-HT1A receptor antagonist, ∼20 min before the injection of buspirone (5 mg/kg), and the protocol was repeated. The timing to start to collect data after intraperitoneal injection of buspirone and p-MPPI was determined based on former studies (4, 25, 27).
Animals were placed in a plethysmographic chamber as previously described (10, 11, 23). Oxygen consumption (V̇o2) and CO2 production (V̇co2) were measured using the open-circuit method (10). After a 5-min recording of baseline breathing, mice were then given a 2-min poikilocapnic hypoxic challenge (10% O2-balance N2). The hypoxic gas was then flushed out of the chamber and replaced with 100% O2 for 5 min. After flushing with room air and a 15-min interval, the mice were given a 2-min hypercapnic gas challenge (8% CO2-balance O2). Body temperature was then immediately measured with a 0.5-mm-diameter thermistor (Thermonetics) inserted rectally to a depth of 1 cm.
Data analysis.
Ventilatory parameters were measured continuously throughout the testing period and scored by computer using a respiratory-based software program (LabView programming by Innovative Computer Engineering, Cleveland, OH), as previously described (10, 11, 23). The following variables were calculated and analyzed: inspiratory tidal volume (Vt; μl), breath frequency (f: breaths/min), minute ventilation (V̇e; ml/min), V̇o2 (ml/min), V̇co2 (ml/min), and respiratory quotient (RQ; V̇co2/V̇o2). Vt, V̇e, V̇co2, and V̇o2 were adjusted by body weight (g). Sighs, sniffs, and breathing during grooming were excluded from the analysis (10, 11, 23). In this study, apnea was defined as an end-expiratory pause of ≥2 average breath durations. A minimum of three instances of apnea were needed to classify a given set of breaths as periodic breathing (11).
Analysis of the ventilatory behavior.
Six separate three-way ANOVAs were used to test data for f, Vt/g, and V̇e/g both for absolute and percent change from baseline values. The factors for these ANOVAs were strain, gas challenge, and drug dosage.
For all of the ANOVAs, drug dosage (vehicle; 1.5, 3.0, 5.0, and 10.0 mg/kg of buspirone; and buspirone + p-MPPI) was treated as a single, six-level, repeated-measures factor. Gas challenge was also treated as a single repeated-measures factor. Strain was treated as a between-groups factor. This study was concerned with strain × drug and/or strain × gas challenge interactions. If a given interaction showed statistical significance, post hoc probing was performed using t-tests of estimated marginal means (simple main effects) using Sidak's correction for multiple comparisons. The stability of breathing for the first 1 min during reoxygenation was assessed by calculating coefficients of variation [CV = (SD/mean)·100] for the f of each mouse and illustrated in Poincaré plots. For the Poincaré plots, f values against n + 1 are shown. The value for f was the breath-by-breath frequency as calculated by 60/(inspiratory time + expiratory time). The apnea length was included in the expiratory time. The CVs for the reoxygenation period were then used in a two-factor ANOVA (strain × dose).
Finally, a logistic regression model was constructed to evaluate the relationship between the presence of periodic breathing and buspirone dosage, excluding p-MPPI and buspirone injection, in the B6 mice. In addition to the logistic regression model, transformed χ2 values for 2 × 2 tables were performed comparing strain and the presence of periodic breathing for each drug dose (12). Periodic breathing was treated as a binary variable for the purposes of this analysis (with 0 indicating normal breathing and 1 indicating periodic breathing). Effect sizes for each of the χ2 tests were produced via fourfold correlation coefficients. For any test, differences with P < 0.05 were considered significant. All results are expressed as means ± SD.
Experimental protocols for arterial blood-gas analysis.
Twelve B6 and 10 A/J mice were used to obtain samples for baseline arterial blood-gas analysis. All animals were implanted the arterial catheter in the left carotid artery. After 24 h of recovery period, half of the animals in each strain were received the intraperitoneal injection of buspirone (5 mg/kg), and another half mice were given vehicle. Twenty-five minute later, arterial blood-gas analyses were performed with the mice unanesthetized and freely moving in room air by collecting 0.1 ml of arterial blood using an i-STAT blood gas analyzer (Abbott Point of Care, East Windsor, NJ). The differences between treatments and strains were detected through the use of a two-way ANOVA and post hoc unpaired t-test.
Surgical procedure.
An arterial catheter was implanted in the left carotid artery for sampling of arterial blood. For this surgical procedure, anesthesia was induced and maintained using isoflurane administered through a face mask. The left common carotid artery was carefully exposed with a 1.0-cm midline incision in the ventral neck region. Inferior to the carotid bifurcation remained, and the vagus nerve was not damaged. A 60-cm Micro-Renathane catheter (model MRE025, Braintree Scientific, Braintree, MA) filled with heparinized saline (20 U/ml) was inserted ∼0.6 cm into the common carotid artery, and it was routed under skin to exit on the dorsal surface of the neck. The catheter was attached to the single channel plastic swivel (375/25PS, Instech Laboratories, Plymouth Meeting, PA) and perfused by the infusion pomp (PHD 2000, Harvard Apparatus, Holliston, MA) with heparinized saline (20 U/ml) at a rate of 7 μl/h. After surgery, mice were transferred to a round cage that allows the mice to move freely and have ad libitum access to food and water at all times. To obtain the arterial blood sample, the catheter is removed from swivel and connected the 1-ml syringe with 26-gauge needle. This approach permitted sample to be obtained without restricting mice.
RESULTS
Ventilatory behavior during room air breathing.
With regard to the metabolic variables, V̇o2/g [F(5, 50) = 3.162, P = 0.015], V̇co2/g [F(5, 50) = 3.162, P = 0.015], and V̇e/V̇co2 [F(5, 50) = 2.587, P = 0.037] showed dose × strain interactions, while V̇e/V̇o2 [F(5, 50) = 0.592, P = 0.706], RQ [F(5, 50) = 2.046, P = 0.088], and body temperature [F(5, 50) = 1.381, P = 0.247] did not. V̇o2/g tended to increase as buspirone dose increased in the B6 mice, whereas this measure was observed as steady in A/J mice (Table 1).
Table 1.
Ventilatory behavior during resting breathing with vehicle and doses of buspirone in B6 and A/J strains
| A/J | Vehicle | 1.5 mg/kg | 3.0 mg/kg | 5.0 mg/kg | 10 mg/kg | Buspirone + p-MPPI |
|---|---|---|---|---|---|---|
| f, breaths/min | 182.9±12.4 | 243.9±8.6*§ | 248.2±8.6§ | 273.2±8.5§ | 276.1±13.6§ | 215.5±16.5 |
| Vt/body wt, μl/g | 1.57±0.22* | 1.69±0.12* | 1.63±0.22 | 1.61±0.09 | 1.62±0.14 | 1.5±0.15* |
| V̇e/body wt, ml·min−1·g−1 | 0.29±0.06* | 0.41±0.03†‡ | 0.4±0.05§ | 0.44±0.03§ | 0.45±0.06§ | 0.32±0.05* |
| V̇o2/body wt, ml·min−1·g−1·10−2 | 7.91±1.02 | 8.16±0.64 | 7.67±0.6 | 7.56±0.35† | 7.71±1.3 | 8.4±1.23 |
| V̇co2/body wt, ml·min−1·g−1·10−2 | 6.12±0.88 | 5.86±0.8 | 5.43±0.41 | 6.27±0.36 | 6.14±0.69 | 5.98±0.81 |
| Body temperature, °C | 36.1±0.1 | 35.8±0.4 | 35.8±0.2 | 35.9±0.2 | 35.9±0.1 | 35.9±0.1 |
| RQ | 0.78±0.09 | 0.72±0.14 | 0.71±0.07 | 0.83±0.05 | 0.81±0.13 | 0.73±0.17 |
| V̇e/V̇o2 | 3.65±0.66 | 5.09±0.64 | 5.26±0.59 | 5.81±0.52 | 5.93±1.12 | 3.91±0.67 |
| V̇e/V̇co2 | 4.76±1.08 | 7.22±1.62* | 7.4±0.44†‡ | 7.0±0.68‡ | 7.39±1.32 | 5.54±1.29 |
| B6 | Vehicle | 1.5 mg/kg | 3.0 mg/kg | 5.0 mg/kg | 10 mg/kg | Buspirone + p-MPPI |
|---|---|---|---|---|---|---|
| f, breaths/min | 171.3±12.6 | 201.6±17.5* | 233.5±14.0§ | 261.9±13.5§ | 275.0±10.7§ | 195.0±19.5 |
| Vt/body wt, μl/g | 1.23±0.17* | 1.45±0.16* | 1.51±0.17 | 1.67±0.15§ | 1.61±0.18‡ | 1.19±0.21* |
| V̇e/body wt, ml·min−1·g−1 | 0.21±0.02* | 0.29±0.03†§ | 0.35±0.06§ | 0.44±0.04§ | 0.44±0.05§ | 0.23±0.06* |
| V̇o2/body wt, ml·min−1·g−1·10−2 | 7.15±0.76 | 7.65±1.14 | 9.28±2.03 | 9.10±1.08† | 8.35±1.09 | 8.24±1.35 |
| V̇co2/body wt, ml·min−1·g−1·10−2 | 5.46±0.44 | 6.33±1.83 | 6.71±1.51 | 7.20±1.02 | 5.98±1.48 | 5.14±2.19 |
| Body temperature, °C | 36.1±0.2 | 36.3±0.2 | 36.0±0.2 | 36.1±0.4 | 36.2±0.3 | 36.1±0.3 |
| RQ | 0.77±0.08 | 0.82±0.13 | 0.73±0.11 | 0.79±0.10 | 0.71±0.09 | 0.61±0.17 |
| V̇e/V̇o2 | 2.98±0.66 | 3.88±0.70 | 3.90±0.59 | 4.87±0.82 | 5.34±0.80 | 2.91±0.85 |
| V̇e/V̇co2 | 3.86±0.61 | 4.95±1.5* | 5.39±0.74† | 6.16±1.05‡ | 7.67±1.70§ | 5.15±2.15 |
Values are mean ± SD. B6, C57BL/6J; V̇o2, O2 consumption; V̇co2, CO2 production; body wt, body weight; RQ, respiratory quotient; f, respiratory frequency; Vt, tidal volume; V̇e, minute ventilation. Significant differences between strains:
P < 0.05;
P < 0.01. Significant differences from vehicle-treated animals:
P < 0.05,
P < 0.01.
Statistically significant interactions for dose by strain were also observed for f [F(5, 50) = 4.501, P = 0.002], Vt/g [F(5, 50) = 4.110, P = 0.003], and V̇e/g [F(5, 50) = 4.464, P = 0.002]. As buspirone dose increased, a general tendency for f and V̇e/g to increase was observed. The values of f and V̇e/g were increased in both the A/J and B6 mice with doses of ≥1.5 mg/kg of buspirone compared with vehicle (P < 0.001 and P = 0.013, for A/J and B6 mice, respectively). At doses of ≥3 mg/kg of buspirone, statistically significant differences between the two strains in V̇e/g were no longer apparent, because the B6 mice responded much more than the A/J mice. Vt was unaffected in the A/J mice by increasing doses of buspirone, whereas this measure increased in the B6 mice at doses >3 mg/kg. In both strains, p-MPPI reversed the respiratory stimulant effect of buspirone on f, Vt/g, and V̇e/g (Table 1).
Modulation for chemosensitivity and posthypoxic frequency and Ventilatory Behavior (STP or PHFD).
The three-way ANOVAs testing interactions between dose × gas challenge × strain were significant for f [F(15, 150) = 4.800, P < 0.001], percent change of f [F(15, 150) = 4.558, P < 0.001], percent change of Vt [F(15, 150) = 2.753, P = 0.005], V̇e/g [F(15, 150) = 2.170, P = 0.010], and percent change of V̇e/g [F(15, 150) = 4.524, P = 0.001], but they were not significant for Vt [F(15, 150) = 1.497, P = 0.113]. Differences were addressed separately for the hypoxic, reoxygenation, and hypercapnic gas challenge periods using estimated marginal means with Sidak's correction for multiple comparisons at the post hoc level.
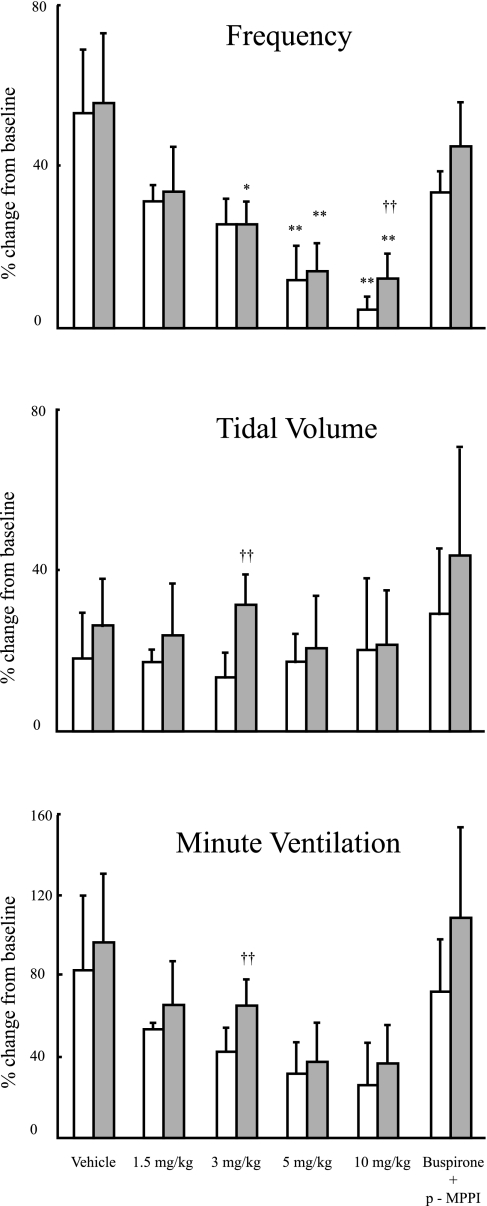
In vehicle-treated animals, hypoxic ventilatory responsiveness, expressed as percent change from baseline values, including f, Vt, and V̇e, was similar between strains when injected with vehicle. Hypoxic response when measured in terms of f was significantly decreased compared with vehicle with a dose of ≥5 mg/kg of buspirone in A/J mice and of ≥3 mg/kg of buspirone in B6 mice. Although hypoxic response when measured in terms of V̇e showed a tendency to decrease, hypoxic response in V̇e was not affected by any doses of buspirone in either strain. (Fig. 1)
Fig. 1.
Hypoxic responsiveness in frequency (top), tidal volume (middle), and minute ventilation (bottom) for A/J (open bars) and C57BL/6J (B6; filled bars) mice injected with vehicle (saline), 4 doses of buspirone (1.5, 3, 5, and 10 mg/kg), or buspirone in addition to 4-iodo-N-{2-[4-(methoxyphenyl)-1-piperazinyl]ethyl}-N-2-pyridinylbenzamide (p-MPPI) (5 mg/kg each). Values are means ± SD. Results are presented as percent change from resting breathing in room air compared with the first 30 s of breathing after exposure to hypoxia. Significant difference between strains: †P < 0.05; ††P < 0.01. Significant difference between a dose and vehicle: *P < 0.05; **P < 0.01.
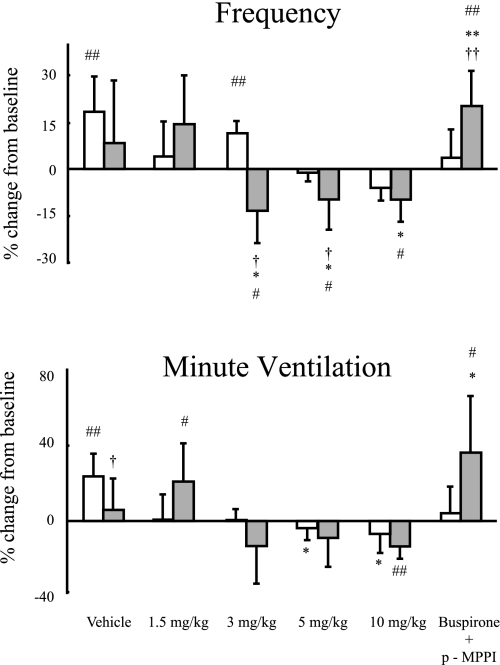
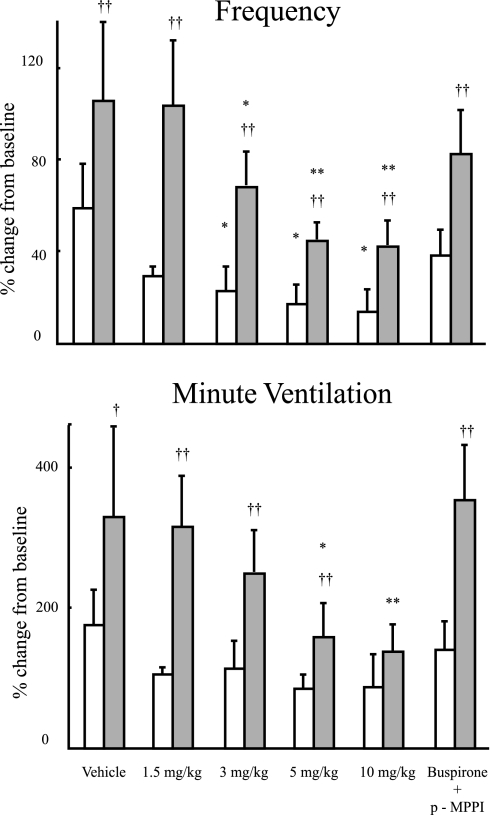
Vehicle-treated A/J mice showed STP (Fig. 2, bottom ). At a dose of ≥3 mg/kg of buspirone, B6 mice showed PHFD (Fig. 2, top). The B6 mice that were given simultaneous injections of buspirone and p-MPPI showed STP (Fig. 2, bottom); B6 with saline does not have any tendency to STP or PHFD. In vehicle-treated animals, hypercapnic response as measured by f and V̇e/g was significantly higher in B6 compared with A/J mice. The B6 mice had responses in terms of both f and V̇e/g that were significantly decreased at doses ≤3 and 5 mg/kg of buspirone, respectively. Differences between strains were found throughout the hypercapnic challenge for f and V̇e/g, although there was no difference between strains at 10 mg/kg of injected buspirone for V̇e/g (Fig. 3).
Fig. 2.
Percent change in frequency (top) and percent change in minute ventilation (bottom) are shown. For instance, for the top panel, the presence of posthypoxic frequency decline (PHFD) would be values below zero; for the bottom panel those of short-term potentiation (STP) would be above zero. Mean values ± SD for the for A/J (open bars) and B6 (filled bars) are shown for mice injected with vehicle (saline), 4 doses of buspirone (1.5, 3, 5, and 10 mg/kg), or buspirone in addition to p-MPPI (5 mg/kg each). Significant difference between strains: †P < 0.05; ††P < 0.01. Significant difference between a dose and vehicle: *P < 0.05; **P < 0.01: significant difference compared with baseline: #P < 0.05; ##P < 0.01. Vehicle-treated A/J mice showed STP. At a dose of ≥3 mg/kg of buspirone, B6 mice showed PHFD; however, the B6 mice that were given simultaneous injections of buspirone and p-MPPI showed STP.
Fig. 3.
Hypercapnic responsiveness in frequency (top) and minute ventilation (bottom) for A/J (open bars) and B6 (filled bars) mice injected with vehicle (saline), 4 doses of buspirone (1.5, 3, 5, and 10 mg/kg), or buspirone in addition to p-MPPI (5 mg/kg each). Results are presented as percent change from resting breathing in room air compared with the first 30 s of breathing after exposure to hypercapnia. Significant difference between strains: †P < 0.05; ††P < 0.01. Significant difference between a dose and vehicle: *P < 0.05; **P < 0.01.
Effects of buspirone on posthypoxic breathing.
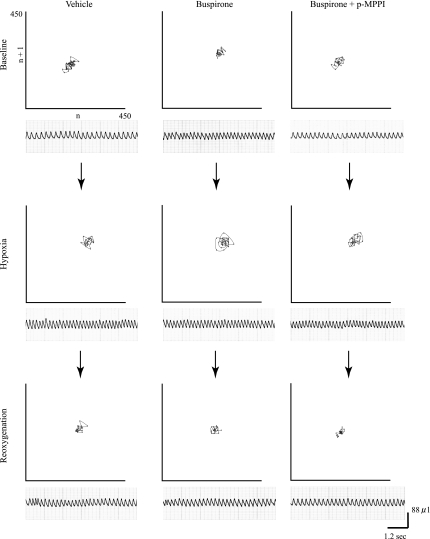
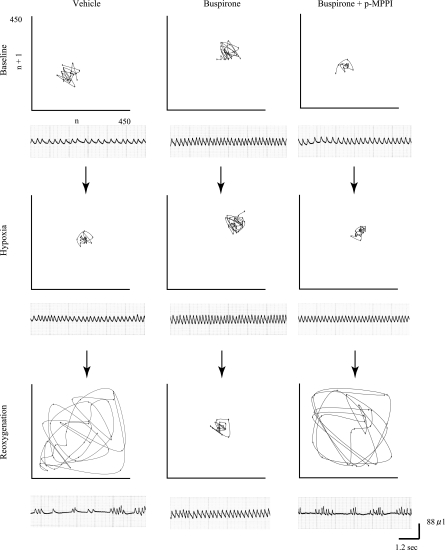
An example of breathing pattern differences between the two strains is illustrated via Poincaré plots in Fig. 4 (A/J) and Fig. 5 (B6). If breathing is stable, the data points would show a tendency toward convergence to one point, and if breathing is irregular, the data points are distributed (5, 9). Figure 5 shows visually that breathing with buspirone tended to be stable in the A/J regardless of treatment. In the B6, however, convergence is least evident on reoxygenation after vehicle and after buspirone plus p-MPPI.
Fig. 4.
Poincaré plots of n vs. n + 1 for breath frequency for each gas exposure in a typical A/J mouse. Baseline, resting breathing in room air; hypoxia, breathing during the first 30 s of hypoxic exposure; reoxygenation, breathing during the first minute after reoxygenation. Left, vehicle. Middle: 5 mg/kg buspirone. Right: 5 mg/kg of both buspirone and p-MPPI. Below each plot are the voltage outputs from the plethysmography chambers used to generate the data points.
Fig. 5.
Poincaré plots of n vs. n + 1 for breath frequency for each gas exposure in a typical B6 mouse. Baseline, resting breathing in room air, Hypoxia: breathing during the first 30 s of hypoxic exposure; reoxygenation, breathing during the first minute after reoxygenation. Left: vehicle. Middle: 5 mg/kg buspirone. Right: 5 mg/kg of both buspirone and p-MPPI. Below each plot are the voltage outputs from the plethysmography chambers used to generate the data points.
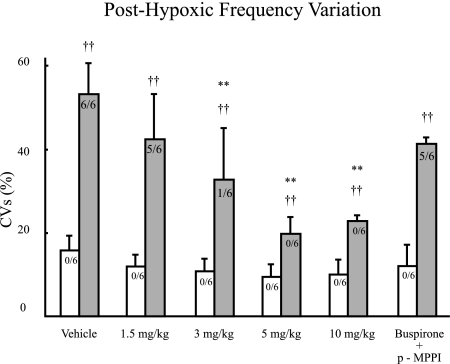
The ANOVA using CVs calculated from f for posthypoxic breathing showed a significant strain × dose interaction [F(5, 50) = 7.448, P < 0.001]. At the post hoc level, the B6 mice demonstrated higher CVs for posthypoxic breathing when vehicle was compared with doses of 3, 5, and 10 mg/kg of buspirone, but this was not shown for 1.5 mg/kg of buspirone and 5 mg/kg of buspirone and p-MPPI. The results of this ANOVA and these post hoc tests indicate that doses of >1.5 mg/kg of buspirone improved posthypoxic unstable breathing in the B6 and that p-MPPI reversed this improvement. The A/Js mice showed no differences between vehicle and any drug dose (Fig. 6).
Fig. 6.
Coefficients of variation (CVs) for frequency during the first minute of reoxygenation after a 2-min hypoxic challenge for A/J (open bars) and B6 (filled bars) mice injected with vehicle (saline), 4 doses of buspirone (1.5, 3, 5, and 10 mg/kg), or buspirone in addition to p-MPPI (5 mg/kg each). Values are means ± SD. Numbers on the bars indicate the incidence of periodic breathing for each strain dose combination. Significant difference between strains: †P < 0.05; ††P < 0.01. Significant difference between a dose and vehicle: *P < 0.05; **P < 0.01.
A series of χ2 tests were used to test the difference between strains with regard to the presence of periodic breathing for each drug injection. The results of these tests are summarized in Table 2. The results of the χ2 tests and fourfold correlation coefficients indicate that as the dosage of buspirone increased the likelihood of finding periodic breathing in the B6 decreased.
Table 2.
χ2 and χ2 effect sizes comparing the difference between A/J and B6 mice in terms the presence of periodic breathing for each drug injection
| χ2 | Significance | Effect Size† | |
|---|---|---|---|
| Vehicle | 12.00 | 0.001 | 1.00 |
| 1.5 mg/kg | 8.57 | 0.000 | 0.85 |
| 3.0 mg/kg | 1.09 | 0.300 | 0.30 |
| 5.0 mg/kg | 0.00 | 1.000 | 0.00 |
| 10.0 mg/kg | 0.00 | 1.000 | 0.00 |
| 5.0 mg/kg Buspirone and p-MPPI | 8.57 | 0.000 | 0.85 |
*Transformed for 2 × 2 tables.
As measured by fourfold correlation coefficient.
A logistic regression showed a statistically significant relationship between buspirone dose and incidence of periodic breathing (Press' Q = 22.533, P < 0.01). The model describing this relationship is as follows:
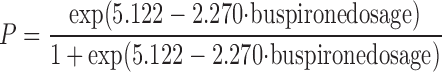 |
The results of this logistic regression indicate that the odds ratio of a B6 mouse demonstrating periodic breathing decreased by 0.103 [95% confidence interval = (0.017, 0.644)] for every milligram per kilogram of buspirone administered.
Arterial blood-gas analysis.
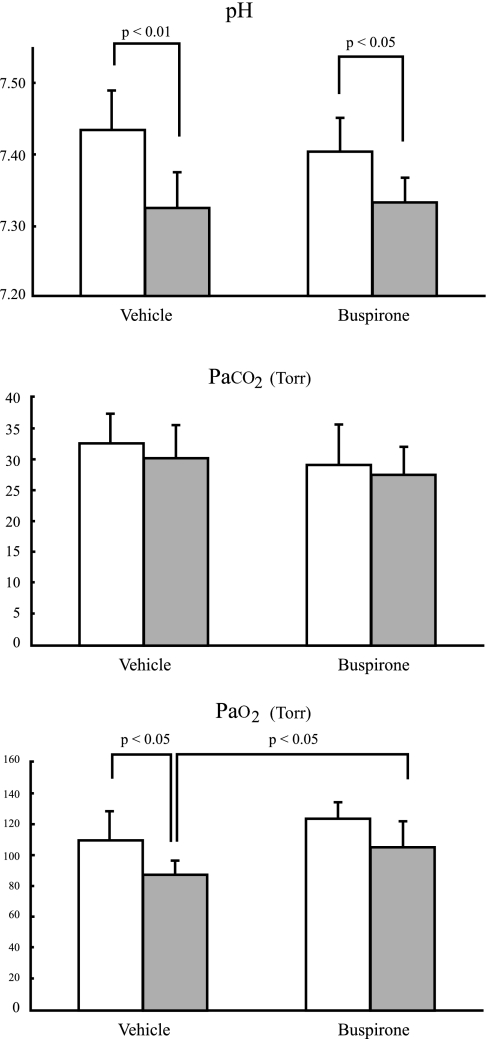
As shown in Fig. 7, arterial Po2 (PaO2) for the buspirone-treated B6 mice were significantly higher than that for saline-treated B6 mice (buspirone vs. saline: 104.8 ± 16.5 vs. 87.5 ± 8.3 Torr;, P < 0.05), whereas arterial Pco2 (PaCO2) (27.4 ± 4.8 vs. 30.2 ± 5.4 Torr; P = 0.357) and pH (7.34 ± 0.03 vs. 7.33 ± 0.05; P = 0.703) were similar between treatments. In the A/J strain, pH (buspirone vs. saline: 7.40 ± 0.05 vs. 7.44 ± 0.05; P = 0.349), PaCO2 (29.0 ± 6.6 vs. 32.5 ± 5.0 Torr; P = 0.374), and PaO2 (123.6 ± 10.3 vs. 109.6 ± 18.7 Torr; P = 0.180) were similar between treatments. In regard to the strain differences, there was a statistical difference only in pH with both buspirone and saline injections (P < 0.01 and P < 0.05 respectively) and PaO2 with saline injection (P < 0.05).
Fig. 7.
Arterial blood-gas analysis including pH, arterial Pco2 (PaCO2), and arterial Po2 (PaO2) for A/J (open bars) and B6 (filled bars). Values are means ± SD. Statistically significant differences are indicated in the figure; not noted are the insignificant differences.
DISCUSSION
The present study shows that buspirone stimulated breathing at rest, including f and V̇e, in both B6 and A/J mice in a dose-dependent manner. Whereas hypoxic ventilatory responsiveness was not significantly affected by buspirone in either strain, hypercapnic ventilatory responsiveness in B6 mice was significantly decreased by buspirone with doses ≥5 mg/kg. Although some doses of buspinone produced PHFD in B6 mice, they also eliminated periodic breathing and reduced in the CV for f. Moreover, pretreatment with p-MPPI reversed the effects of buspirone seen in both strains, indicating that a selective action of buspirone on ventilatory behavior other than posthypoxic periodic and irregular breathing was not an aberration seen only in one strain.
Buspirone is clinically approved as an antianxiety agent. Although buspirone has no significant affinity for benzodiazepine receptors and does not affect GABA binding, buspirone has a moderate affinity for brain dopamine-2 (14, 21) receptors and can bind to 5-HT7 receptors as well. Although these actions could be important in other settings, in this study, p-MPPI, a selective 5-HT1A receptor antagonist, reversed the effects of buspirone; therefore, we attribute the effects of buspirone to an activation of 5-HT1A receptors. Furthermore, in both strains, its actions not only affected posthypoxic ventilatory behavior but acute chemoresponsiveness as well.
Our data indicated that systemic administration (intraperitoneal injection) of buspirone stimulated the breathing in both B6 and A/J strains in terms of f and V̇e. These findings are supported by some previous in vivo and in vitro studies. Intraperitoneal injection of buspirone (10 and 20 mg/kg) to unanesthetized, intact, freely moving rats increased f, Vt, and V̇e evaluated by a similar plethysmographic technique (16). Garner et al. (8) have demonstrated that intravenous administration of buspirone (0.32 mg/kg) to anesthetized cats increased respiratory rate, tidal phrenic activity, and minute phrenic activity (8). The in vitro brain stem preparation of the newborn rat treated with 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT) as well as buspirone increased its f (18, 19). One of the possible mechanisms for improving the posthypoxic unstable breathing in B6 mice could have been a reduced apneic threshold for PaCO2. Indeed, in previously mentioned literature, the study of Garner et al. demonstrated that buspirone decreased the apneic threshold PaCO2, which was determined by reduction in PaCO2 levels until breathing ceased and shifted the CO2 response curve to the left of the control CO2 response curve (8).
Enhanced chemosensitivity, especially in the hypercapnic response, is associated with the occurrence of periodic breathing in patients with heart failure. In this study, we found that doses of ≥5 mg/kg of buspirone significantly depressed the hypercapnic response in the B6 strain. A reduced hypercapnic response would act to stabilize posthypoxic respiratory behavior in B6 mice. In agreement with the present findings, former studies have demonstrated that microdialysis of the 5-HT1A receptor agonist (8-OH-DPAT) into the medullary raphe region in adult unanesthetized rats and piglets led to significant decreases in the hypercapnic ventilatory response (17, 26). In regard to hypoxic ventilatory responsiveness, although f responses were significantly decreased with doses of ≥5 mg/kg buspirone, the Vt was not affected by buspirone in both strains. Thus V̇e was not significantly affected by buspirone, a finding similar to that of Taylor and colleagues (17, 26).
Another element proposed to reduce the occurrence of periodic breathing is STP, a phenomenon in which V̇e remains elevated above baseline values after hypoxic exposure, in contrast to PHFD in which V̇e or f falls below baseline values after hypoxic exposure (6, 22). The absence of STP or conversely the presence of PHFD is reasoned to promote recurrent apneas and unstable breathing (1, 20, 22, 30) and seems to be coordinated in part by pontine A5 neurons (6). In our study, B6 mice treated with ≥3 mg/kg of buspirone exhibited PHFD; however, posthypoxic periodic breathing of B6 mice was improved. Moreover, A/J mice treated with 10 mg/kg of buspirone did not exhibit posthypoxic periodic breathing despite exhibiting PHFD. Similarly to this findings, Price et al. (23) indicated that neuronal nitric oxide synthase (nNOS) inhibition produces PHFD in A/J mice; however, A/J mice treated with a nNOS inhibitor did not exhibit periodic breathing. Hence, treatment with buspirone is another demonstration that in this mouse model the appearance of periodic breathing can be independent of PHFD.
Buspirone increased V̇e in strains with and without posthypoxic ventilatory instability, an indication of a common property in the two strains. However, the B6 strain responded to buspirone with a greater increase in resting f than the A/J strain, and contrary to the B6 strain, hypercapnic ventilatory responsiveness in A/J mice was not affected by any dose of buspirone. To explain this drug × strain effect, one might consider pharmacological issues such as uptake or metabolism, differences in binding, or differences in genetic background affecting 5-HT1A receptor functions in the central nervous system.
Contrary to the former study from our group that has demonstrated that the B6 and A/J strains had a similar V̇e at the resting room air breathing and hypercapnic response (10), vehicle-treated A/J mice showed significantly higher minute ventilation at resting room air breathing and lower hypercapnic ventilatory responsiveness than vehicle-treated B6 mice. A difference in vehicle or vehicle pH between the two studies might be responsible. We speculate that the differences between strains represents the stress of surgery, rather than a trait present in the absence of injury or inflammation.
Before the dynamic gas challenges, buspirone increased V̇e in both strains, but there were no statistical differences between treatments for PaCO2 values in either strain. Thus a reduced hypercapnic responsiveness in buspirone-treated B6 animals compared with saline-treated animals did not correlate with a measurable change in baseline PaCO2 values, indicating direct effects of buspirone on hypercapnic responsiveness above eucapnia.
There are limitations to this study. First, we did not measure dynamic changes in body temperature during the challenges. One study showed that microdialysis of 5-HT1A receptor agonist lowered core body temperature by ∼0.4°C (26). Although continuous monitoring of temperature was not performed, we measured body temperature immediately after testing, and there was no difference in body temperature between strains and treatment; hence, we suspect our results were not affected by body temperature. Second, because we only changed the gas concentration inside the chamber, the oxygen (PaO2), carbon dioxide (PaCO2), and pH environment in these animals during the rapid cycling of gases is not known. The continuous measurements of arterial blood gases are impractical for detecting dynamic changes within the internal environment. Third, pH in the B6 treated with both saline and buspirone were lower than that in A/J. Both B6 and A/J mice were given 24-h recovery period after implantation of catheters; thus there may be a strain differences in the recovery from surgical stress. Fourth, Wong et al. (29) have demonstrated that buspirone increased 5-HT1A receptor occupancy in the dorsal raphe 60 min after intravenous injection of buspirone to rat. Thus it is not clear whether the time period of 25 min after intraperitoneal injection of buspirone in the present study is long enough to optimize buspirone effects on the respiratory central nerve system. As well the site of action needs to be further examined.
In conclusion, buspirone in a dose-dependent manner improves unstable breathing after reoxygenation in the B6 model of periodic breathing, consistent with a reduced hypercapnic responsiveness and without altering hypoxic responsiveness or producing STP. Furthermore, these effects appear mediated by 5-HT1A receptor activation. In addition, there were strain differences among B6 and A/J mice in the response of buspirone regarding its respiratory stimulant effect and hypercapnic responsiveness. Our theory is that the derivation of unstable breathing occurs through pathways in a network where activation of 5-HT1A receptors play an important role in determining loop gain and the degree of stabilized breathing over time.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-052452 and the Veterans Affairs Research Service.
DISCLOSURES
M. Yamauchi was supported by a traveling grant from Fuji Respironics, Danny Risberg, President.
Acknowledgments
We are grateful to Dr. Thomas E. Dick for help in the review of this work.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmed M, Serrette C, Kryger MH, Anthonisen NR. Ventilatory instability in patients with congestive heart failure and nocturnal Cheyne-Stokes breathing. Sleep 17: 527–534, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Andaku DK, Mercadante MT, Schwartzman JS. Buspirone in Rett syndrome respiratory dysfunction. Brain Dev 27: 437–438, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, Woodard EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci 25: 4550–4559, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Negro CA, Hsiao CF, Chandler SH, Garfinkel A. Evidence for a novel bursting mechanism in rodent trigeminal neurons. Biophys J 75: 174–182, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol 121: 87–100, 2000. [DOI] [PubMed] [Google Scholar]
- 7.El-Khatib MF, Kiwan RA, Jamaleddine GW. Buspirone treatment for apneustic breathing in brain stem infarct. Respir Care 48: 956–958, 2003. [PubMed] [Google Scholar]
- 8.Garner SJ, Eldridge FL, Wagner PG, Dowell RT. Buspirone, an anxiolytic drug that stimulates respiration. Am Rev Respir Dis 139: 946–950, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Gonsenhauser I, Wilson CG, Han F, Strohl KP, Dick TE. Strain differences in murine ventilatory behavior persist after urethane anesthesia. J Appl Physiol 97: 888–894, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol 91: 1962–1970, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol 92: 1133–1140, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Jaccard J, Becker MA. Statistics for the Behavioral Science. Pacific Grove, CA: Brooks/Cole, 1997.
- 13.Lalley PM, Bischoff AM, Richter DW. Serotonin 1A-receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett 172: 59–62, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Lechin F, van der Dijs B, Jara H, Orozco B, Baez S, Benaim M, Lechin M, Lechin A. Effects of buspirone on plasma neurotransmitters in healthy subjects. J Neural Transm 105: 561–573, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 164: 2147–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Mendelson WB, Martin JV, Rapoport DM. Effects of buspirone on sleep and respiration. Am Rev Respir Dis 141: 1527–1530, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol 96: 1909–1919, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Monteau R, Di Pasquale E, Hilaire G. Further evidence that various 5-HT receptor subtypes modulate central respiratory activity: in vitro studies with SR 46349B. Eur J Pharmacol 259: 71–74, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Morin D, Hennequin S, Monteau R, Hilaire G. Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res 535: 281–287, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med 165: 1251–1260, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Ohlsen RI, Pilowsky LS. The place of partial agonism in psychiatry: recent developments. J Psychopharmacol 19: 408–413, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Price ER, Han F, Dick TE, Strohl KP. 7-nitroindazole and posthypoxic ventilatory behavior in the A/J and C57BL/6J mouse strains. J Appl Physiol 95: 1097–1104, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA. Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. J Pharmacol Exp Ther 292: 704–713, 2000. [PubMed] [Google Scholar]
- 25.Sugimoto Y, Takashima N, Noma T, Yamada J. Inhibitory effects of the 5-HT(1A) receptor agonist buspirone on stress-induced hyperglycemia in mice: involvement of insulin and a buspirone metabolite, 1-(2-pyrimidinyl)piperazine (1-PP). Biol Pharm Bull 28: 733–735, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566: 543–557, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci 23: 4182–4189, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr 130: 89–94, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Wong H, Dockens RC, Pajor L, Yeola S, Grace JE Jr, Stark AD, Taub RA, Yocca FD, Zaczek RC, Li YW. 6-Hydroxybuspirone is a major active metabolite of buspirone: assessment of pharmacokinetics and 5-hydroxytryptamine1A receptor occupancy in rats. Drug Metab Dispos 35: 1387–1392, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Younes M The physiologic basis of central apnea and periodic breathing. Curr Pulmonol 10: 265–326, 1989. [Google Scholar]