Abstract
The effects of lung injury on pulmonary recruitment are incompletely understood. X-ray computed tomography (CT) has been a valuable tool in assessing changes in recruitment during lung injury. With the development of preclinical CT scanners designed for thoracic imaging in rodents, it is possible to acquire high-resolution images during the evolution of a pulmonary injury in living mice. We quantitatively assessed changes in recruitment caused by intratracheal bleomycin at 1 and 3 wk after administration using micro-CT in 129S6/SvEvTac mice. Twenty female mice were administered 2.5 U of bleomycin or saline and imaged with micro-CT at end inspiration and end expiration. Mice were extubated and allowed to recover from anesthesia and then reevaluated in vivo for quasi-static compliance measurements, followed by harvesting of the lungs for collagen analysis and histology. CT images were converted to histograms and analyzed for mean lung attenuation (MLA). MLA was significantly greater for bleomycin-exposed mice at week 1 for both inspiration (P < 0.0047) and exhalation (P < 0.0377) but was not significantly different for week 3 bleomycin-exposed mice. However, week 3 bleomycin-exposed mice did display significant increases in MLA shift from expiration to inspiration compared with either group of control mice (P < 0.005), suggesting increased lung recruitment at this time point. Week 1 bleomycin-exposed mice displayed normal shifts in MLA with inspiration, suggesting normal lung recruitment despite significant radiographic and histological changes. Lung alveolar recruitment is preserved in a mouse model of bleomycin-induced parenchymal injury despite significant changes in radiographic and physiological parameters.
Keywords: X-ray computed tomography, bleomycin, pulmonary fibrosis, lung recruitment
acute respiratory distress syndrome (ARDS) results in the heterogeneous loss of alveolar recruitment and subsequent tissue and systemic hypoxia, requiring mechanical ventilation to support gas exchange until the lung is able to recover from the inciting insult (21, 30). Unfortunately, optimal ventilatory strategies have not been fully identified, resulting in potentially severe consequences. Mechanical ventilation may exacerbate lung injury via several biophysical events occurring singularly or collectively, and include 1) repetitive shear injury induced by recruitment and derecruitment of alveolar units during tidal volume ventilation, 2) hyperinflation of normally recruited lung parenchyma during tidal volume ventilation, and 3) overdistension of normally recruited alveolar units at end expiration by the application of positive end-expiratory pressure (PEEP) (6, 21, 27). Recent work has applied high-resolution computed tomography (CT) measurements of mean lung attenuation (MLA) to assess the physiological effects of various lung recruitment strategies on alveolar distension. Many of these studies have been clinical in nature, but only a limited number have focused on changes associated with tidal volume ventilation where the majority of lung injury has been postulated to occur (6, 7, 29). Development of a methodology capable of assessing lung recruitment in small animal models with and without underlying lung pathology would provide an experimental and translational platform to enhance the understanding of alveolar recruitment-related lung injury.
Recently, micro-CT scanners have been introduced that are designed for preclinical investigations in small animals (24, 26). The majority of the commercial systems have limited utility in pulmonary imaging because they are unable to produce high-resolution images during active respiratory motion and require a breath-hold maneuver for acceptable imaging. However, these problems have been addressed with a system designed explicitly for imaging the thorax in the mouse and rat (1). This system provides the ability to noninvasively assess lung injury at a single time point or longitudinally to characterize the effect of disease or of an experimental intervention in a single animal. Few studies have employed imaging-based analysis of lung injury in small animals.
Intratracheal instillation of bleomycin has been commonly used in rodents as a simple, reproducible model of acute and subacute lung injury (11, 14). The lung injury induced by bleomycin has a characteristic progression with early injury characterized by pulmonary inflammation with neutrophilic alveolar infiltrates that peak at 7 days after drug administration (11). The inflammatory infiltrates subside during the second week and are replaced with a fibrotic pneumonitis and significant deposition of collagen within the pulmonary interstitium (14).
The objective of our study was to evaluate changes in lung recruitment associated with bleomycin-induced injury by imaging mice at end inspiration and end exhalation during tidal volume breathing and compare these findings with more established physiological and histological end points such as collagen deposition and changes in pulmonary compliance. Our hypothesis was that the characteristic progression of bleomycin-induced parenchymal lung injury would result in recognizable changes in the pattern of alveolar recruitment as measured by changes in MLA during tidal volume ventilation.
MATERIALS AND METHODS
Experimental design.
All experimental procedures conformed to National Research Council guidelines and were approved by the Duke University Institutional Animal Care and Use Committee. Twenty pathogen-free female 129S6/SvEvTac mice (Taconic Labs, Hudson, NY), 24.7 ± 1.6 g (mean ± SD), were kept in a pathogen-free vivarium and fed food and water ad libitum. The mice were sedated with isoflurane, then intraperitoneally injected with ketamine (75 mg/kg) and xylazine (11 mg/kg), suspended on a specially designed instillation board at 70° by their front incisors, and perorally intubated with a 22-gauge Jelco intravenous catheter trimmed to 2.1 cm (Medex Medical, Lancashire, UK) using a modification of the procedure described by Brown et al. (2). Following intubation mice were instilled with bleomycin (Mayne Pharma, Paramus, NJ), 2.5 U/kg, in 50 μl sterile saline (0.9% NaCl) or sterile saline alone using a catheter inserted into the endotracheal tube with delivery of the instillation fluid at the distal tip of the endotracheal tube. Animals were treated in groups of five, with bleomycin- and saline-treated animals comprising each group, and then imaged 7 days or 21 days following treatment. A total of five treated and five control mice were imaged for each time point.
Animal preparation of micro-CT imaging.
Mice were imaged as previously described (1). Animals were sedated with isoflurane and intraperitoneally (ip) injected with ketamine (75 mg/kg) and xylazine (11 mg/kg) to achieve prolonged sedation. The animals were then perorally intubated as described above. Mice were placed in a Plexiglas cradle and suspended vertically for imaging. Sedation was maintained with isoflurane 2.5% administered via a custom-built mechanical ventilator (9, 10). ECG was monitored by placement of electrodes on the animal's footpads, and body temperature was maintained using a rectal thermistor controlling heating lamps placed around the mouse. Ventilation was provided via a constant-pressure ventilator at a respiratory rate of 120 breaths/min and 30% inspired O2. Inspiratory pressure was adjusted to 9 cmH2O using a water manometer. Inspiratory time was set to 24% (0.120 s) of the breath cycle with opening of the ventilator expiratory valve occurring at 0.130 s and remaining open until the initiation of the next breath. This breath cycle resulted in PEEP of 5 cmH2O (9, 10). Ventilator air pressures were checked at the beginning and end of imaging for each animal and adjusted as necessary. Following imaging, animals were recovered from anesthesia on a heating pad to maintain body temperature. Extubation occurred when the animal was spontaneously breathing and had recovery of pinch and righting reflexes. The oropharynx was carefully suctioned before removal of the endotracheal tube.
Micro-CT image acquisition.
Micro-CT images were acquired on a custom system designed explicitly for cardiopulmonary imaging (1). X-ray parameters were as follows: 80 kVp, 220 mA, and 12-ms exposure per projection. Three hundred eighty projections were acquired at 0.5° increment between projections for a total rotation angle of 190°. Total imaging time was ∼8 min per data set. The entire image acquisition is controlled by custom software (LabView, National Instruments) that allows acquisition at any point in the ventilatory cycle. We employ the idea of a “biological imaging sequence” in which two complete data sets at end inspiration and end expiration were collected for each mouse by prospective respiratory gating of the imaging system at 0.120 and 0.480 s from the inspiratory ventilator trigger, respectively, with a 0.010-s window for image acquisition. The resulting images are effectively free of distortion arising from respiratory motion. Moreover, the data sets are effectively registered, enabling the more sophisticated histogram processing described below. The radiation dose associated with each data set was 32 cGy.
The two-dimensional (2D) projection images were used to reconstruct tomograms by Feldkamp algorithm (4) using a commercial software package (Cobra EXXIM, EXXIM Computing, Livermore, CA). Data were reconstructed with Parker weighting (20) as isotropic 512 × 512 × 512 arrays with effective digital sampling of 100 μm along all three axes.
Image processing.
Reconstructed images were converted to Hounsfield units (HU) by scaling air selected from a region outside of the animal to −1,000 HU and water to 0 HU using a water-filled phantom secured to the abdomen of each animal via a conversion file written for MATLAB (The MathWorks, Natick, MA). No artifacts related to presence of the phantom were noted in the reconstructed images. Files were then imported to ImageJ (available from http://rsb.info.nih.gov/ij/) and cropped from the first rib to the tip of the right lower lobe. Images were modified as previously described (25) using the threshold operation in ImageJ to exclude tissue <500 HU, which excluded all tissue except for the bony structures. A convex hull algorithm was then applied to the resulting images, forming an elliptical surface surrounding the outer aspect of the ribs, and included the area bounded by this ellipse. This procedure was performed for each slice in the axially oriented image stacks and was used as a binary mask to digitally exclude all tissue outside of the bounded space. The resulting thoracic images include all of the trachea below the first rib, all of the intrathoracic anatomic structures, and the cephalad portions of the liver, as well as a thin strip of intercostal tissues.
Histogram analysis.
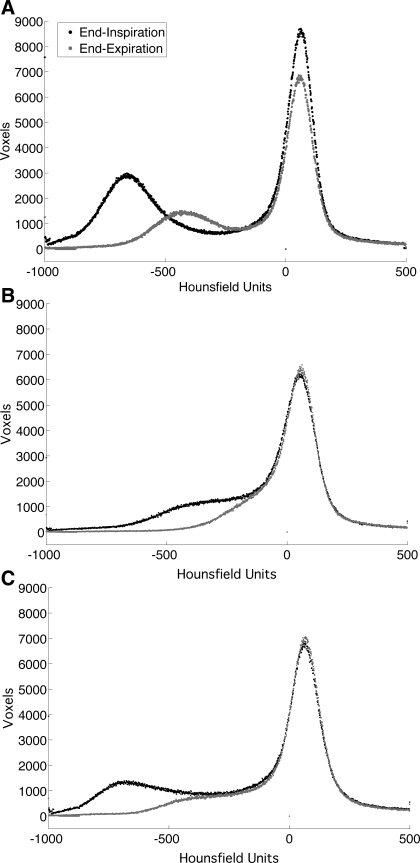
MLA was determined from the frequency histograms of the thoracic images resulting from the processing described above using the histogram function of ImageJ. Full-stack histograms were produced for each end-inspiratory and end-expiratory data set using a window from −1,000 to 500 HU with 1 to 1 binning. The resulting histograms displayed two prominent features, a peak at low HU that shifted with lung inflation corresponding to voxels containing lung parenchyma, and a higher-attenuation peak that did not substantially change with lung inflation, which corresponded to voxels containing the soft tissue structures of the mediastinum, portions of the liver, and intercostal tissues (Fig. 1A). Marked changes occurred in the lung parenchyma portion of the histograms depending on timing of the ventilatory cycle and degree of lung injury. Lung injury resulted in a substantially greater MLA at end exhalation, resulting in an inability to distinguish the lung and soft tissue curves (Fig. 1, B and C). To permit analysis of the expiratory pulmonary parenchymal histograms, we subtracted end-inspiratory histograms from end-expiratory histograms, resulting in cancellation of voxels corresponding to the soft-tissue structures since these tissues minimally changed in density from inspiration to expiration, allowing the visualization of the pulmonary voxels previously incorporated in the soft tissue portion of the frequency histogram.
Fig. 1.
Representative end-inspiration (black tracing) and end-expiration (gray tracing) histograms from control (A), week 1 bleomycin-exposed (B), and week 3 bleomycin-exposed mice (C). Peak centered near 0 Hounsfield units (HU) is composed of the soft tissues of the mediastinal structures and the chest wall. The portions of the histogram that change with inhalation are derived from voxels containing lung parenchyma. Note increased density of the pulmonary voxels in bleomycin-exposed mice, particularly with end exhalation.
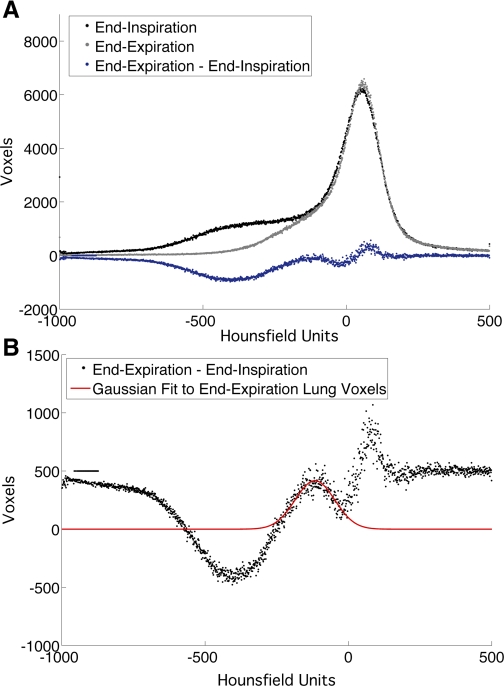
Gaussian curve fitting was performed for the region of the frequency histogram corresponding to the end-inspiratory and end-expiratory pulmonary parenchyma on the subtraction histograms described above (Fig. 2). Estimation of Gaussian mean and width at half curve height was performed using the curve-fitting toolbox in MATLAB, and values of each curve were compared across experimental groups. Determination of MLA shift was performed by subtracting end-inspiratory MLA from end-expiratory MLA. Skewness and kurtosis were determined for each curve by examining the histogram curve of interest ±250 HU from MLA using MATLAB.
Fig. 2.
A: representative subtraction histogram (blue tracing) derived from subtracting end-inspiration histogram (black tracing) from end-expiration histogram (gray tracing). B: enlargement of subtraction histogram with modeled Gaussian average (red tracing) over end-expiration portion of the curve. Note the whole curve has been displaced by 500 voxel units to facilitate curve modeling. The inverse subtraction histogram was used to model the end-inspiration portion of the curve.
Fraction of air within the lung tissue was calculated with a modification of the method described by Gattinoni et al. (7) using Eq. 1:
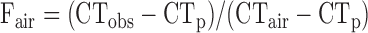 |
(1) |
where Fair is the value of the fraction of air in the lung, including the large airways within the region of interest, CTobs is the observed MLA in HU for the region of interest, CTp is the lung density in HU of the parenchymal portions of the lung taken from the mean value of the density of the mediastinal structures, and CTair is the density of air in HU defined as −1,000 HU.
Cavalieri lung volume estimates.
Parenchymal lung volume measurements were performed as previously described (25). Briefly, 10 random stratified sections were selected from each processed data set, with initial section selected from a table of random numbers used only once. A series of digital points 1 pixel square in area were applied to each section at a density of 300 pixels/point using the “Grid” plug-in for ImageJ. Points overlying the thoracic cage and overlying the lung parenchyma were counted for each section, while total number of voxels in the data set were tabulated using the “voxel counter” plug-in for ImageJ (both plug-ins available from http://rsb.info.nih.gov/ij/). Lung volumes were determined using Eq. 2 (18):
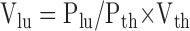 |
(2) |
where Vlu is the calculated lung volume, Plu is the total number of points overlying the lung parenchyma, Pth is the number of points overlying the entire thorax, and Vth is the volume of the thoracic cage measured by summation of the voxels in the thoracic cage multiplied by volume of the individual voxels. Tidal volumes were determined by subtracting end-expiratory lung volumes from end-inspiratory lung volumes.
Quasi-static compliance measurements.
Immediately following the completion of imaging, quasi-static compliance measurements were performed using a computer-controlled small animal ventilator (FlexiVent, SCIREQ, Scientific Respiratory Equipment, Montreal, Quebec, Canada). Mice were sedated with intraperitoneal pentobarbital sodium (50 mg/kg), and tracheotomy was performed using PE-10 tubing, which was secured with two ligatures around the trachea to provide an air-tight seal and prevent migration of the tubing, followed by paralysis with pancuronium (0.8 mg/kg ip). Animals were ventilated in the supine position at a respiratory rate of 120 breaths/min, and PEEP was set to match the level applied during imaging. Before quasi-static compliance measurements, the mouse lungs were inflated to total lung capacity using 30-cmH2O pressure held for 3 s. Lungs were inflated from PEEP to 30 cmH2O and back in seven increments for inflation and deflation with a 3-s pause at each increment. Compliance was calculated using the software supplied with the ventilator from the slope of the Salazar-Knowles (23) equation fit to the deflation limb of the pressure-volume curve at 5 cmH2O. Animals were killed with a lethal dose of pentobarbital sodium following compliance measurements.
Histology.
After completion of quasi-static compliance measurements, the chest cavity was opened and the right lung was tied at the hilum with silk ligature, removed, and frozen in liquid nitrogen and stored at −80°C until further analysis. The left lung was inflated with 10% phosphate-buffered formalin to 20 cmH20 and held for 30 min. The trachea was tied off, and the samples were placed in formalin for further fixation. Samples were then trimmed of nonpulmonary tissue, the left lung was embedded in paraffin, and 6-μm coronal sections were taken from the midlung and stained with hematoxylin and eosin (H and E) or Masson's trichrome stain in adjacent sections.
Collagen measurements.
Tissue collagen was measured using the Sircol Assay (Bicolor, Newtownabbey, UK) according to the manufacturer's instructions. Briefly, the right lung of each mouse was minced and placed in 3 ml of 0.5 M acetic acid and homogenized with a Teflon pestle and glass mortar on ice until all tissue was disintegrated and incubated on a shaker table at 4°C overnight. Samples were then pelleted, and 100 μl of the supernatant was incubated with 1 ml of the sircol dye reagent (sirius red) for 30 min at room temperature. Samples were again pelleted, and the supernatant was discarded. Pellets were resuspended in 1 ml of the alkali reagent, diluted 1:10, and absorbance was measured at 563 nm. A calibration curve using known concentrations of a reference collagen solution supplied with the assay kit was linear to 50 μg/ml and was used to calculate the collagen concentrations in the experimental samples.
Statistics.
Data are presented as means ± SD. One-way ANOVA was performed for all comparisons, with Tukey-Kramer testing for multiple comparisons within groups when statistical significance was present. All statistical calculations were performed using the statistics toolbox in MATLAB with significance defined as P < 0.05.
RESULTS
Animals.
All mice survived to their respective imaging time point. Control mice had weight changes of −0.56 ± 0.50 and +0.34 ± 0.60 g at weeks 1 and 3, respectively, compared with the baseline weight, while bleomycin-exposed mice had expected weight loss of −2.48 ± 1.24 and −2.06 ± 1.69 g at weeks 1 and 3, respectively. All mice were imaged successfully and were recovered from anesthesia postimaging for measurements of pulmonary compliance later that same day.
Imaging.
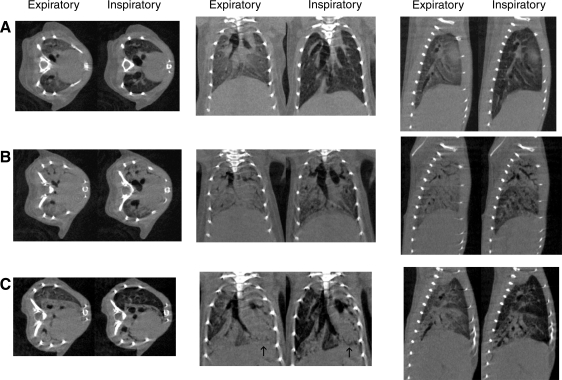
Representative inspiratory and expiratory images are shown in Fig. 3, A–C, for control mice and mice at 7-day and 21-day post-bleomycin administration, respectively. In control mice, primary imaging changes from expiration to inspiration included enlargement of the central airways, depression of the diaphragm (seen on coronal and sagittal images), and reduction in the radiographic density of the lung parenchyma (Fig. 3A). At 7 days post-bleomycin administration, mice developed dense parenchymal opacities with a primarily apical and central distribution. At inspiration, the basal segments of the lung inflated incompletely and remained radiographically denser than normal lung tissue. In addition, the apical and central areas of injured lung appeared to minimally inflate and remained opacified (Fig. 3B). At 21 days, the inflammatory radiographic changes have regressed in the less affected portions of the lung, leaving near normal-appearing tissues abutting densely consolidated portions of lung. At inspiration, the less involved areas appeared to expand normally, while the areas of consolidation appeared to have minimal change in volume (Fig. 3C).
Fig. 3.
Representative micro-computed tomography (micro-CT) images of control mouse (A), week 1 mouse (B), and week 3 mouse (C). Note darkening of lung parenchyma, diaphragmatic depression, and enlargement of the airways present in control mouse (A). In week 1 mouse (B), there is dense consolidation in the expiratory images with ground-glass opacities at end inspiration in the peripheral and lower lung fields with persistent consolidation apically. In addition there is limited movement of the diaphragm, suggesting reduction in lung volumes at end inspiration. Week 3 mouse shows areas of clearing with near normal-appearing lung adjacent to densely consolidated lung that displays minimal change in appearance with lung inflation. Also note asymmetric movement of the diaphragm on coronal images (arrows), suggesting limited inflation in the consolidated left lung.
Histogram analysis.
Histograms were constructed for each mouse at end exhalation and end inspiration. Histograms from control animals revealed separation in MLA between end-inspiratory and end-expiratory pulmonary tissue density. Subtraction of histograms also demonstrated clear resolution of the two radiographic densities (Fig. 1A). Radiographic density corresponding to soft tissue and mediastinal structures showed no change in HU between inspiration and expiration. Total number of voxels in the soft-tissue region of the histogram showed small declines with expiration.
Histograms from bleomycin-exposed mice with imaging at 1 wk revealed increased MLA in both end inspiration and end exhalation. At end exhalation, the lung tissue developed a radiographic density close to the soft-tissue structures, making it difficult to separate the two tissues visually (Fig. 1B). However, by subtracting the end-inspiratory histogram from the end-expiratory histogram, it was possible to identify the end-expiratory voxels (Fig. 2). Analysis of week 3 bleomycin-exposed mice revealed a normalization of MLA at end inspiration; however, end-expiratory MLA showed a nonsignificant trend toward increased lung density (Fig. 1C).
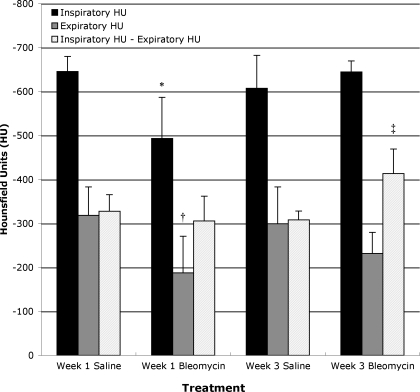
MLA for the end-inspiratory lung in control mice was −646 ± 34 and −607 ± 75 HU for weeks 1 and 3, respectively, while end-expiratory MLA was −318 ± 65 and −299 ± 84 HU, respectively (Fig. 4). In bleomycin-administered mice, MLA at end inspiration was −493 ± 93 and −645 ± 25 HU at weeks 1 and 3, respectively, while expiratory lung density was −188 ± 84 and −232 ± 48 HU at weeks 1 and 3, respectively. MLA was significantly greater for bleomycin-exposed mice at week 1 for both inspiration (P < 0.0047) and exhalation (P < 0.0377) (Fig. 4).
Fig. 4.
Mean lung attenuation (MLA) at end-inspiration, end-exhalation, and shift in MLA from expiration to inhalation ± SD in saline- and bleomycin-exposed mice at weeks 1 and 3. Note significantly increased MLA at both end inspiration and end expiration in bleomycin-exposed mice at week 1. There is also a significant increase in MLA shift from end inspiration to end expiration in week 3 bleomycin-exposed mice. *P < 0.0047 compared with other end-inspiratory values. †P < 0.0378 compared with other end-expiratory values. ‡P < 0.005 compared with other shifts in MLA.
Although the MLA in 1-wk bleomycin-exposed mice was significantly greater at both inspiration and exhalation than control values, the shift in MLA from exhalation to inspiration was no different in the 1-wk bleomycin-exposed mice compared with either of the two control groups (306 ± 56 HU in bleomycin-exposed mice vs. 328 ± 38 and 308 ± 20 HU for control mice at weeks 1 and 3, respectively). In week 3 bleomycin-exposed mice, the MLA shift was significantly greater than either week 1 or week 3 control mice (413 ± 56 HU, P < 0.005, Fig. 4). There were no significant differences between the two control groups for any of the measured parameters. Calculations of Fair mirror changes in MLA and are presented in Table 1.
Table 1.
Fractional content of air in lungs measured by micro-CT in control and bleomycin-exposed mice at 1 and 3 weeks
| Fraction of Air Comprising Observed Lung Volume |
||
|---|---|---|
| End Inspiration | End Exhalation | |
| Control (1 wk) | 66.6±3.2% | 35.7±6.1% |
| Bleomycin (1 wk) | 52.2±8.8%* | 23.3±7.9%† |
| Control (3 wk) | 63.0±7.1% | 33.9±7.9% |
| Bleomycin (3 wk) | 66.5±2.4% | 27.5±4.6% |
Values are means ± SD; n = 5 mice for each group. Micro-CT, micro-computed tomography.
P < 0.005.
P < 0.038.
Assessment of skewness and kurtosis of the curves revealed minimal positive skewness at both inspiration and exhalation in control and bleomycin-exposed mice, with the exception of the week 1 control group, which had weakly negative values for both inhalation and exhalation (Table 2). There was no significant change in skewness associated with bleomycin treatment at any lung inflation or time point. Kurtosis was 1 or less at end inspiration for all treatment groups, indicating curves that approximate the normal distribution; however, kurtosis did increase substantially with exhalation. There were no statistical differences in kurtosis associated with bleomycin treatment (Table 2).
Table 2.
Skewness and kurtosis data for inspiratory and expiratory lung histograms in control and bleomycin-exposed mice at weeks 1 and 3
| Control Mice |
Bleomycin-Exposed Mice | |||||||
|---|---|---|---|---|---|---|---|---|
| Inspiratory |
Expiratory | Inspiratory | Expiratory | |||||
| Skewness | Kurtosis | Skewness | Kurtosis | Skewness | Kurtosis | Skewness | Kurtosis | |
| Week 1 | −0.19±0.10 | 1.75±0.07 | −0.75±0.93 | 3.30±0.53 | −0.50±0.47 | 2.12±0.69 | −0.46±0.40 | 2.58±0.53 |
| Week 3 | −0.30±0.18 | 1.91±0.20 | −1.13±0.26 | 3.58±0.68 | 0.29±0.13 | 1.89±0.24 | −1.24±0.90 | 4.94±3.58 |
Values are means ± SD; n = 5 for each group. No significant differences were found between control and bleomycin-exposed mice within or between time points.
Lung parenchymal volumes.
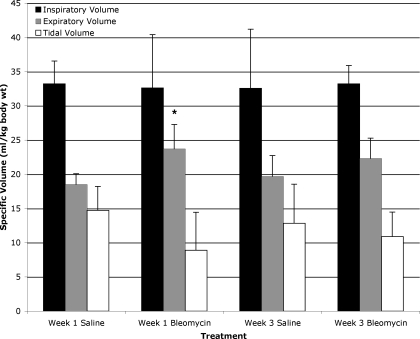
To account for differences in body weight in mice at the start of the experiment, the lung volume data are normalized to body weight at the time of bleomycin administration and presented as specific lung volumes. We elected to use starting weight due to the substantial weight loss in the bleomycin-exposed mice, which is unlikely to change anticipated absolute lung volumes. Inspiratory specific lung volumes in saline exposed mice were 33.2 ± 3.3 and 32.5 ± 8.7 ml/kg for weeks 1 and 3, respectively, while bleomycin-exposed mice had values of 32.6 ± 7.8 and 33.2 ± 2.7 ml/kg for weeks 1 and 3, respectively (Fig. 5). Interestingly, specific lung volumes at end expiration were significantly greater in bleomycin-exposed mice at week 1 with a value of 23.7 ± 4.3 ml/kg compared with week 1 saline-exposed mice but did not meet significance with week 3 saline-exposed mice (18.5 ± 1.6 and 19.7 ± 3.0 ml/kg, respectively, P < 0.042). Week 3 bleomycin-exposed mice had end-expiratory lung volumes of 22.3 ± 2.9 ml/kg, which did not achieve statistical significance when compared with week 1 or week 3 saline-exposed mice. There was a nonstatistically significant trend toward reduced tidal volume in the week 1 bleomycin-exposed mice (Fig. 5).
Fig. 5.
Lung parenchymal volumes at end-inspiration, end-expiration, and calculated tidal volumes ± SD for saline- and bleomycin-exposed mice at weeks 1 and 3. Values are normalized for body weight at time of bleomycin or saline administration. End-expiratory lung parenchymal volumes are significantly increased in week 1 bleomycin-exposed mice compared with week 1 saline-exposed mice, *P < 0.042. There were no significant differences between week 3 bleomycin-exposed mice and control mice.
Quasi-static compliance measurements.
Quasi-static pressure-volume measurements were completed for all mice before death. Bleomycin-exposed mice displayed a significant reduction in compliance compared with control mice. Lung compliance continued to trend downward from week 1 to week 3 in bleomycin-exposed mice, but this change did not reach statistical significance (0.067 ± 0.009 vs. 0.051 ± 0.010 ml/cmH2O, P > 0.05; Table 3).
Table 3.
Quasi-static compliance measurements in saline and bleomycin-exposed mice at 1 and 3 wk
| Compliance, ml/cmH2O | |
|---|---|
| Saline (1 wk) | 0.104±0.008 |
| Bleomycin (1 wk) | 0.067±0.009* |
| Saline (3 wk) | 0.103±0.019 |
| Bleomycin (3 wk) | 0.051±0.010* |
Values are means ± SD; n = 5 mice for each group.
P < 0.00003 compared with saline-exposed mice. There is no significant difference between weeks 1 and 3 bleomycin-exposed mice.
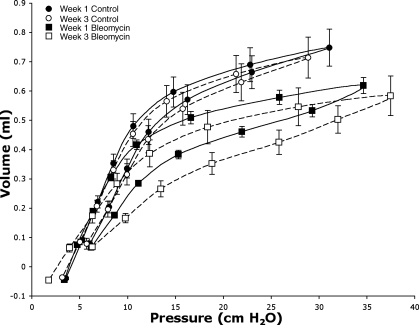
Inspection of the pressure-volume curves (Fig. 6) reveals small change in compliance at low lung volumes in week 1 injured animals but a marked decline in compliance at higher lung volumes compared with control mice. The decline in compliance progresses by week 3 at higher lung volumes in injured mice, while there is loss of compliance at lower lung volumes as well. In addition, there is an increase in hysteresis between the inspiratory and expiratory limbs of the pressure-volume curves in the injured mice.
Fig. 6.
Quasi-static pressure-volume loops from control mice (•, week 1; ○, week 3) and bleomycin-exposed mice (▪, week 1; □, week 3). Note flattening of curve at higher pressures and increased hysteresis in bleomycin-exposed mice, demonstrating loss of compliance; n = 5 for all curves.
Collagen measurements.
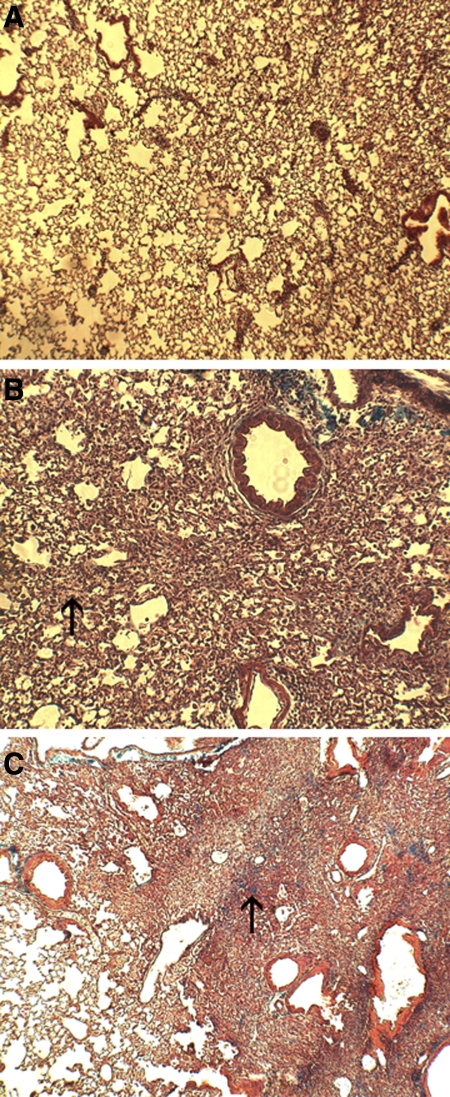
As anticipated, total lung collagen did not change between control mice and week 1 bleomycin-exposed mice (8.59 ± 0.99, 6.73 ± 0.65, and 7.83 ± 1.24 mg/lung for saline-exposed mice at weeks 1 and 3 and bleomycin-exposed mice at week 1, respectively) but increased by 53% in the week 3 bleomycin-exposed group (10.29 ± 1.45 mg/lung, P < 0.0011) compared with the week 3 control mice. Histologically, mice showed little change between control mice and week 1. Primarily there was interstitial infiltration of inflammatory cells consistent with the early inflammatory injury previously described in this model. At week 3, however, severe interstitial pneumonitis was present with early collagen deposition and loss of identifiable architecture within the affected lung parenchyma (Fig. 7).
Fig. 7.
Histological samples from control (A), week 1 bleomycin-exposed (B), and week 3 bleomycin-exposed mice (C). Note thickening of the interstitium at week 1 (arrow, B) due to cellular infiltration, with progressive pneumonitis at week 3 characterized by severe architectural distortion and early collagen deposition (arrow, C).
DISCUSSION
We have developed a quantitative in vivo micro-CT-based technique suitable for the assessment of lung injury in small animals. We demonstrate characteristic radiographic changes associated with the progression of bleomycin-induced lung injury in living mice and quantify alterations in lung recruitment and lung parenchymal volumes that correspond to different periods in the progression of bleomycin-induced lung injury.
CT imaging of week 1 lung injury reveals rightward shift in MLA corresponding with the consolidation and ground-glass opacities present radiographically. Total specific lung parenchymal volumes are preserved at end inspiration and increased at end expiration due to space-occupying consolidation related to the early inflammatory injury following bleomycin exposure (Table 1). Despite the observed injury, MLA shift with inspiration is preserved, suggesting normal increase in fraction of aeration from exhalation to inhalation, although absolute fraction of aeration is depressed at both imaging points. In contrast, imaging at week 3 reveals an increased MLA shift and increased transition in aeration from exhalation to inspiration, resulting in normal fraction of aeration at end inspiration. These findings suggest alveolar derecruitment at end exhalation that may be related to the development of fibrosis at this time point as demonstrated by increases in total collagen content. The increased MLA shift may represent regions of lung that are undergoing shear injury due to repetitive alveolar recruitment and derecruitment.
Several prior studies have examined the radiographic changes associated with bleomycin-induced lung injury in small animals with similar findings (3, 11, 13, 15, 16). However, few studies have examined the radiographic changes associated with tidal volume ventilation in lung injury models.
Neumann et al. (19) examined the rate of lung recruitment and derecruitment using porcine models with administration of oleic acid, repeated lavage to produce surfactant washout, or lipopolysaccharide to induce lung injury. These authors reported that MLA was greater in the oleic acid model at both end inspiration and end exhalation, but the MLA shift was ∼300 HU for all three models, similar to the findings observed in our week 1 bleomycin-exposed mice. Ford et al. (5) examined changes in lung attenuation in anesthetized but otherwise healthy free-breathing C57BL/6 mice. They reported MLA at end inspiration was −548 HU, modestly greater than the values we determined in this study for control mice by 50–75 HU, whereas end-expiratory MLA was −467 HU in the study of Ford et al. (5), ∼150 HU less dense than our values from control mice at end expiration. The cause of the end-inspiratory differences in MLA between the two studies is likely related to inspiratory pressure on the ventilator being set slightly above normal physiological pressures in our mice, which may have resulted in a modest increase in lung volume at end inspiration. The differences in MLA at end exhalation may be related to several factors. First, inbred strain differences may have an effect on end-expiratory MLA as Mitzner et al. (17) has previously shown significant differences in functional reserve capacity (FRC) between A/J and C57BL/6 mice, while Reinhard and colleagues (22) have previously shown that FRC is 10% larger in female C57BL/6 mice compared with 129Sv/Im mice. Therefore, it is likely that the female 129S6/SvEvTac mice we studied may normally have a lower FRC than the male C57BL/6 mice used in the study of Ford et al. (5), as reflected by an increased end-expiratory MLA. Second, mice in this study were ventilated at 120 breaths/min with an inspiratory time of 0.120 s, while most free-breathing mice in the study of Ford et al. (5) had respiratory rates closer to 150 breaths/min with measurements of inspiratory time closer to 0.170 s that encompassed the entire inspiratory segment of the breath. Consequently expiratory time is 0.380 s in the present study, while expiratory times in the free-breathing mice are likely closer to 0.230 s, potentially resulting in greater lung deflation and so higher end-expiratory MLA in the present study. Third, there may be differences in MLA between our study and that of Ford et al. (5) due to normalization of lung density and conversion of the image density to HU. However, if this were the case, we would anticipate a fixed offset in MLA between the two studies. In fact the MLA shift in the study of Ford et al. (5) was ∼100 HU, while MLA shift in the present study was close to 300 HU, which is unlikely to be related to HU scaling of the images. Finally, mouse positioning may have had some effect on lung volumes, with the upright positioning used in this study resulting in changes in compliance and so changes in lung inflation compared with the prone imaging used in the study by Ford et al. (5). However, we performed duplicate measurements of compliance in a mouse placed in the imaging cradle, and we found that quasi-static compliance was 0.0918 and 0.0954 ml/cmH2O in the vertical position compared with 0.0943 and 0.0942 ml/cmH2O for the supine position. These findings suggest that body position may have little effect on lung compliance in anesthetized mice.
Quasi-static compliance in injured mice at both weeks 1 and 3 was significantly decreased despite evidence of preserved lung recruitment as measured radiographically. This seemingly paradoxical result may be explained by two factors. First, MLA is a measure of lung density and shifts in MLA with inflation reflect aeration within a given region of interest. However, regional inflation may be heterogeneous, particularly in injured lung (29), and so MLA within a given region may not reflect changes in tidal volume. Recent work by Terragni et al. (29) employed a similar histogram subtraction technique to quantify changes in lung ventilation in combination with regional lung density analysis and demonstrated significant heterogeneity in tidal volume recruitment in patients with ARDS. Second, inflation pressures during imaging were limited to 9 cmH2O while examination of the pressure-volume curves reveals small differences in lung volume between control and injured mice at these pressures. Consequently, differences in compliance may be too subtle to be detected radiographically at physiological pressures in this model of lung injury.
The strengths of the present study are the quantitative nature of the analysis, which allows for reproducible and physiologically useful assessments of the injury caused by bleomycin administration; the semiautomated image analysis, which requires minimal image processing before generating the desired histograms; and the minimally invasive nature of the technique, which may be repeated in the same animal over time. Although not performed in this experiment, we have previously serially imaged individual mice up to three times, 1 wk apart (unpublished observation), and so it may be possible to longitudinally examine the changes related to bleomycin exposure in an individual mouse.
The primary limitation of this technique is the inability to directly correlate the regions of lung evaluated with histogram analysis with the radiographic images. Recent studies have used a deformable image registration algorithm to quantify the expansion of specific regions of lung during inflation (8). It is possible that poorly but significantly inflated regions of lung are excluded from the analysis due to canceling during the histogram subtraction, which may be apparent on direct inspection of the registered CT images. Second, the image resolution at 100 μm/voxel is unable to resolve individual alveoli but presents an average of approximately 21–42 individual alveoli per voxel at end inspiration using the extremes of the estimated alveolar sizes in mice (35–45 μm in diameter in C57BL/6J and C3H/HeJ strains, respectively) as determined by Soutiere et al. (28). Consequently, within a given voxel, alveolar size may vary with some alveoli being hyperinflated with recruitment, while others remain poorly inflated after lung injury. However, given the finding of recruitment to similar MLA at week 3 between control and bleomycin-exposed mice, it seems unlikely that this phenomenon plays a significant role in this model. A final limitation of this technique is the inability to exclude the soft tissue and mediastinal structures from the CT images before generating the corresponding histogram. While automated segmenting algorithms have been described for use with CT data sets in fibrotic lung injury models (8, 12), we felt the resulting images did not provide adequate separation between the most radiographically dense lung tissue and adjacent soft tissue structures, resulting in exclusion of portions of the most radiographically dense lung from the resulting images. Manual segmentation is labor intensive and, with over 200 images in some data sets, not practical for larger studies.
In conclusion, we have developed a physiologically meaningful, quantitative method for the analysis of radiographic changes caused by the administration of bleomycin in a mouse model of pulmonary fibrosis. Using this technique, we demonstrate increased end-expiratory lung parenchymal volumes in bleomycin-injured mice compared with control mice. In addition, there is preserved lung recruitment in regions of lung which display significantly increased MLA at week 1, while week 3 bleomycin-exposed mice show increased lung recruitment with tidal ventilation, which is likely related to alveolar derecruitment at end exhalation. The data provided are complementary but distinctly different from more traditional measures of lung injury such as collagen content and pulmonary compliance. This imaging-based technique should be applicable to a wide variety of lung injury models.
GRANTS
This work was supported by the Duke Center for In Vivo Microscopy, a National Institutes of Health/National Center for Research Resources/National Cancer Institute National Biomedical Technology Resource award (P41 RR-005959, U24-CA-092656), and National Institute of Environmental Health Sciences Center Award ES-011961.
Acknowledgments
We thank Dr. Steve Young for thoughtful comments during the writing of this manuscript. We also thank Dr. David Schwartz who kindly provided assistance with the pathology processing.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Badea C, Hedlund LW, Johnson GA. Micro-CT with respiratory and cardiac gating. Med Phys 31: 3324–3329, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RH, Walters DM, Greenber RS, Mitzner W. A method of endotracheal intubation and pulmonary functional assessment for repeated studies in mice. J Appl Physiol 87: 2362–2365, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Cavanaugh D, Travis E, Price R, Gladish G, White RA, Wang M, Cody DD. Quantification of bleomycin-induced murine lung damage in vivo with micro-computed tomography. Acad Radiol 13: 1505–1512, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A 1: 612–619, 1984. [Google Scholar]
- 5.Ford NL, Martin EL, Lewi JF, Veldhuizen RAW, Drangova M, Holdsworth DW. In vivo characterization of lung morphology and function in anesthetized free-breathing mice using micro-computed tomography. J Appl Physiol 102: 2046–2055, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354: 1775–1786, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164: 1701–1711, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero T, Castillo R, Noyola-Martinez J, Torres M, Zhou X, Guerra R, Docy D, Komaki R, Travis E. Reduction of pulmonary compliance found with high-resolution computed tomography in irradiated mice. Int J Radiat Oncol Biol Phys 67: 879–887, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hedlund LW, Cofer GP, Owen SJ, Johnson GA. MR-compatible ventilator for small animals: computer-controlled ventilation for proton and noble gas imaging. Magn Reson Imaging 18: 753–759, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Hedlund LW, Johnson GA. Mechanical ventilation for imaging the small animal lung. ILAR J 43: 159–174, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Hirose N, Lynch DA, Cherniack RM, Doherty DE. Correlation between high resolution computer tomography and tissue morphometry of the lung in bleomycin-induced pulmonary fibrosis in the rabbit. Am Rev Respir Dis 147: 730–738, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging 20: 490–498, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Johnson K Imaging techniques for small animal imaging models of pulmonary disease: micro-CT. Toxicol Pathol 35: 59–64, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol 83: 111–119, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch D Imaging of diffuse parenchymal lung diseases. In: Interstitial Lung Disease (4th ed.), edited by Schwarz MI, King TE. London: Decker, 2003, p. 75–113.
- 16.Lynch DA, Hirose N, Cherniack R, Doherty DE. Bleomycin-induced lung disease in an animal model: correlation between computed tomography-determined abnormalities and lung function. Acad Radiol 4: 102–107, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Mitzner W, Brown R, Lee W. In vivo measurement of lung volumes in mice. Physiol Genomics 4: 215–221, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Mouton PR Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Baltimore, MD: The Johns Hopkins Univ. Press, 2002.
- 19.Neumann P, Berglund JE, Fernandez-Mondejar E, Magnusson A, Hedenstierna G. Dynamics of lung collapse and recruitment during prolonged breathing in porcine lung injury. J Appl Physiol 85: 1533–1543, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Parker DL Optimal short scan convolution reconstruction for fan beam CT. Med Phys 9: 254–257, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. Lancet 361: 332–340, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Reinhard C, Eder G, Fuchs H, Ziesenius A, Heyder J, Schulz H. Inbred strain variation in lung function. Mamm Genome 13: 429–437, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964. [DOI] [PubMed] [Google Scholar]
- 24.Schuster DP, Kovacs A, Garbow J, Piwnica-Worms D. Recent advances in imaging the lungs of intact small animals. Am J Respir Cell Mol Biol 30: 129–138, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Shofer S, Badea C, Auerbach S, Schwartz DA, Johnson GA. A microCT-based method for the measurement of pulmonary compliance in healthy and bleomycin-exposed mice. Exp Lung Res 33: 169–183, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon BA, Christensen GE, Low DA, Reinhardt JM. Computed tomography studies of lung mechanics. Proc Am Thorac Soc 2: 517–521, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slutsky AS, Hudson LD. PEEP or no peep—lung recruitment may be the solution. N Engl J Med 354: 1839–1841, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Soutiere SE, Tankersley CG, Mitzner W. Differences in alveolar size in inbred mouse strains. Respir Physiol Neurobiol 140: 283–291, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Terragni PP, Rosbach G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175: 160–166, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]