Abstract
The retrotrapezoid nucleus (RTN) contains 2,000 glutamatergic neurons that innervate selectively the respiratory centers of the pontomedullary region. These cells are at the ventral medullary surface in a previously identified chemosensitive region. RTN neurons are highly sensitive to acid in vitro and vigorously activated by inputs from the carotid body and from the hypothalamus in vivo. Mutations of the transcription factor Phox2b cause the congenital hypoventilation syndrome (CCHS), a disease characterized by extremely reduced chemoreflexes and the loss of breathing automaticity during sleep. RTN neurons express Phox2b and develop poorly in a mouse model of CCHS, which lacks chemoreflexes. Based on these and other data, I propose that the RTN is a critical nodal point for the homeostatic regulation of arterial Pco2 and that the nucleus operates as follows. RTN always contributes a major fraction of the tonic excitatory drive to the respiratory centers. RTN neurons derive their activity from two sources: a chemosensory drive (intrinsic chemosensitivity and inputs from the carotid bodies) and synaptic inputs from higher brain centers (non-chemosensory drive). Under anesthesia or non-rapid eye movement sleep, the chemosensory drive to RTN neurons dominates, and, under these circumstances, the excitatory input from RTN to the respiratory controller is required for breathing automaticity. During waking and exercise, RTN contributes a reduced fraction of the total excitatory drive to the respiratory controller, but this fraction remains essential for CO2 homeostasis because of its exquisite chemosensitivity. The working hypothesis could explain the breathing deficits experienced by CCHS patients.
Keywords: respiration, retrotrapezoid nucleus, central respiratory chemoreceptors, central chemoreflex, congenital central hypoventilation syndrome, Phox2b, medulla oblongata
more than a century ago, Haldane and Priestley (28) demonstrated that arterial Pco2 is very tightly regulated and only deviates (downward) from its set point during severe hypoxia. They showed that a trivial increase in arterial Pco2 is enough to stimulate breathing vigorously in awake man, and they concluded their paper by stating that “CO2 is the factor that drives breathing.” At present, we know that CO2 stimulates breathing by acidifying the carotid bodies and central nervous system entities called central chemoreceptors (86). We also know a great deal about the neural circuit that generates the respiratory rhythm and produces the orderly activation of the various respiratory motor outflows [henceforth called central pattern generator (CPG)] (5, 18, 39, 74, 79). However, we still know relatively little about the homeostatic control of breathing, i.e., the neurobiological mechanisms that maintain Pco2 stability in the face of large behavior-related changes in CO2 production (19, 50). Despite the long-standing evidence that feed-forward mechanisms emanating from higher centers are critical to this process (15), we seem to be still under the charm of Haldane and Priestley's view that CO2 is the factor that drives breathing (emphasis mine). Accordingly, recent research has emphasized the molecular and cellular nature of central chemoreceptors (19, 76) and somewhat neglected the integrative aspects of respiratory homeostasis.
This review is based on the Carl Ludwig Distinguished Lecture that I had the honor of delivering at the Experimental Biology meeting in San Diego in 2008. Its focus is the retrotrapezoid nucleus (RTN), which my laboratory views as the modern reincarnation of the ventral surface chemoreceptors (42, 48). In this lecture, I suggest that the RTN is of critical importance for the regulation of blood gases (including but not limited to CO2 homeostasis), for breathing automaticity during sleep, and, possibly, for the sensation of asphyxia (air hunger).
CENTRAL RESPIRATORY CHEMORECEPTORS: BACK TO THE VENTRAL MEDULLARY SURFACE
Brain Pco2 is almost certainly detected via changes in pH (the “reaction” theory) (42) but how pH is detected, where pH is detected, and how brain acidification stimulates breathing (the central chemoreflex) are still controversial issues. A relatively recent theory postulates that central respiratory chemoreception results from the cumulative effect of pH on the CPG itself and many of its tonic drives (37, 57). Opinions vary greatly as to the number of participating neurons, but the list of candidates includes at present several monoaminergic cell groups (serotonergic neurons, pontomedullary catecholaminergic cells), neurons located at the ventral medullary surface and within the nucleus of the solitary tract, every type of spino-medullary respiratory neurons tested so far including the motoneurons, hypothalamic orexinergic neurons, and cerebellar neurons (4, 19, 37, 38, 75, 81, 88, 92). Arguments for this theory, which I called previously the “distributed chemoreception theory” (27), can be found in several recent reviews (64, 76).
The other theory, henceforth called “the specialized chemoreceptor theory” (27), is the earliest one in historical terms (for review, see Ref. 42) and the one favored in this review. This theory postulates that, in its intact state in vivo, the CPG is not or only weakly pH responsive and that the central chemoreflex is primarily due to a small number of specialized acid-sensitive neurons (the central respiratory chemoreceptors) that drive the CPG synaptically. From the early 1960s to the early 1980s, the main central respiratory chemoreceptors were thought to reside at the ventral surface of the rostral medulla oblongata (42, 47). Although cellular evidence for the existence of ventral surface chemoreceptors kept slowly accruing after that point (66, 73, 80), concurrent experimental work in slices also revealed that a high proportion of neurons recorded in the brain stem (30–100% depending on the region or the type of neuron investigated) respond to pH to some degree by either an activation or an inhibition (4, 19, 37, 38, 75, 81, 92). This fact has been and still is interpreted as evidence for the widespread presence of central respiratory chemoreceptors in the brain stem and elsewhere (4, 19, 37, 38, 75, 81, 92). This interpretation has been very difficult to either prove or disprove experimentally because many groups of putative central chemoreceptor neurons (catecholaminergic, serotonergic neurons, orexinergic) produce widespread effects on neuronal excitability in the brain. Therefore, even if the deletion of such cells, genetic or otherwise, attenuates the chemoreflex, this attenuation only shows that these cells intervene somewhere within the blackbox that lies between the chemosensory stimulus and the respiratory outflow. Such evidence does not prove that the cells in question detect pH changes in vivo.
Strong evidence in favor of the specialized chemoreceptor theory came recently from the most ancient approach to neuroscience, namely human pathophysiology. The congenital central hypoventilation syndrome (CCHS), a rare developmental disease caused by mutations of the transcription factor Phox2b, is characterized by a virtually complete loss of central respiratory chemoreception, loss of air hunger (the aversive sensation caused by an increase in arterial Pco2), and typically complete sleep apnea but usually maintained breathing during waking and exercise (2, 6, 89). Phox2b is a homeobox gene that governs the differentiation and survival of a subset of pontomedullary neurons, selected visceral afferents, and many autonomic efferents (Brunet & Pattyn, 2002). The fact that CCHS patients lack central chemosensitivity, often entirely, and yet breathe relatively normally when awake or exercising, indicates that CCHS patients must have a relatively intact CPG. If their CPG is intact and their chemoreflex absent, central respiratory chemoreception therefore cannot derive to a significant degree from the pH-sensitivity of the CPG itself but must derive from the pH-sensitivity of other neurons that activate the CPG synaptically. Because Phox2b is not expressed above the pons nor in the cerebellum, these regions most likely develop normally when Phox2b is mutated and such regions are also unlikely to be major contributors to central respiratory chemoreception. Because Phox2b-expression is not required for the differentiation of serotonergic neurons (6), it is also likely that serotonergic neurons are relatively intact in CCHS and, therefore, that the loss of the chemoreflex cannot be primarily explained by a deficit of those cells. Thus the CCHS suggests that many brain regions in which “candidate” chemoreceptors have been postulated to exist (motoneurons, the CPG, the serotonergic neurons, the cerebellum, the orexinergic neurons of the hypothalamus) may not be essential for the stimulation of breathing by CO2. A contrario, the CCHS supports the notion that chemosensitivity, breathing automaticity during sleep, and air hunger depend on a subset of specialized neurons located in regions where Phox2b is expressed. This reasoning limits the candidates to presumably nonserotonergic cells located in the pontomedullary region outside the CPG. This interpretation does not require that the “missing” neurons be (the only) central chemosensors, although this interpretation is the most parsimonious. It is possible that the missing neurons could be merely funneling information from a variety of central chemoreceptors to the CPG.
One of the hypotheses developed in this review is that the loss of the Phox2b-expressing neurons of the RTN could account for the respiratory deficits of CCHS patients (14, 91).
THE RTN
The name “retrotrapezoid nucleus” was coined by Feldman and colleagues in 1989 to describe a sparse population of neurons located under the facial motor nucleus in very close proximity to the ventral surface of the medulla oblongata (8, 87). These neurons were originally detected because of their projections to more caudal regions of the ventral respiratory column. The same laboratory also provided anatomical evidence of a polysynaptic pathway between RTN and phrenic motoneurons, which suggests that the RTN somehow controls inspiratory activity (13). Since the early 1990s, Nattie and his colleagues provided abundant evidence that RTN contributes to central respiratory chemoreception in awake and anesthetized animals (19, 64). In particular, this laboratory showed that chemical stimulation of RTN with glutamate or thyrotropin-releasing hormone increases breathing, whereas chemical inhibition of the nucleus with muscimol inhibits breathing and the chemoreflex (19, 64, 94, 94). Based on these experiments, Nattie et al. described the probable anatomical extent of the RTN (9), and they suggested that the region contains a group of neurons that drive breathing, are probably chemosensitive, and mediate some portion of the chemoreflex (64). Their interpretation was also supported by evidence that the RTN region contains neurons that respond to acidification with above-average sensitivity in slices and that neurons within the RTN region express the early gene c-Fos in animals exposed to high levels of CO2 (e.g., Refs. 66, 73, 80).
Our laboratory identified in 2004 a population of neurons that is likely to be responsible for the respiratory effects caused by acidifying or otherwise altering the region previously defined as RTN (25, 53). We have determined that these neurons can be identified by a specific combination of markers, namely the combined presence of the transcription factor Phox2b and the vesicular glutamate transporter 2 and the absence of tyrosine-hydroxylase (91). RTN neurons (around 2,000 per brain in the rat) form a loose column that extends from the caudal end of the trapezoid body rostrally to a region slightly caudal to the facial motor nucleus over a distance of roughly 1.8 mm (Fig. 1A) (95). The location of these neurons is in good register with the region of the cat ventral medullary surface called area M by Mitchell and colleagues (see Fig. 6 of Ref. 82). The following sections focus primarily on the biology of RTN neurons, and the evidence is analyzed in the context of a new working hypothesis of breathing automaticity and CO2 homeostasis. This hypothesis borrows essential ideas from the ventral surface chemoreceptor theory developed by Mitchell and Loeschcke (42) and from Eldridge's views on the role of central command in the regulation of breathing and blood gases (15, 16).
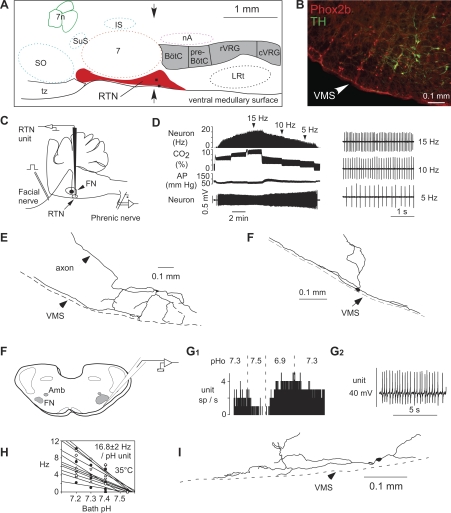
Fig. 1.
location and general characteristics of retrotrapezoid nucleus (RTN) neurons. A: schematic but correctly scaled drawing of a parasagittal section through the pontomedullary region of the adult rat showing the location of the RTN. BötC, Bötzinger region of the ventral respiratory column; preBötC, pre-Bötzinger region; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group; IS, inferior salivary nucleus; LRt, lateral reticular nucleus; nA, nucleus ambiguus pars compacta; SO, superior olive; tz, trapezoid body; 7, facial motor nucleus; 7n, seventh nerve. The two black dots are the cell bodies of the neurons shown in E and F. B: coronal section at the level indicated by the arrows in A showing the distribution of neurons that express Phox2b (left side of brain). The chemoreceptors are the Phox2b-positive neurons that do not express tyrosine-hydroxylase (TH). The cells that express both markers are the C1 neurons, which regulate blood pressure. C: method used to record from RTN neurons in vivo. D: effect of changing end-expiratory CO2 on the activity of an RTN neuron recorded in vivo after intracerebral injection of the glutamate blocker kynurenic acid. The neuron still encodes the level of arterial CO2 despite the fact that the drug has silenced the activity of the central pattern generator (CPG; evidence that it has is not shown in the figure). E and F: structure of two RTN neurons recorded in vivo illustrating the fact that a major portion of the dendritic domain of these cells resides within the marginal layer of the ventral medullary surface. F: method used to record from RTN neurons in rat brain slices (FN, facial motornucleus; Amb, rostral portion of nuc ambiguus). G: effect of pH on a single RTN neuron recorded at room temperature (G1, integrated rate histogram; G2, excerpt of original recording showing the regularity of the cell's discharge). H: relationship between discharge rate and extracellular pH for a sample of 11 RTN neurons recorded at 35°C. I: structure of an RTN neuron recorded in a slice (rat). Note cell proximity to ventral medullary surface.
ROLE OF THE RTN: THE WORKING HYPOTHESIS
Figure 2 describes our working hypothesis. This hypothesis is not yet fully documented and a brief list of the essential missing pieces of evidence will be provided at the end of the review to emphasize this point. We postulate that the RTN is not part of the network that generates the respiratory rhythm and motor pattern (the CPG) and that, under all circumstances, RTN neurons generate a large portion of the excitatory drive to the CPG. Because the RTN is both uncommonly sensitive to brain extracellular pH and strongly activated by inputs from peripheral chemoreceptors, this nucleus is viewed as primarily responsible for the stability of arterial Pco2.
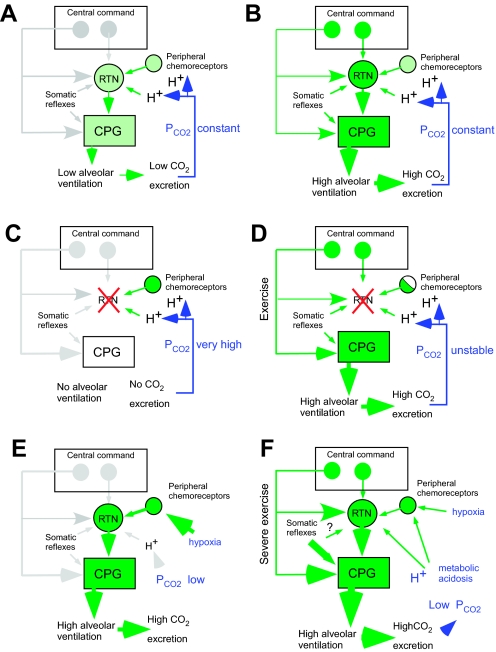
Fig. 2.
The role of RTN in breathing: a working hypothesis. More detailed explanations of the figures are to be found in the text. A: role of RTN during sleep. During sleep, central command pathways are hypothesized to be virtually inactive. Breathing is driven by CO2 via RTN. B: role of RTN during high metabolic states (waking, exercise). RTN is upregulated by central command but so is the CPG; therefore, RTN contributes only a fraction of the excitatory drive to the CPG. RTN is no longer indispensable for breathing, but it still regulates Pco2 by virtue of the fact that its activity is powerfully and uniquely controlled by pH and by inputs from the carotid bodies. C: in the absence of RTN, we postulate that breathing is not possible during sleep. D: in the absence of RTN, breathing is still possible during high metabolic states (waking, exercise) because of central command and reflex influences on the CPG that bypass RTN. However, the absence of RTN eliminates the chemoreflexes and causes arterial Pco2 instability. C and D may account for the respiratory symptoms of the CCHS. E: during hypoxia such as caused by altitude, RTN is driven by its carotid body input, which overcomes the ability of this nucleus to maintain arterial Pco2 constant. F: exercise. Central command upregulates RTN and the CPG. During strenuous exercise, the ability of RTN to maintain arterial Pco2 is overridden by the direct effect of metabolic acidosis on RTN, by the strong stimulation of RTN from peripheral chemoreceptors, and by powerful somatic reflexes (metabotropic reflexes, central pathways unknown).
The working hypothesis has two additional and critical features. First, the activity of RTN neurons is regulated by descending inputs from regions of the brain located rostral to the pons. I will refer to these descending inputs as central command to distinguish them from cardiopulmonary or other reflex inputs to the same cells. The expression “central command” has been originally used in a more restrictive sense to describe descending excitatory inputs that adjust cardiorespiratory responses to exercise specifically (15, 16). At this time we do not know whether the central command of exercise operates, even in part, via RTN; we only know that RTN neurons can be driven from the hypothalamus (vide infra). The central command of RTN neurons is likely to be stimulatory in most physiological contexts (exercise, fear, arousal from sleep, postural changes, feeding, increased body temperature) but may be inhibitory in other contexts such as, hypothetically, diving or hypothermia when CO2 stability must be superseded by more critical physiological requirements (respectively, protection from watery environment and heat preservation) (90, 97).
The second postulate of the model is that the proportion of the total excitatory drive that the CPG receives from RTN increases when the energy expenditure (hence the production of CO2 and the need for lung ventilation) decreases. When energy expenditure is low (anesthesia, rest in a thermoneutral environment, non-rapid eye movement sleep), the central command inputs that normally adjust breathing intensity to behavior or to various homeostatic requirements should be least active (Fig. 2A). Under such conditions, the model postulates that the CPG is driven primarily by the RTN and that the activity of RTN neurons depends primarily on 1) their intrinsic membrane properties and 2) their chemical drives (i.e., their intrinsic pH sensitivity and their inputs from peripheral chemoreceptors). Thus, under such resting conditions, Haldane and Priestley's 1905 pronouncement would be closest to being correct: CO2 is the (as opposed to descending control from higher brain centers) factor that drives breathing, and one might add that CO2 drives breathing through RTN. Conversely, under physiological conditions requiring a higher level of metabolism (wakefulness, feeding, exercise, etc.), the CPG and the RTN are both, and probably independently, driven by multiple sources of reflex and feed-forward inputs (Fig. 2B). Under such conditions, the model requires that RTN still contribute a significant fraction of the excitatory drive to the CPG, notably via its inputs from the hypothalamus. Although no longer the exclusive source of excitatory drive to the CPG and, therefore, no longer indispensable for breathing automaticity, RTN neurons would still remain essential for the regulation of blood gases because the excitatory input that they contribute to the CPG is very sensitively regulated by both central pH and by peripheral chemoreceptors. The model predicts that, during non-REM sleep (Fig. 2A), breathing automaticity should be exquisitely dependent on an appropriate level of arterial Pco2 as detected both by RTN neurons and by the carotid bodies. The model also predicts that the loss of the RTN neurons should cause apnea during sleep (Fig. 2C) and blood gas instability under all other circumstances (Fig. 2D). By and large, these are the respiratory deficits observed in the CCHS (2, 6, 14, 84, 89).
During hypoxia and strenuous exercise, oxygen availability is maintained at the expense of Pco2 stability. The model correctly predicts that, under hypoxia, the ability of RTN to regulate Pco2 would be overridden by the strong excitatory input that these cells receive from peripheral chemoreceptors (Fig. 2E). The model also appropriately predicts that, during extreme exercise, the ability of RTN to regulate Pco2 would be overridden by the direct effect of metabolic acidosis on these neurons and by the strong synaptic input that they receive from peripheral chemoreceptors (Fig. 2F). The experimental evidence that supports this model is detailed below.
RTN NEURONS AS CENTRAL RESPIRATORY CHEMORECEPTORS: CELLULAR EVIDENCE
The evidence has been reviewed recently (25) and will be therefore briefly summarized before the main remaining uncertainties are considered. In vivo, RTN neurons are silent at low levels of end-expiratory CO2 (∼4%; arterial pH 7.5), they accelerate to a maximum of ∼8–14 Hz within a 2–3% increase in end-expiratory CO2 above their CO2 threshold, and they reach a plateau discharge above 7–8% CO2 (26, 53). Their CO2 threshold is typically below that of the phasically active neurons of the CPG regardless of the anesthetic, and, unlike those cells, RTN neurons discharge tonically over a large range of Pco2 (26). The CO2 response of RTN neurons in vivo is not driven by the CPG because administration of a blocker of ionotropic glutamatergic receptors silences the CPG and blocks excitatory synaptic inputs to RTN neurons (e.g., input from the carotid bodies) but does not change the response of these neurons to hyperoxic hypercapnia (53) (Fig. 1D).
In brain slices (age P7–P12), RTN contains acid-activated neurons that express the same two markers as the CO2-sensitive neurons recorded in vivo [the transcription factor Phox2b and the vesicular glutamate transporter 2 (VGLUT2)] and have the same unusual structure characterized by large numbers of very superficial dendrites (Fig. 1, E–G, I) (53, 91). The firing threshold of RTN chemosensitive neurons is typically around pH 7.5 in vitro (Fig. 1H) (26), and these cells respond to increases in Pco2 via changes in pH (53) according to the “reaction theory” (42). The response of RTN neurons to pH in coronal slices of neonate brain is ∼2.2 Hz per 0.1 pH unit at 37°C (Fig. 1H) (26), which is comparable albeit slightly lower than their pH sensitivity in the adult in vivo when measured against arterial pH (3.9 Hz per 0.1 unit arterial blood pH). This discrepancy is unexplained but may have the following several causes. A portion of the dendrites of RTN cells is amputated by preparing slices, making the cells less responsive to pH. The pH sensitivity of brain stem neurons may be less during the early postnatal period than in adulthood (10, 101). Finally, the CO2 response of RTN neurons in vivo may not be exclusively intrinsic. It could be partially mediated by inputs from unrelated central nervous system pH-sensitive neurons that operate via transmitters other than glutamate, GABA, glycine, or ATP. In slices, RTN neurons discharge with extreme regularity (Fig. 1G2), which is also the case in vivo under conditions of reduced synaptic activity (Fig. 1C) (26). The acid sensitivity of RTN neurons in vitro does not require the release of glutamate, GABA, ATP, or UTP because the addition of the appropriate receptor antagonists is without effect (51). Although ATP or UTP is released from the ventral medullary surface by hypercapnia and hypoxia, the function of the released nucleotides is unknown (21). Bath alkalization through a physiological pH range (pH 6.9–7.5) causes an outward current in RTN neurons that has the characteristics of a “leak” or “resting” potassium current (53). The pH-sensitive TWIK-related acid-sensitive potassium channels (TASK1 and TASK3), which are widely expressed in the ventrolateral medulla, do not contribute significantly to the pH sensitivity of RTN (54, 103). Appropriately, mice lacking both of these channels have a normal central chemoreflex, and the pH-sensitivity of RTN neurons is also normal in these mutants (54).
High sensitivity to pH in vivo and in vitro is a necessary but not a sufficient attribute of central respiratory chemoreceptors. Central respiratory chemoreceptors should also have the proper synaptic connections with the breathing network, and their activation should produce a vigorous activation of breathing (76). These additional criteria are particularly well fulfilled in the case of RTN neurons since these neurons innervate selectively the pontomedullary region that contains the various components of the respiratory rhythm and pattern generator (78), and anatomical evidence suggests that some of their targets within these regions are neurons that control the diaphragm (13).
Although RTN neurons have anatomical and physiological characteristics that are consistent with a central chemoreceptor role, definitive evidence that their acid sensitivity is an intrinsic property has yet to be produced. The pH-sensitive resting potassium conductance observed in RTN neurons in the presence of TTX is still unidentified. To demonstrate that this conductance underlies the pH sensitivity of RTN neurons would require one first to identify the responsible channel and then to demonstrate that the pH sensitivity of these neurons disappears after selective downregulation of this channel. This type of experiment has been performed only in the case of the serotonergic neurons. In this case, TASK channel knockout has been shown to attenuate considerably the pH sensitivity of serotonergic neurons recorded extracellularly in slices and to reduce the pH-sensitive whole cell resting current to a similar extent (54). However, TASK channel knockout changes neither the chemoreflex nor the pH sensitivity of RTN neurons (54). Although this evidence argues against the notion that generic serotonergic neurons are essential central chemoreceptors (more on this issue later), it does not advance the question of whether the pH sensitivity of RTN neurons is intrinsic. The fact that blockers of the most common form of synaptic transmission (GABA, glycine, glutamate, and purinergic) do not change the response of these cells to pH (51, 53) does not exclude the possibility that RTN neurons respond to pH via the release of other transmitters such as catecholamines, serotonin, or unknown peptides also present in the slice. Finally, TTX only blocks action potential-dependent transmitter release, and one can still speculate that the effect of pH on RTN neurons results partially or totally from the release of some unknown substance by nearby components of the blood-brain barrier, such as vascular endothelial cells, pericytes, or glial cells. The very same issues are also pending in the case of the other central chemoreceptor candidates.
In summary, the properties of RTN neurons are consistent with those of central chemoreceptors, but the fact that their response to pH is intrinsic has not yet been proven. Furthermore, the precise targets of RTN neurons within the CPG remain to be defined, and no information exists as to how synaptic transmission between RTN neurons and various components of the CPG operates.
RTN NEURONS ARE A CHEMOSENSORY INTEGRATING CENTER
RTN neurons are strongly activated by carotid chemoreceptor stimulation via a pathway that probably consists of only two glutamatergic neurons: the carotid body afferents and neurons located in the commissural part of the nucleus of the solitary tract (94). The activity of RTN neurons is therefore presumably driven directly by the level of acidification of the surrounding parenchyma and indirectly by the composition of arterial blood gases, as detected by peripheral chemoreceptors. As such, RTN neurons have the properties of a chemosensory integrating center, and their output reflects both brain and blood Pco2 levels as well as blood oxygenation. This convergence supports the concept of a “common central pathway” interposed between chemosensors (central and peripheral chemoreceptors) and the “respiratory controller” (CPG). This concept had already been proposed in the early 1980s based on the results of experiments in which the ventral medullary surface was impaired by cooling (17, 42). The concept is compatible with the fact that, during non-rapid eye movement sleep, the apneic threshold can be highly dependent on carotid body stimulation by CO2 (85).
LESIONS OR INHIBITION OF RTN BOTH DEPRESS BREATHING AND ATTENUATE THE CHEMOREFLEX
This section describes a few key studies in which the RTN region has been acutely inhibited or chronically lesioned. The degree to which these experiments support the working hypothesis is evaluated.
In anesthetized rats, bilateral inhibition of the RTN region with muscimol eliminates the phrenic nerve discharge and the chemoreflexes (94). Thus, under such conditions, breathing seems to be entirely dependent on active neurons located in this region (94). The destruction of ∼70% of the Phox2b+-TH− neurons of RTN raises the average apneic threshold of anesthetized rats close to 8% end-expiratory CO2, and this rise correlates with the percentage loss of this type of neuron (95). Thus the phrenic nerve discharge appears to depend on the activity of the RTN Phox2b+-TH− neurons, cells that have been previously shown to be robustly activated by CO2 in vivo (91) and by pH in slices (54). The working hypothesis predicts that, after destruction of a large percentage of these cells, the surviving neurons would have to be driven harder to initiate a PND, meaning that, under anesthesia, the surviving cells would require a higher level of CO2 to activate the CPG. This is consistent with the rise in apneic threshold observed in rats in which a substantial number of these cells were detroyed (95). The evidence is therefore compatible with the working hypothesis: under anesthesia, breathing could be driven by the Phox2b+-TH− neurons of RTN, which are, in turn, driven by CO2.
In unanesthetized rats, lesions of the RTN region using a saporin-substance P conjugate decrease breathing at rest and reduce the stimulation of breathing by CO2 regardless of the state of vigilance (61). The lesioned rats also have a higher resting arterial Pco2 than the controls (+4 Torr; P < 0.05). These results are also consistent with our working hypothesis given the additional assumption that the RTN lesions performed by Li and Nattie (61) partially destroyed the Phox2b+-TH− neurons of RTN. Indeed, the working hypothesis predicts that partial lesions of RTN should produce a reduced respiratory drive, which should in turn cause a slight elevation of resting Pco2 at rest. The working hypothesis also predicts that partial lesions of these neurons should reduce the respiratory stimulation caused by elevating CO2. Finally, partial lesions of RTN neurons should also reduce breathing both during SW sleep and during quiet waking, also as observed (61). Despite the fact that the Phox2b+-TH− express very low levels of NK1R immunoreactivity, these cells are highly responsive to substance P both in vitro and in vivo (52), and, as expected from this characteristic, they are destroyed by a saporin-based toxin that is very closely related to that used by Li and Nattie (95). The assumption that the RTN lesions performed by Li and Nattie destroyed some of the Phox2b+-TH− neurons of RTN is therefore well supported by our later study (95), and the assumption that the lesions performed by these authors were partial is also supported by the large anatomical spread of these neurons (95).
Experiments in goats indicate that the rostral surface of the ventral medulla (including area M, which presumably also overlaps with the RTN in this species) is critical to sustain breathing under anesthesia (references in Ref. 65). These authors have also shown that area M is more important to maintain breathing during SW sleep than during waking, especially in the absence of inputs from the carotid bodies (65).
Last but not least, a large majority of the Phox2b+ neurons of RTN fail to develop or degenerate prenatally in a genetic model of CCHS, the Phox2b27Ala/+ mouse, and this mouse has no central chemoreflex at birth and an abnormal breathing causing its eventual death (14). No other histological defect has yet been identified in this mouse. In particular, the carotid bodies of these mutant mice appeared structurally intact, the number of Phox2b-expressing neurons located in the nucleus of the solitary tract was normal, and the number of catecholaminergic and serotonergic neurons was also normal. These mice also have a normal complement of NK1R-immunoreactive neurons within the pre-Bötzinger complex region, an accepted measure of the integrity of the inspiratory rhythm generator (23, 34). The Phox2b27Ala/+ mouse demonstrates that RTN neurons are among a small minority of brain neurons whose prenatal development is impaired by the Phox2b mutation most commonly present in CCHS patients. Although this mouse may have other still unrecognized defects, such as abnormal function of peripheral chemoreceptors, the evidence at this point is consistent with the possibility that the integrity of the RTN is required both for breathing and for the stimulation of breathing by CO2 in the neonate.
In summary, under anesthesia, RTN neurons seem driven mostly by CO2, and their activity appears indispensable to breathing. At birth, the integrity of the RTN may also be required for respiration and the chemoreflex, as suggested by the Phox2b27Ala/+ mouse model. Whether the RTN is equally important in the unaesthetized adult, especially during slow-wave sleep, remains uncertain, although evidence in goats suggests that this could be the case (65). Our knowledge concerning the function of RTN remains uncertain at present because some of the most critical experiments have not yet been performed. For example, the consequences of abruptly silencing a majority of RTN neurons in unanesthetized animals are unknown. Our model predicts that this would be fatal if done during sleep. Furthermore, the consequences of a complete or near complete lesion of RTN in the adult are also unknown. It is a general observation in neurology and neuroscience that over 80% loss of a neuronal pathway must occur before serious deficits are observed, the most famous example being Parkinson's disease. With a 70% kill rate of the presumably relevant RTN neurons, we observed significant deficits under anesthesia, but the animals remained viable and did not display any obvious respiratory distress (95). The degree of RTN lesion achieved by Li and Nattie, who demonstrated significant breathing deficits in unanesthetized rats (61), is unknown.
RTN IS NOT THE UNIQUE SOURCE OF EXCITATORY DRIVE TO THE CENTRAL RESPIRATORY NETWORK
Our model does not require that RTN be the sole source of excitatory drive to the CPG. The model only postulates that this drive is indispensable to breathing during sleep and is present, although not indispensable for survival, at all other times. There is indeed considerable evidence that the CPG receives inputs that bypass RTN. The monoaminergic systems of the lower brain stem provide the proof of principle. For example, serotonergic terminals are found in every subdivision of the pontomedullary network, and both serotonin and the peptides that serotonergic cells release (substance P and TRH) modify the discharge or the membrane properties of many types of respiratory neurons down to the motoneurons (41, 44, 72, 77, 96). Several pontomedullary catecholaminergic cell groups (A5 and locus coeruleus, possibly C1 cells) also influence the respiratory network via spinal and medullary projections (29, 104), and cholinergic inputs, possibly from the pontomesencephalic region, exert strong influences on breathing that are presumably not mediated by RTN (83). This evidence indicates that RTN is not the only source of excitatory drive to the CPG and the motoneurons and, therefore, that RTN is not the “final common pathway” for the control of breathing. However, the relative importance of these inputs vs. those that are funneled through RTN is unknown.
Additional evidence, albeit circumstantial, can be added to this list. For example CCHS patients typically breathe unassisted during waking; their breathing increases reasonably normally during moderate exercise, and their breathing can be stimulated, even during sleep, by muscle afferent stimulation (7, 22, 84). These observations suggest that the CPG can be activated by pathways that are not funneled through regions that mediate the chemoreflexes, especially during exercise, and our model reflects this assumption (Fig. 2). However, because CCHS is a developmental disease in which compensatory mechanisms almost certainly develop, the above reasoning does not exclude that a component of exercise hyperpnea could be funneled through RTN in the intact brain via central command or reflex mechanisms. Whether RTN plays any role in the hyperpnea of exercise is unknown. This possibility is especially worth considering because RTN neurons may control most particularly expiratory activity (34), and they receive strong excitatory inputs from a region involved in the central command of exercise: the hypothalamus (16).
RTN IS UPREGULATED BY DESCENDING INPUTS FROM THE HYPOTHALAMUS
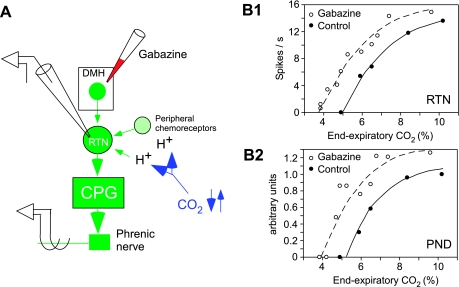
Activation of the medial hypothalamus stimulates breathing, although the physiological context under which these neurons are recruited (thermogenesis, exercise, stress, all of the above) remains to be defined (16, 46, 55, 97). As shown in Fig. 3, the activation of neurons located in the dorsomedial hypothalamic region by microinjection of the GABAa-receptor antagonist gabazine produces a left and upward shift of the relationship between phrenic nerve discharge and end-expiratory CO2 and a similar shift of the relationship between RTN activity and end-expiratory CO2. These results (n = 7 neurons; unpublished results of Fortuna and Guyenet) suggest that the activity of RTN neurons is upregulated by descending inputs from the hypothalamus. Anatomical findings suggest that the pathway from the hypothalamus to the RTN could even be direct (78).
Fig. 3.
Central command of RTN. A: experimental scheme. An RTN neuron is recorded alongside the phrenic nerve discharge in a paralyzed and vagotomized rat anesthetized with isoflurane. The GABAa-receptor antagonist gabazine (30 nl; 150 pmol) is injected into the dorsomedial hypothalamic region to activate neurons located in this region. End-expiratory CO2 is stepped up or down. B1: relationship between the activity of one RTN neuron and end-expiratory CO2 measured at steady state. B2: simultaneously measured relationship between the PND (rectified and integrated activity per unit of time in arbitrary units) and end-expiratory CO2. Note the parallel upregulation of the activity of the RTN neuron and of PND. The causality between the activation of RTN and that of PND is suspected but has not yet been demonstrated experimentally.
RTN NEURONS RECEIVE FEEDBACK FROM THE CPG AND FROM THE LUNG
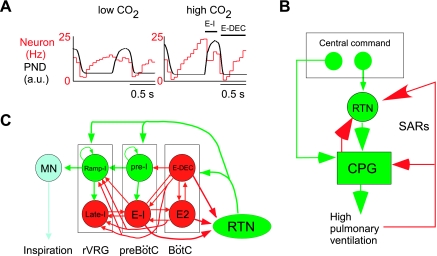
In the absence of lung input (vagotomy), RTN neurons retain a central respiratory modulation that increases with the level of CO2 (Fig. 4A) (26). This modulation indicates that these neurons receive inputs from the CPG. These inputs seem predominantly inhibitory and may be responsible for the saturation of the discharge rate of RTN neurons that is observed at high levels of CO2 (Fig. 3B1) (26). These inhibitory inputs seem to originate from neurons with early inspiratory, postinspiratory, and expiratory augmenting discharges, and they recombine in various proportions on different RTN neurons, causing these cells to adopt a variety of respiratory patterns (Fig. 4C) (26). In vagotomized anesthetized mammals, the phrenic nerve discharge (PND) is a saturable function of Pco2 (Fig. 3B2) (17, 26). This saturation could be explained by our hypothesis that, under these conditions, the CPG is being driven by RTN chemoreceptors and RTN neurons are, in turn, inhibited by the CPG (Fig. 4). The biological significance of this feedback is not clear. It could conceivably be a mechanism that protects the inspiratory motor output against excessive activation. An alternate theoretical explanation is that the voluntary (cortical) drive of the CPG bypasses RTN and that cortical activation of the CPG may require the temporary suppression of the involuntary (homeostatic and RTN-dependent) mechanism of breathing. It is also possible that this feedback arrangement could underlie the “rhythmogenic” role attributed to the RTN in certain neonate preparations (18, 34, 68, 70).
Fig. 4.
Feedback regulation of RTN neurons by lung inputs and by the CPG. A: example of an RTN neuron recorded in a vagotomized rat anesthetized with halothane (unpublished experiment; for further experimental details see Ref. 26). The firing probability of the neuron, represented here as an event-triggered activity histogram triggered on the upstroke of the PND, fluctuates during the central respiratory cycle. The discharge probability shows two dips that increase in amplitude when end-expiratory CO2 is elevated, causing an increase of the PND. The first dip occurs during the early inspiratory phase (E-I), and the other coincides with the postinspiratory phase (E-DEC). These dips are interpreted as phasic inhibitions, which originate from E-I and E-DEC neurons that are part of the central respiratory pattern generator (CPG). B: model of network that generates inspiratory activity (modified after Ref. 79) and its hypothetical inhibitory connections with the RTN. These connections are based on the type of observations shown in A (26). In turn, RTN presumably activates many types of CPG neurons, but the exact targets are not known. C: schematic illustration of the feedbacks to which RTN is thought to be subjected. The feedback from the CPG probably accounts for the fact that the relationships between arterial Pco2 and RTN and between arterial Pco2 and PND are both saturable as illustrated in Fig. 3. The biological significance of this arrangement needs to be further clarified. At the simplest neurophysiological level, this feedback arrangement could conceivably underlie the “rhythmogenic” role attributed to the RTN in certain neonate preparations (18, 34, 68, 70). At a more global level, the role of this feedback could be to achieve the temporary suppression of the RTN when breathing is under voluntary control. Finally, the inhibitory feedback from the lungs to RTN may be a component of a protective reflex designed to limit lung expansion. In B and C, green lines and arrows signify excitatory neurons or pathways, and the color red codes for inhibition. E2 (also known as E-AUG), expiratory augmenting neurons; L-I, late inspiratory neuron; MN, motoneuron; Ramp-I, inspiratory premotor neuron; SARs, slowly-adapting lung stretch receptors.
A large subset of RTN neurons receive polysynaptic inhibitory inputs from the slowly adapting lung receptors (SARs) responsible for the Hering-Breuer reflex (49). The inhibitory input from SARs could also be viewed as a feedback that limits the excitatory influence of RTN neurons on the CPG under conditions when respiration is being strongly activated by other sources of drive (Fig. 4B).
IS RTN PART OF THE CPG?
The model (Fig. 2) postulates that, at least in the adult, the RTN is not part of the CPG. However, this issue is not entirely settled. Arguments in favor of this view will be presented first, followed by the objections.
In vivo, RTN neurons exhibit respiratory patterns that are very different from those of neurons previously associated with respiratory rhythm and pattern generation (20, 26). The respiratory patterns of RTN neurons are complex, typically phase-spanning, and their respiratory modulation becomes pronounced only when the central respiratory drive is high (Fig. 4A) (26). By contrast, the respiratory neurons that participate in respiratory rhythm or pattern generation typically have “on-off” discharges, the timing of which remains relatively invariant as the central respiratory drive increases. There are exceptions to this rule, for example expiratory premotor neurons can have a low tonic discharge below the apneic threshold, like RTN neurons (32). However, unlike expiratory motor and premotor neurons, RTN neurons do not develop an expiratory-augmenting discharge at higher levels of respiratory drive (20). Accordingly, we interpret the respiratory modulation of RTN neurons as a feedback from the CPG (Fig. 4, B and C), which may be responsible for the fact that the relationship between phrenic discharge and end-expiratory CO2 is saturable in vagotomized animals (Fig. 3B2)(26). We also explain the tonic discharge of RTN neurons below the apneic threshold by a direct effect of pH on their activity.
The second argument against a role of RTN in rhythm or pattern generation in the adult is that, in both anesthetized and awake animals, inhibition or lesion of RTN neurons do not cause anomalies of the breathing rhythm or pattern contrary to when the dorsal pons or the pre-Bötzinger regions are lesioned (1, 23, 40, 56, 58, 60, 63). Additionally, RTN neurons discharge tonically in slices when activated by acidification (Fig. 1G2). There is no evidence that these cells are capable of generating bursts on their own, a property often associated with respiratory rhythm-generating neurons (11, 67, 71). Finally, CCHS patients do not seem to have notable disturbances of respiratory rhythm or pattern generation. Again, given the assumption that these patients have a pronounced and somewhat selective lesion of the RTN, the nature of their symptoms also argues against a role of RTN in respiratory rhythm or pattern generation.
However, there is also some evidence that RTN could have a role in rhythm generation. This evidence derives primarily from studies of the lower brain stem of neonate rats (postnatal age 0–2 days) maintained in vitro. In such preparations, neurons located in the general region of the RTN have been found that discharge phasically and in synchrony with the respiratory motor outflow [parafacial respiratory group (pfRG)] (69, 70). These neurons discharge twice per respiratory cycle, including just before the phrenic nerve, and, for this and other reasons, these cells are viewed as a master inspiratory oscillator that entrains the pre-Bötzinger complex (18, 34, 68, 70) or as a rhythm generator (oscillator) dedicated to expiratory motor function (18, 69, 93). The proponents of these interpretations have suggested that pfRG neurons and the neurons that we define as RTN chemoreceptors could be the same, but no supportive histological evidence has yet surfaced (20).
The second evidence that suggests that RTN neurons could be an integral part of the circuitry that underlies respiratory rhythm and pattern generation is the Phox2b27Ala/+ mouse, which has an abnormally slow and, in some cases, erratic respiratory rhythm at birth in addition to a loss of respiratory chemosensitivity (14). This rhythm disturbance could be due to other, still unrecognized, neuronal defects besides the loss of RTN neurons or it could simply be the normal response of the CPG to the quasi complete absence of excitatory drive from the RTN.
In short, the evidence generated in adult rats and in neonate slices (P7-P12) suggests that RTN neurons are not involved in respiratory rhythm generation, and this interpretation is congruent with the respiratory deficits of the CCHS and, to some extent, with the breathing characteristics of its genetic model, the Phox2b27Ala/+ mouse. However, the possibility that some of the Phox2b-expressing RTN neurons have rhythmogenic properties, especially during the early neonatal period, cannot be excluded at this time. It is also possible that the RTN region contains neurons with a different phenotype, which have the rhythmogenic properties postulated by other investigators (18, 69, 93). Finally, a rhythmogenic role is not inherently incompatible with a role as central chemoreceptors.
CONTROL OF BREATHING BY SEROTONERGIC NEURONS: THE ROLE OF RTN AND THE SEROTONIN PARADOX
Overwhelming anatomical and cellular evidence indicates that serotonergic neurons, as a class, activate or stabilize breathing via actions at multiple levels of the respiratory network down to the motoneurons (41, 41, 44, 72, 77, 96). Equally persuasive evidence supports the conclusion that the serotonergic system facilitates the central respiratory chemoreflex, i.e., the respiratory stimulation caused by inhalation of CO2. For instance, a substantial reduction of this reflex is observed in rats with selective lesions of serotonergic neurons and in transgenic mice in which serotonergic neurons have failed to differentiate during development (31, 62). Although these experiments demonstrate that serotonergic neurons are required for the chemoreflex to operate optimally, the mechanisms that underlie the serotonergic facilitation of this reflex are unclear. Three possibilities that are neither inclusive nor mutually exclusive will be considered here. The first is that serotonergic neurons activate breathing and the chemoreflex because these cells are part of a system of wake promoting neurons. The second possibility is that the serotonergic neurons are central chemoreceptors. The third possibility is that the serotonergic system stimulates the chemoreflex by activating the RTN.
All serotonergic neurons, regardless of location, have an activity pattern in vivo that is more or less dependent on the arousal state: their activity is greatest during active waking and decreases during sleep (33). Chemoreceptor stimulation produces a powerful arousing response, and any form of arousal engages multiple descending wake-promoting systems, foremost among them, the locus coeruleus, orexinergic, histaminergic, and serotonergic neurons (43, 45). A general arousal caused by stimulating central chemoreceptors could therefore contribute significantly to the rise in extracellular serotonin caused by hypercapnia in vivo, even under anesthesia (36).
The second possibility is that serotonergic neurons are central respiratory chemoreceptors and therefore capable of detecting rises in arterial CO2 in vivo via their intrinsic pH sensitivity (31, 64, 75, 76, 76). This view is indirectly supported by the following observations: serotonergic neurons are typically and sometimes even vigorously activated by acidification in brain slices or cell culture (75, 102), acidification of the raphe (raphe obscurus, generally) by microdialysis usually stimulates breathing (30, 40, 64) but not always (12), and finally, as mentioned already, serotonin overflow increases with hypercapnia in vivo (36). However, only a small minority of serotonergic neurons (0–25% depending on location, species and, perhaps, presence or absence of anesthesia) actually respond to hypercapnia in vivo (53, 99, 100). CO2-responsive neurons were detected in the raphe obscurus, pallidus, or dorsalis of awake cats (99, 100), and unresponsive neurons were found in the parapyramidal region of anesthetized rats (53). The responsive cells (in cats) were not definitively identified as serotonergic by histological means, and these responsive cells became either insensitive to CO2 (2/4) during slow-wave sleep or their sensitivity to CO2 was decreased fivefold (2/4 neurons) (99), suggesting that the excitatory effects of CO2 could have resulted from a behavioral change. Given the above-mentioned single-unit recording evidence, one must conclude first that CO2 detection in vivo is not a general property of serotonergic neurons, contrary to a prior hypothesis (75) and second that this property is, at best, restricted to a relatively small subset of serotonergic neurons located in the raphe obscurus, the raphe pallidus, and the dorsal raphe (53, 99, 100). The discrepancy between the high pH sensitivity of serotonergic neurons in vitro and their infrequent and state-dependent response to arterial CO2 elevation in vivo is a paradox in search of an explanation. One possibility is that most serotonergic neurons, while intrinsically pH-sensitive, reside in regions where interstitial fluid pH is buffered by the blood-brain barrier against variations of arterial CO2 (3, 59). The paradox of having central respiratory chemoreceptors located in an environment where pH is highly buffered against arterial CO2-induced pH changes has not gone unnoticed (59). The ventral medullary surface may be a region where the buffering capacity of the blood-brain barrier is low or nonexistent. Indeed, there is no RTN paradox: the response of RTN cells to CO2 elevation in vivo is commensurate with their pH sensitivity in vitro (26). The minority of serotonergic neurons that respond to CO2 in vivo may also reside in such an unprotected glial environment, although the state dependence of their activation by arterial CO2 will still have to be explained (99).
As stated previously, it is clear that serotonergic neurons regulate breathing at multiple levels down to the motoneurons (41, 41, 44, 72, 77, 96), and recent evidence indicates that one such level is the RTN (52). Indeed, serotonin itself and two co-transmitters of serotonergic neurons (substance P and TRH) excite RTN neurons in vivo and in vitro, and serotonergic neurons from every subdivision of the midbrain and pontomedullary raphe innervate the RTN region (52). The activation of RTN neurons by the raphe may therefore contribute to the fact that this reflex is attenuated by genetic deletion of the serotonergic neurons (31). The serotonergic input to RTN could also explain why raphe inhibition potentiates the reduction of the chemoreflex caused by directly inhibiting RTN in awake rats (40) and why raphe obscurus stimulation potentiates the effect of RTN acidification on respiration (12).
In brief, the overall function of the serotonergic system is still difficult to conceptualize in any context, including breathing. Via their projections to RTN and other parts of the respiratory network, various groups of serotonergic neurons may contribute to modulate respiration in a variety of contexts such as physical exercise, feeding, or general cortical activation (33). Finally, serotonergic neurons are undoubtedly important for central chemoreception, but whether this is related to the ability of these neurons to detect changes in arterial CO2 remains in question.
CONCLUSIONS
The evidence available at this time suggests that the RTN could be a key nodal point for CO2 homeostasis and that this nucleus may function generally along the lines suggested by Loeschke 25 years ago for the hypothetical ventral surface chemoreceptors (42). RTN neurons appear to fit the definition of central respiratory chemoreceptors, although their synaptic regulation by brain stem and suprabulbar structures could be as or more important to CO2 homeostasis than their chemosensory regulation. The mechanism responsible for the carotid body-independent activation of RTN neurons by rising levels of arterial CO2 is not definitively established. Although no experimental evidence contradicts the notion that RTN neurons are activated by CO2 in vivo purely by virtue of their intrinsic sensitivity to pH, this interpretation requires additional testing. Several pertinent research objectives can be identified. The first would be to reinvestigate the relationship between arterial CO2 elevation and brain interstitial fluid acidification, the precise role of the various components of the blood-brain barrier in regulating this process and whether interstitial fluid acidification is indeed different in brain areas where recent research has identified candidate chemoreceptors (RTN, raphe, NTS, ventrolateral respiratory group, etc.). Another important issue would be to identify the channel(s) or receptors responsible for the pH sensitivity of RTN neurons and other putative central chemoreceptors, wherever they might be. This objective is especially important because the cell-specific deletion of such pH-sensitive molecules is probably the only way to test definitively the importance of the various chemoreceptor candidates to central respiratory chemoreception.
The proposed dual function of RTN, namely to drive respiration and to stabilize Pco2, has noteworthy analogies with the role of the neighboring C1 adrenergic neurons in blood pressure control since the latter neurons both drive the vasomotor outflow and reflexly stabilize arterial pressure (24). The evidence reviewed herein indicates that RTN is not a final common pathway through which every source of excitatory drive is funneled to the CPG. The C1 cells are not the only source of excitatory drive to sympathetic vasomotor preganglionic neurons, but these cells seem to be nonetheless critical for blood pressure stabilization because of their powerful inputs from arterial baroreceptors (24, 35). Likewise, the model of respiratory control proposed in Fig. 2 does not require that the RTN be the final common pathway for all respiratory drives to play central stage in stabilizing arterial Pco2. All that is required is that RTN contribute a sufficiently large proportion of that excitatory drive under all circumstances and that RTN neurons be sufficiently responsive to arterial Pco2 to produce a high gain reflex regulation of breathing. The high pH sensitivity of RTN neurons combined with their strong and direct inputs from the carotid bodies may endow these neurons with the required sensitivity to arterial CO2. The feed-forward control that RTN neurons receive from the hypothalamus and presumably from many other brain areas may be present to ensure that these neurons always generate a sufficiently large fraction of the excitatory drive to the CPG to enable them to stabilize Pco2. As illustrated in Fig. 2, E and F, the proposed mechanism also appropriately enables oxygen preservation to take precedence over Pco2 stabilization during hypoxia or strenuous exercise. Finally, the development or survival of RTN neurons in rodents is selectively vulnerable to a mutation of the transcription factor Phox2b that causes most cases of CCHS (14), suggesting that the respiratory symptoms of the disease may likewise be caused by various degrees of loss of RTN neurons.
Many issues still need to be resolved to validate the proposed model. First and foremost, the consequences of an acute, specific, and total inhibition of RTN neurons need to be assessed in unanesthetized neonate and adult animals. This type of experiment will be required to determine without ambiguity whether RTN neurons are indeed indispensable to breathing during sleep, whether these cells are indeed essential for CO2 stabilization at all times, or whether they mediate the hyperpnea of exercise or emotions.
With respect to the CCHS, neuropathological studies will have to be carried out, first to verify that the RTN does exist in humans and second to ascertain that this nucleus is selectively affected by the disease, as seems to be the case in its mouse model. CCHS patients have many other neurological deficits besides sleep apnea and depressed chemoreflexes (89, 98). It will therefore be important to identify which other neurons besides RTN might be damaged by Phox2b polyalanine expansion and to assess whether these neurons could also potentially account for the respiratory deficits. Finally, the reason why RTN neurons are especially vulnerable to this type of mutation will also require clarification in hope that understanding this mechanism may eventually lead to a therapeutic intervention.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-28785 and HL-74011 to P. G. Guyenet.
REFERENCES
- 1.Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol 82: 469–479, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33: 459–461, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Arita H, Ichikawa K, Kuwana S, Kogo N. Possible locations of pH-dependent central chemoreceptors: intramedullary regions with acidic shift of extracellular fluid pH during hypercapnia. Brain Res 485: 285–293, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Bayliss DA, Talley EM, Sirois JE, Lei QB. TASK-1 is a highly modulated pH-sensitive 'leak' K+ channel expressed in brainstem respiratory neurons. Respir Physiol 129: 159–174, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Brunet JF, Pattyn A. Phox2 genes: from patterning to connectivity. Curr Opin Genet Dev 12: 435–440, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chen ML, Keens TG. Congenital central hypoventilation syndrome: not just another rare disorder. Pediatr Respir Rev 5: 182–189, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett 105: 34–40, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol 130: 121–137, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101: 1097–1103, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Del Negro CA, Koshiya N, Butera RJ Jr., Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Botzinger complex inspiratory neurons in vitro. J Neurophysiol 88: 2242–2250, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus (ROb) does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus (RTN). J Appl Physiol. In press. [DOI] [PMC free article] [PubMed]
- 13.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105: 1067–1072, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldridge FL Central integration of mechanisms in exercise hyperpnea. Med Sci Sports Exerc 26: 319–327, 1994. [PubMed] [Google Scholar]
- 16.Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211: 844–846, 1981. [DOI] [PubMed] [Google Scholar]
- 17.Eldridge FL, Millhorn DE, Waldrop TG. Input-output relationships of the central respiratory controller during peripheral muscle stimulation in cats. J Physiol 324: 285–295, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity plasticity chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortuna MG, West GH, Stornetta RL, Guyenet PG. Bötzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci 28: 2506–2515, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci 25: 1211–1218, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gozal D Congenital central hypoventilation syndrome: an update. Pediatr Pulmonol 26: 273–282, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires pre-Botzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyenet PG The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Guyenet PG, Bayliss DA, Mulkey DK, Stornetta RL, Moreira TS, Takakura AT. The retrotrapezoid nucleus and central chemoreception. Adv Exp Med Biol 605: 327–332, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol 32: 225–266, 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilaire G Endogenous noradrenaline affects the maturation and function of the respiratory network: possible implication for SIDS. Auton Neurosci 126–127: 320–331, 2006. [DOI] [PubMed]
- 30.Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol 97: 2303–2309, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iscoe S Control of abdominal muscles. Prog Neurobiol 56: 433–506, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev 40: 45–52, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res 683: 1–24, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Kanamaru M, Homma I. Compensatory airway dilation and additive ventilatory augmentation mediated by dorsomedial medullary 5-hydroxytryptamine 2 receptor activity and hypercapnia. Am J Physiol Regul Integr Comp Physiol 293: R854–R860, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem: spinal cord preparation of the neonatal rat. J Physiol 492: 277–292, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol 572: 525–537, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci 28: 2353–2365, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577: 307–318, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol 461: 213–234, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeschcke HH Central chemosensitivity and the reaction theory. J Physiol 332: 1–24, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 441: 589–594, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226–229, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Marshall JM Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543–594, 1994. [DOI] [PubMed] [Google Scholar]
- 46.McDowall LM, Horiuchi J, Dampney RA. Effects of disinhibition of neurons in the dorsomedial hypothalamus on central respiratory drive. Am J Physiol Regul Integr Comp Physiol 293: R1728–R1735, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol 18: 523–533, 1963. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell RA, Loeschcke HH, Severinghaus JW, Richardson JW, Massion WH. Regions of respiratory chemosensitivity on the surface of the medulla. Ann NY Acad Sci 109: 661–681, 1963. [Google Scholar]
- 49.Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol 580: 285–300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mortola JP, Frappell PB. Ventilatory responses to changes in temperature in mammals and other vertebrates. Annu Rev Physiol 62: 847–874, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci 26: 7230–7233, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol 89: 153–162, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci 126–127: 332–338, 2006. [DOI] [PubMed]
- 58.Nattie EE Chemoreception and tonic drive in the retrotrapezoid nucleus (RTN) region of the awake rat: bicuculline and muscimol dialysis in the RTN. Adv Exp Med Biol 499: 27–32, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Nattie Chemoreceptors EE, breathing, pH. In: Seldin and Giebisch's The Kidney, Vols. 1–2, edited byAlpern RJ, Hebert SC. New York: Elsevier, 2007, p. 1587–1600.
- 60.Nattie EE, Li A. Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respir Physiol 97: 63–77, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556: 235–253, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nattie EE, Li A, Stjohn WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol 71: 1364–1375, 1991. [DOI] [PubMed] [Google Scholar]
- 64.Nattie G, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol 605: 343–347, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Ohtake PJ, Forster HV, Pan LG, Lowry TF, Korducki MJ, Whaley AA. Effects of cooling the ventrolateral medulla on diaphragm activity during NREM sleep. Respir Physiol 104: 127–135, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol 93: 427–439, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Onimaru H, Arata A, Homma I. Intrinsic burst generation of preinspiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Exp Brain Res 106: 57–68, 1995. [DOI] [PubMed] [Google Scholar]
- 68.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onimaru H, Homma I, Feldman JL, Janczewski WA. Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol 100: 2094–2098, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol 96: 55–61, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respir Physiol 110: 71–85, 1997. [DOI] [PubMed] [Google Scholar]
- 75.Richerson GB Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med 9: 542–548, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Morschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol 143: 307–319, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol 73: 96–100, 1992. [DOI] [PubMed] [Google Scholar]
- 81.Scheid P; guest editors Putnam RW, Dean JD, Ballantyne D. Special issue: Central chemosensitivity. Respir Physiol Neurobiol 129: 1–278, 2001. [DOI] [PubMed] [Google Scholar]
- 82.Severinghaus JW, Mitchell RA, Richardson BW, Singer MM. Respiratory control at high altitude suggesting active transport regulation of CSF pH. J Appl Physiol 18: 1155–1166, 1963. [DOI] [PubMed] [Google Scholar]
- 83.Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci 28: 519–528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shea SA, Andres LP, Shannon DC, Banzett RB. Ventilatory responses to exercise in humans lacking ventilatory chemosensitivity. J Physiol 468: 623–640, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith CA, Chenuel BJ, Henderson KS, Dempsey JA. The apneic threshold during non-REM sleep in dogs: sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol 103: 578–586, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol 100: 13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989. [DOI] [PubMed] [Google Scholar]
- 88.Solomon IC, Edelman NH, O'Neill MH. CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol 88: 1996–2007, 2000. [DOI] [PubMed] [Google Scholar]
- 89.Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol 129: 247–255, 2001. [DOI] [PubMed] [Google Scholar]
- 90.Stephenson R A theoretical analysis of diving performance in the Weddell seal (Leptonychotes weddelli). Physiol Biochem Zool 78: 782–800, 2005. [DOI] [PubMed] [Google Scholar]
- 91.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons. J Physiol 578: 831–841, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taccola G, Secchia L, Ballanyi K. Anoxic persistence of lumbar respiratory bursts and block of lumbar locomotion in newborn rat brainstem spinal cords. J Physiol 585: 507–524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesions of retrotrapezoid Phox2b-expressing neurons raises the apneic threshold in rats. J Physiol 586: 2975–2991, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Talley EM, Lei QB, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 294: R477–R486, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Trang H, Girard A, Laude D, Elghozi JL. Short-term blood pressure and heart rate variability in congenital central hypoventilation syndrome (Ondine's curse). Clin Sci (Lond) 108: 225–230, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience 79: 161–169, 1997. [DOI] [PubMed] [Google Scholar]
- 101.Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999. [DOI] [PubMed] [Google Scholar]
- 102.Wang WG, Tiwari JK, Bradley SR, Zaykin AV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol 138: 19–35, 2003. [DOI] [PubMed] [Google Scholar]
- 104.Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurones. Respir Physiol Neurobiol 153: 126–138, 2006. [DOI] [PubMed] [Google Scholar]