Abstract
The clinical importance of vascular reactivity as an early marker of atherosclerosis has been well established, and a number of established and emerging techniques have been employed to provide measurements of peripheral vascular reactivity. However, relations between these methodologies are unclear as each technique evaluates different physiological aspects related to micro- and macrovascular reactive hyperemia. To address this question, a total of 40 apparently healthy normotensive adults, 19–68 yr old, underwent 5 min of forearm suprasystolic cuff-induced ischemia followed by postischemic measurements. Measurements of vascular reactivity included 1) flow-mediated dilatation (FMD), 2) changes in pulse wave velocity between the brachial and radial artery (ΔPWV), 3) hyperemic shear stress, 4) reactive hyperemic flow, 5) reactive hyperemia index (RHI) assessed by fingertip arterial tonometry, 6) fingertip temperature rebound (TR), and 7) skin reactive hyperemia. FMD was significantly and positively associated with RHI (r = 0.47) and TR (r = 0.45) (both P < 0.01) but not with reactive hyperemic flow or hyperemic shear stress. There was no correlation between two measures of macrovascular reactivity (FMD and ΔPWV). Skin reactive hyperemia was significantly associated with RHI (r = 0.55) and reactive hyperemic flow (r = 0.35) (both P < 0.05). There was a significant association between reactive hyperemia and RHI (r = 0.30; P < 0.05). In more than 75% of cases, vascular reactivity measures were not significantly associated. We concluded that associations among different measures of peripheral micro- and macrovascular reactivity were modest at best. These results suggest that different physiological mechanisms may be involved in changing different measures of vascular reactivity.
Keywords: flow-mediated dilatation, reactive hyperemia, pulse wave velocity, endothelial function
in the primary prevention of cardiovascular disease, the assessment of traditional risk factors and the subsequent calculation of the Framingham risk factors are useful initial steps in the stratification of cardiovascular risks. However, a considerable number of at-risk patients cannot be identified on the basis of these conventional risk factors (9). This has prompted the search for new markers of subclinical disease. In particular, reactive hyperemia following limb ischemia has emerged as a promising methodology to assess vascular abnormality and cardiovascular risks (23). Identification of such abnormalities and risks in accessible peripheral arteries provides a conventional means for early detection of presymptomatic vascular disease. Among the various measures of peripheral vascular reactivity, endothelium-dependent flow-mediated dilatation (FMD) is considered a biomarker of endothelial function and has been increasingly used in a number of research investigations (4, 16). This technique is based on the premise that physiological increases in blood flow, more precisely shear stress, induce vasodilation that is mediated by an increased nitric oxide release (4). However, flow-mediated dilatation requires high levels of technical expertise and an expensive ultrasound machine and may be burdened by considerably large intra- and interobserver variability. As such, even though the prognostic value of brachial artery FMD has been demonstrated in several studies (15, 16), many have questioned that this methodology will ever be applied and implemented in routine clinical practice (1, 24, 27).
Recently, a number of alternative or complementary approaches to evaluate macro- and microvascular reactivity following brachial ischemia have been introduced. These techniques monitor various physiological aspects in response to reactive hyperemia (e.g., skin blood flow, fingertip temperature, finger pulse wave amplitude). Some are measures of macrovascular function [e.g., pulse wave velocity (PWV)] and others are indexes of microvascular functions (e.g., reactive hyperemia, skin reactive hyperemia). Currently, it is not known if and how well these measures of peripheral vascular reactivity are related. Such information is critical as an increasing number of studies have reported that measures of peripheral vascular reactivity provide prognostic information incremental to conventional risk factors (10, 15, 23) and that the knowledge regarding the interrelationships could facilitate their integration into clinical and research practice.
Accordingly, the primary aim of the present investigation was to determine the interrelationships among different noninvasive measures that have been used to assess micro- and macrovascular reactivity. We reason that such information is critical to compare and contrast the findings from various studies using different measure of vascular reactivity. To minimize the influence of chronic diseases and risk factors that could differentially affect vascular reactivity and to study the associations of different methodologies in more physiological rather than pathophysiological states, we recruited and studied apparently healthy normotensive subjects in the present study. The selection of the subject population seems reasonable given the wide interindividual variabilities in vascular reactivity measures we observed in similar populations in our previous studies (5, 7). The working hypotheses were that macrovascular reactivity measures (e.g., FMD, ΔPWV) and microvascular reactivity (e.g., reactive hyperemic flow, skin reactive hyperemia, fingertip pulse wave amplitude, fingertip temperature) would be significantly correlated to each other and that measures of macrovascular reactivity would not be associated with indexes of microvascular reactivity.
METHODS
Subjects
Forty apparently healthy subjects (28 men, 12 women) aged 19–68 yr were studied. All subjects were normotensive, nonobese nonsmokers and free of overt cardiovascular diseases as assessed by medical history. None of the subjects were taking any cardiovascular acting medications. Subjects were recruited from The University of Texas at Austin, the city of Austin, and the surrounding community using flyers and direct mailings.
All procedures involved in the study were explained, and written informed consents were obtained from the subjects. The study was reviewed and approved by the local Institutional Review Board.
Protocol
Subjects were required to fast and abstain from coffee and alcohol at least 4 h before the start of the study, and measurements were made with subjects lying in the supine position. All the experiments were conducted in the morning between 0800 and 1200. Subjects were instructed not to perform any strenuous physical activity for 24 h before the study. After height, weight, and blood pressure (standing, sitting, and supine position) were measured, the subjects were instructed to lie down in a quiet, temperature-controlled laboratory room for 30 min. The temperature of the room was maintained between 22 and 25°C.
To accommodate all of the techniques assessed in the present study, subjects underwent two randomized testing sessions on the same day. There was a resting period of at least 30 min between the two sessions, and a total time of ∼3 h was needed to complete the entire protocol. During the resting period, ECG electrodes were placed on the chest and a blood pressure cuff was secured on the forearm for occluding blood flow. Additionally, multiple probes were placed, including a temperature probe [for temperature rebound (TR)] on the index finger, a finger plethysmograph probe [for reactive hyperemia index (RHI)] on the middle finger, and a laser-Doppler probe (for skin reactive hyperemia) on the little finger (see Measurements). Fingers were carefully positioned so that they did not touch each other. During session 1, an ultrasound probe was placed on the brachial artery for the measurement of FMD, and during session 2, Doppler flowmeters were placed on the brachial and radial arteries for the measurement of PWV. Extra care was taken to ensure that the subject's arm position as well as the probe position stayed constant throughout the testing session. All the testing was conducted on the subjects' left arm.
Measurements
FMD/reactive hyperemia/hyperemic shear stress.
Brachial artery diameters and blood flow velocity measurements were performed using a Doppler ultrasound machine (HDI 5000CV, ATL Instruments, Bothel, WA) equipped with a high-resolution linear-array transducer while subjects rested in supine position (7). A customized transducer holding device was used to secure a transducer in place. A longitudinal image of the brachial artery was acquired 5–10 cm proximal to the antecubital fossa, and baseline blood flow and diameter measurements were made. After the acquisition of baseline measurements, blood flow was occluded with a blood pressure cuff placed on the forearm and inflated to 100 mmHg above baseline systolic blood pressure for 5 min. After cuff deflation, ultrasound-derived measurements of the brachial artery diameters and blood flow were made for 3 min. Blood flow measurements were taken during the first 45 s after cuff deflation to obtain peak blood flow and shear stress, and then the brachial artery image was quickly switched to acquire the diameter for the next 135 s to capture peak arterial diameter. The transition between different acquisition modes was instantaneous and was facilitated by the preset built in the ultrasound machine. All ultrasound brachial images were recorded and analyzed by the same investigator. Ultrasound images (ECG R-wave gated) were transferred to a digital viewing software (Vascular Research Tool Brachial Analyzer, Medical Imaging Applications, Coralville, IA) for later analyses. FMD was calculated using the equation: (maximum diameter − baseline diameter)/baseline diameter × 100 (4). FMD was also expressed as the diameter change normalized for shear stress (data not shown as the results were similar between unadjusted and adjusted FMD).
Brachial artery blood flow was measured with duplex ultrasound and was analyzed manually by using software integral to the ultrasound machine. Blood flow was calculated as MBV × π × (brachial arterial radius)2 × 60, where MBV is mean blood velocity (in cm/s), and 60 is used to convert from milliliters per second to milliliters per minute. Reactive hyperemic flow was calculated as peak blood flow/baseline blood flow. Hyperemic shear stress was calculated using the following equation: 8 × blood viscosity (assumed to be 0.035 dyn×s/cm2) × peak blood velocity/baseline brachial artery diameter (at end diastole) (20).
RHI.
Finger plethysmograph (ENDOPAT 2000, Itamar Medical, Caesarea, Israel) were used to measure changes in pulse wave amplitude in response to reactive hyperemia (see above). This device measures peripheral arterial tone with a probe that has a pneumatic cuff, which encapsulates the fingertip and assesses digital volume changes with each pulse wave (18). The probes were placed on the middle finger of both hands, and the data were recorded continuously before, during, and after cuff deflation. The data were automatically analyzed with a computerized automated algorithm built in the machine, and the result was expressed as RHI. RHI is the ratio of the average pulse wave amplitude during the 1-min period following the release of blood pressure cuff to the average pulse wave amplitude during 210-s baseline period (18). The coefficient variation of RHI is 12%.
TR.
Digital thermal monitoring (DTM) is a noninvasive measurement of vascular responsiveness (Vendys 5000, Endothelix, Houston, TX) that assesses changes in fingertip temperature in response to blood flow changes in the fingertips (17). The device consists of a computer-based thermometry system, and two fingertip thermocouple probes designed to minimize the area of skin-probe contact and fingertip pressure. DTM was performed according to an automated operator-independent protocol. Fingertip skin temperature was measured by the probes placed on the index finger of both hands. During ischemia, the temperature drops toward room temperature, and on cuff release, the temperature rapidly returns to and exceeds the baseline fingertip skin temperature. The increase in fingertip temperature after cuff deflation above the starting finger temperature (designated as the TR) was used as an index of vascular reactivity.
Skin reactive hyperemia.
Cutaneous reactive hyperemia following limb ischemia was measured as previous described in detail (28). Skin blood flow was measured noninvasively using a laser-Doppler monitor (moorLAB, Moor Instruments, Devon, UK). Laser-Doppler flow measures moving blood cells in the underlying skin. Low-power laser light transmitted via an optic fiber to the tissue is scattered by moving blood cells causing Doppler frequency shifts that are measured as Doppler flux signals. The laser-Doppler probe was placed over the skin at the little finger level, and the data were collected continuously. The skin blood flow was expressed in an arbitrary perfusion unit. Skin reactive hyperemia was calculated as (peak blood flow − baseline blood flow)/baseline blood flow × 100.
PWV.
PWV was calculated from the measurement of pulse transit time (or time delay) and the distance traveled between two arterial recording sites. Two identical transcutaneous Doppler flowmeters (810-A, Parks Medical, Aloha, OR) were used to obtain the pulse wave between the brachial and radial artery. Transit time was determined from the time delay between the proximal and the distal foot waveforms. The foot of the wave was identified as the commencement of the sharp systolic upstroke. Distance traveled by the pulse wave was assessed in duplicate with a random zero-length measurement over the surface of the body with a nonelastic tape measure. PWV was calculated from distance (in cm) divided by transit time (in s). According to the Bramwell-Hill equation to calculate PWV, one of the most important factors affecting PWV is the diameter of arteries (2). Because FMD is obtained by tracking changes in arterial diameter from baseline to postischemic condition, changes in PWV during the same time span might be related to peripheral vascular reactivity, more specifically FMD (11). PWV was reported in this paper as percent change in PWV from the baseline to peak value (ΔPWV).
Statistical Analyses
Pearson product-moment correlational analyses were used to determine the relation among vascular reactivity measures. One-way ANOVA was used to determine the group differences stratified by the tertiles and quatiles of FMD, as well to examine the differences in vascular reactivity measures between sessions 1 and 2. Significance was set a priori at P < 0.05. All data are expressed as means ± SD.
RESULTS
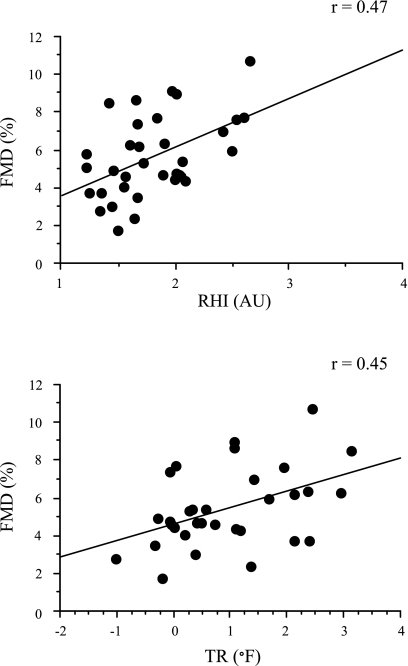
Subjects were relatively young and apparently healthy as demonstrated by mean blood pressure and body mass index values that were well within the clinically normal range (Table 1). Mean values of peripheral vascular reactivity measures are displayed in Table 2. There were no systematic differences in peripheral reactivity measures between sessions 1 and 2 (P > 0.05), so the results were combined. Table 3 displays intercorrelations between different measures of peripheral vascular reactivity. FMD was significantly (P < 0.01) and positively associated with RHI (r = 0.47) and TR (r = 0.45) (both P < 0.01) (Fig. 1). FMD was not significantly related to reactive hyperemic flow, hyperemic shear stress, or skin reactive hyperemia (P > 0.05). There was no correlation between two measures of macrovascular reactivity (FMD and ΔPWV). Skin reactive hyperemia was associated with reactive hyperemic flow (r = 0.35) and RHI (r = 0.55) (both P < 0.05). There was a significant association between reactive hyperemia and RHI (r = 0.30; P < 0.05). In >75% of cases (5 of 21 possible associations), peripheral vascular measures were not significantly correlated.
Table 1.
Selected subject characteristics
| Variable | Value |
|---|---|
| n | 40 |
| Men/women | 28/12 |
| Age, yr | 32±13 |
| Height, cm | 173±10 |
| Body mass, kg | 71.7±15.3 |
| Systolic BP, mmHg | 119±12 |
| Diastolic BP, mmHg | 76±8 |
| BMI, kg/m2 | 24±4 |
Values are means ± SD. BP, blood pressure; BMI, body mass index.
Table 2.
Peripheral vascular reactivity measures
| Variable | Value |
|---|---|
| FMD, % | 5.5±2.1 |
| Reactive hyperemic flow, AU | 5.1±1.1 |
| Hyperemic shear stress, dyn/cm2 | 55±14 |
| RHI, AU | 1.9±0.5 |
| TR, oF | 1.1±1.1 |
| Skin reactive hyperemia, AU | 304±284 |
| ΔPWV, % | −18±11 |
Values are means ± SD. FMD, flow-mediated dilatation; RHI, reactive hyperemia index; TR, temperature rebound; PWV, pulse wave velocity; AU, arbitrary units.
Table 3.
Pearson product-moment correlation coefficients among different measures of peripheral vascular reactivity
| Macro |
Macro/Micro | Micro | |||||
|---|---|---|---|---|---|---|---|
| FMD | ΔPWV | Hyperemic Shear Stress | Reactive Hyperemic Flow | RHI | TR | Skin RH | |
| Macro FMD | NS | NS | NS | 0.47 (P < 0.01) | 0.45 (P < 0.01) | NS | |
| ΔPWV | NS | NS | NS | NS | NS | ||
| Macro/Micro Hyperemic shear stress | NS | NS | NS | NS | |||
| Reactive hyperemic flow | 0.30 (P < 0.05) | NS | 0.35 (P < 0.05) | ||||
| Micro RHI | NS | 0.55 (P < 0.01) | |||||
| TR | NS | ||||||
| Skin RH | |||||||
Macro, macrovascular; Micro, microvascular; RH, reactive hyperemia; NS, not significant.
Fig. 1.
Relations between flow-mediated dilatation (FMD) and reactive hyperemia index (RHI) (top), as well as relations between FMD and temperature rebound (TR) (bottom). AU, arbitrary units.
The subjects were stratified into tertiles and quartiles based on brachial artery FMD to determine if subjects with the lowest FMD values had the lowest RHI and TR. However, the group differences did not reach statistical significance.
DISCUSSION
In the present study, interrelationships among various measures of postischemic macro- and microvascular reactivity were determined. Although there has been a one-to-one comparison of peripheral vascular reactivity measures (7, 13, 18), this is the first study to comprehensively determine the interrelationships among various measures of traditional and emerging peripheral vascular reactivity measures in the same subjects. This is critical as differences in subject characteristics and experimental protocols make comparisons of different methodologies difficult. A total of seven different techniques applied to the same subjects were assessed and compared in the present study. The primary finding of the present study is that measures of vascular reactivity were not strongly associated. In most of the techniques assessed (>75% of cases), correlations between vascular reactivity measures did not even reach statistical significance. These results suggest that varying degrees of physiological mechanisms may be involved in evoking different peripheral vascular responses.
Currently, it is not known what physiological factors are responsible for inducing reactive hyperemic responses that are assessed with each technique. Reactive hyperemia is a complex hemodynamic response of the vasculature that aims to accelerate the delivery of oxygen to tissues as well as the removal of metabolic byproducts after a period of ischemia. It has been assumed that in most of the techniques used in the present study, nitric oxide (NO) plays an important role in the dilatory responses. Indeed, several studies have demonstrated a role of NO in evoking FMD (the most physiologically characterized vascular reactivity measure), as the infusion of NO synthase (NOS) blocker abolishes the increase in arterial diameter following reactive hyperemia (14). However, blood vessels of endothelial NOS (eNOS) knockout mice still experience FMD by responding to shear stress, most likely mediated by prostaglandins (25). Nervous system regulation of vascular tone has also been implicated in FMD (26).
Whether NO plays a role in reactive hyperemia (increases in blood flow) remains highly controversial, with stronger evidence suggesting otherwise (6, 19, 27, 29). In the case of the cutaneous circulation, recent findings demonstrate that the skin reactive hyperemic response is not mediated by NO release (29). This could explain the lack of association between FMD and skin reactive hyperemia observed in the present study. Aside from NO, the reactive hyperemic response depends on a number of other physiological factors, including adenosine, prostaglandin, endothelium-derived hyperpolarizing factors, potassium, pH, hydrogen peroxide, the myogenic response, and microvascular structure (6, 21, 26). Considering the involvements of different physiological factors regulating reactive hyperemic responses, nonsignificant correlations we identified in >75% of comparisons among peripheral vascular reactivity measures in the present study may not be too surprising. In addition to physiological factors, measurement locations/sites/tissues at which vascular reactivity is measured in each technique would further contribute to the varying responses.
Endothelium-dependent vasodilation is typically examined in one of two ways: 1) increases in forearm blood flow to intrabrachial artery infusions of endothelial dilators like ACh (5), and 2) brachial artery FMD (3, 4). If the two procedures are measuring similar or common properties of peripheral vascular endothelial vasodilatory capacity, they will be expected to correlate with each other. However, we have previously reported that these two methodologies do not significantly relate to each other (7), a finding that has since been confirmed by other groups (8). Although there are a number of possible explanations for the divergent results produced by the use of these two techniques, one possibility is different levels of major contributions from macro- vs. microvascular properties affecting each technique. The ACh infusion examines resistance vessel endothelium-dependent vasodilation in response to a pharmacological stimulus, whereas the FMD assesses large-conduit artery endothelial function in response to the physiological stimulus of increased shear stress. A similar analogy can be applied to the present findings. That is, a lack of association between different measures of vascular reactivity may be attributed to different levels of contributions from macro- vs. microvascular properties.
There has been a lot of confusion and controversy regarding the relative clinical utility of reactive hyperemia and FMD. For example, a recent report from the Framingham study suggested that hyperemic shear stress may be a better predictor because hyperemic shear stress was more strongly correlated with risk factors than FMD (20). In the present study, FMD was not significantly associated with reactive hyperemia or hyperemic shear stress. A lack of association may be surprising considering that reactive hyperemia and shear stress are thought to be stimuli for FMD. Consistent with the present study, however, a recent study in patients with peripheral artery disease undergoing vascular surgery also reported no significant association between FMD and reactive hyperemia (12). A lack of association may be attributed to the physiological difference that reactive hyperemia is a measure of microvascular function whereas FMD is an index of macrovascular function.
Because of the ease of set-up and accessibility, fingertips have been selected and used to assess vascular reactivity in some emerging methodologies. Pulsatile blood volume changes in the fingertips are evaluated in ENDOPAT, whereas a newer technology, DTM, monitors temperature changes in the fingertip following the cuff release. These techniques are operator independent and easy to apply to larger populations. Because TR and RHI are indexes of microvascular reactivity and FMD is a measure of macrovascular reactivity, we hypothesized that there would not be any significant associations between these measures. In contrast to our working hypothesis, we found that both TR and RHI were significantly associated with FMD. These results suggest that TR and RHI may be used as an alternative approach to FMD. Indeed, such an approach has been recently taken by a number of investigators (10, 30). However, there was no significant correlation when we compared the primary derivatives from these devices. These results indicate that both methodologies are governed by factors that are unique to each. In this context, because brachial arterial blood flow is directed to both muscle and skin, and RHI was more strongly associated with skin vascular reactivity as assessed by laser-Doppler flowmeter, the hyperemic response assessed using the ENDOPAT may be affected to a greater extent by skin blood flow changes and/or its correlates.
Several limitations of this study deserve comments. First, we only studied apparently healthy subjects in the present study. By doing so, we may have limited the “spread” of the data distribution and reduced the strength of correlations. An obvious next step would be to determine which vascular reactivity measures are most sensitive in terms of identifying persons with subclinical atherosclerosis. Second, it is important to emphasize that measurement variabilities inherent in each technique may have invariably reduced the strength of the association.
In summary, different measures of peripheral micro- and macrovascular reactivity were not strongly correlated, suggesting different physiological mechanisms involved. Given significant association with the clinically validated FMD, TR (as measured by the DTM) and RHI (as measured by the ENDOPAT) may be promising technologies to assess peripheral vascular reactivity. These techniques need to be further investigated in the future for their potential clinical utility.
GRANTS
The present study was supported in part by National Institutes of Health (NIH) Award AG-20966. A. E. DeVan was supported by a fellowship award from the NIH (DA-018431).
Acknowledgments
The DTM Vendys 5000 was on a term-limited loan from Endothelix.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bhagat K, Hingorani A, Vallance P. Flow associated or flow mediated dilatation? More than just semantics. Heart 78: 7–8, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond 93: 298–306, 1922. [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
- 5.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in the human forearm. J Appl Physiol 81: 1807–1814, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacological vs. flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol 88: 47–49, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Gori T, Di Stolfo G, Sicuro S, Dragoni S, Lisi M, Parker JD, Forconi S. Correlation analysis between different parameters of conduit artery and microvascular vasodilation. Clin Hemorheol Microcirc 35: 509–515, 2006. [PubMed] [Google Scholar]
- 9.Greenland P, Smith SC Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation 104: 1863–1867, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Haller MJ, Stein J, Shuster J, Theriaque D, Silverstein J, Schatz DA, Earing MG, Lerman A, Mahmud FH. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes 8: 193–198, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hayano J, Ishihara S, Fukuda H, Sakata S, Mukai S. New methodology to assess endothelial function using pulse wave velocity changes following reactive hyperemia (in Japanese). In: Proceedings of Working Group on Clinical Arterial Pulse Wave Conference. Tokyo, Japan: Medical View, 2004.
- 12.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irace C, Ceravolo R, Notarangelo L, Crescenzo A, Ventura G, Tamburrini O, Perticone F, Gnasso A. Comparison of endothelial function evaluated by strain gauge plethysmography and brachial artery ultrasound. Atherosclerosis 158: 53–59, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Karatzis EN, Ikonomidis I, Vamvakou GD, Papaioannou TG, Protogerou AD, Andreadou I, Voidonikola PT, Karatzi KN, Papamichael CM, Lekakis JP. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol 98: 1424–1428, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 111: 310–314, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kozlemeny E Examination of flow mediated dilation with thermometry in case of patients with high cardiovascular risk. Cardiologia Hungarica 35: 11–16, 2005. [Google Scholar]
- 18.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 146: 168–174, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Melkumyants AM, Balashov SA, Klimachev AN, Kartamyshev SP, Khayutin VM. Nitric oxide does not mediate flow induced endothelium dependent arterial dilatation in the cat. Cardiovasc Res 26: 256–260, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Miura H, Wachtel RE, Liu Y, Loberiza FR Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol 101: 545–548, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Sejda T, Pit'ha J, Svandova E, Poledne R. Limitations of non-invasive endothelial function assessment by brachial artery flow-mediated dilatation. Clin Physiol Functional Imaging 25: 58–61, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Thijssen DH, de Groot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol 291: H3122–H3129, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Tschakovsky ME, Pyke KE. Counterpoint: flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Vuilleumier P, Decosterd D, Maillard M, Burnier M, Hayoz D. Postischemic forearm skin reactive hyperemia is related to cardiovascular risk factors in a healthy female population. J Hypertens 20: 1753–1757, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95: 504–510, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Impaired nitric oxide bioavailability and l-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 204: 2693–2704, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]