Abstract
Strength deficits associated with eccentric contraction-induced muscle injury stem, in part, from excitation-contraction uncoupling. FKBP12 is a 12-kDa binding protein known to bind to the skeletal muscle sarcoplasmic reticulum Ca2+ release channel [ryanodine receptor (RyR1)] and plays an important role in excitation-contraction coupling. To assess the effects of FKBP12 deficiency on muscle injury and recovery, we measured anterior crural muscle (tibialis anterior and extensor digitorum longus muscles) strength in skeletal muscle-specific FKBP12-deficient and wild-type (WT) mice before and after a single bout of 150 eccentric contractions, as well as before and after the performance of six injury bouts. Histological damage of the tibialis anterior muscle was assessed after injury. Body weight and peak isometric and eccentric torques were lower in FKBP12-deficient mice compared with WT mice. There were no differences between FKBP12-deficient and WT mice in preinjury peak isometric and eccentric torques when normalized to body weight, and no differences in the relative decreases in eccentric torque with a single or multiple injury bouts. After a single injury bout, FKBP12-deficient mice had less initial strength deficits and recovered faster (especially females) than WT mice, despite no differences in the degree of histological damage. After multiple injury bouts, FKBP12-deficient mice recovered muscle strength faster than WT mice and exhibited significantly less histological muscle damage than WT mice. In summary, FKBP12 deficiency results in less initial strength deficits and enhanced recovery from single (especially females) and repeated bouts of injury than WT mice.
Keywords: mouse, skeletal muscle, damage, force, recovery
the performance of eccentric contractions can result in significant skeletal muscle fiber (9, 19) and sarcomere damage (17), t-tubule disruptions (29, 39), sarcoplasmic reticulum (SR) Ca2+ dysregulation (3, 13), resting cytosolic Ca2+ perturbations (3, 13, 20, 34), mitochondrial disruption (6, 38), and weakness (3, 9, 11, 13–15, 19, 22, 24, 32, 34, 36). Our laboratory (13, 35–37) and others (3) have shown that the strength deficits observed within the first few days of injury stem, in large part, from failure of excitation-contraction (E-C) coupling, at a site somewhere between the dihydropyridine (i.e., L-type Ca2+ channel) and ryanodine receptors (RyR1) (13, 14, 35). The ability of skeletal muscle to produce peak force after a single bout of eccentric contractions can be impaired up to 4 wk (11, 24, 35, 36). This prolonged muscle weakness appears to stem, in part, from the loss of myofibrillar protein (11, 19). Complete restoration of skeletal muscle function requires the upregulation of protein synthesis and satellite cell activity (19, 24).
Current models of skeletal muscle E-C coupling suggest that Ca2+ is released from RyR1s after direct physical interaction with voltage-gated dihydropyridine receptors on the t-tubule (5, 7). However, RyR1s are also known to interact with a number of different molecules that regulate SR Ca2+ channel activity. Therefore, Ca2+ released from RyR1s can be modulated by a number of different ions (e.g., Ca2+, Mg2+), proteins (e.g., calmodulin), enzymes (e.g., calcineurin), redox status, and immunophilins [e.g., 12-kDa FK506 binding protein (FKBP12)]. The observation that eccentric contractions do not alter dihydropyridine and RyR sensitivity (14) suggests that the E-C coupling failure may involve one or more of the ancillary proteins that form complexes with the RyR1.
FKBPs, a family of binding proteins named according to their molecular mass, are known to bind the immunosuppressive drugs FK506 and rapamycin. Skeletal muscle contains both FKBP12 and FKBP12.6, with RyR1 possessing a higher affinity for FKBP12.6. However, FKBP12 is normally bound to RyR1 because of its higher cellular concentration. FKBP12 can be displaced from RyR1 when bound to either FK506 or rapamycin. The loss of FKBP12 binding to RyR1 increases the probability of channel opening (2) and thus is thought to stabilize a closed-channel state (4). Using a skeletal muscle-specific FKBP12 knockout mouse model, we have demonstrated that myotubes from these mice display altered orthograde and retrograde coupling between the dihydropyridine and RyRs (30). EDL muscles from these mice have a rightward shift in the force-frequency relationship, reduced peak isometric force-producing capability, and normal peak caffeine contracture force, consistent with the idea of E-C uncoupling.
We have suggested that E-C uncoupling may serve to protect skeletal muscle from excessive cellular damage and prolonged functional deficits (14). Although the degree of muscle strain influences the magnitude of injury (32), peak force appears to be a primary determinant of muscle injury and strength deficits observed after the performance of eccentric contractions (32). Therefore, skeletal muscle that is prone to E-C uncoupling might be expected to produce lower peak forces during eccentric contractions and exhibit less cellular damage. Because FKBP12 deficiency appears to result in some degree of E-C uncoupling in certain skeletal muscles, we sought to test the hypothesis that skeletal muscle-specific FKBP12 deficiency would reduce strength deficits and muscle damage observed following eccentric contraction-induced muscle injury. To assess the effects of FKBP12 deficiency on muscle injury and recovery, we measured anterior crural muscle [tibialis anterior (TA) and extensor digitorum longus (EDL) muscles] strength in skeletal muscle-specific FKBP12-deficient and wild-type (WT) mice before and after a single bout of 150 eccentric contractions, as well as before and after the performance of six bouts of eccentric contractions.
METHODS
Animals
Skeletal muscle-specific FKBP12-deficient mice were created using embryonic stem cell technology and Cre-LoxP-mediated gene recombination with Cre transgene expression under the regulation of the muscle creatine kinase promoter, as described previously (30). Skeletal muscle-specific knockout mice will be referred to as FKBP12-deficient mice. Mice with the Cre transgene served as controls and will be referred to as WT. Mice were bred at Georgia State University, and genotypes were confirmed using PCR analysis. Based on immunoblotting, the levels of FKBP12 in skeletal muscle tissue are reduced 90%, with the remaining expression associated with nonskeletal muscle cells (30). The mice were housed in groups of 5–10 animals per cage, supplied with food and water ad libitum, and maintained in a room at 20–22°C with a 12-h photoperiod. Mice were euthanized with an overdose of pentobarbital sodium. All animal care and use procedures were approved by the institutional animal care and use committee and met the guidelines set by the American Physiological Society.
Experimental Design
Two types of studies were performed. In the first study, 24 FKBP12-deficient mice 120 ± 35 days old (mean ± SD; 12 males and 12 females) and 24 WT mice 96 ± 24 days old (mean ± SD; 12 males and 12 females) were used to determine the effects of a single bout of 150 eccentric contractions on anterior crural muscle function and muscle damage. This contraction protocol has been shown to induce significant skeletal muscle injury, as evidenced by strength deficits and myofiber damage. The lengthening contraction protocol causes a significant loss of maximum strength (∼50%), stemming from multiple mechanisms (e.g., damage and loss to E-C uncoupling proteins and force-bearing structures), and requires a prolonged period (≤4 wk) of recovery time (11, 35, 36). Isometric torques of this muscle group as a function of stimulation frequency (20–400 Hz) were measured in anesthetized adult mice before and immediately after the eccentric contraction bout. Peak torques were recorded during the 150 maximal lengthening contractions. The recovery of anterior crural muscle strength after injury was also evaluated in vivo by measuring isometric torque as a function of stimulation frequency (20–400 Hz) at 3, 7, and 14 days after injury. The extent of muscle damage was assessed histologically in injured and contralateral control TA muscles at 3, 7, and 14 days postinjury. Isometric force production of the EDL muscle as a function of stimulation frequency (10–300 Hz) was assessed in vitro in uninjured muscles, as well as in muscles immediately after, and at 3, 7, and 14 days after injury.
In the second study, we sought to determine the adaptability of FKBP12-deficient skeletal muscle to eccentric contraction-induced muscle injury. Specifically, eight FKBP12-deficient 100 ± 43-day-old mice (mean ± SD; 4 males and 4 females) and six WT 100 ± 18-day-old mice (mean ± SD; 3 male and 3 female) were used to determine the effects of six bouts of eccentric contractions, with 1-wk recovery between bouts, on anterior crural muscle function and muscle damage. Isometric torques of this muscle group as a function of stimulation frequency (20–400 Hz) were measured in vivo in anesthetized adult mice before and immediately after each of the eccentric contraction bouts. Peak torques were recorded during the 150 maximal eccentric contractions that were performed in each bout. The recovery of anterior crural muscle strength after injury was also evaluated in vivo by measuring isometric torque as a function of stimulation frequency (20–400 Hz) at 3 days following the sixth injury bout. Our laboratory has previously shown that peak muscle damage occurs at 3 days following a single bout of 150 eccentric contractions (19) and that anterior crural muscle function is completely recovered at this time point following five bouts of 150 eccentric contractions (15). Isometric force production of the injured EDL muscle as a function of stimulation frequency (10–300 Hz) was assessed in vitro 3 days after injury.
In Vivo Muscle Strength Analysis and Injury Induction
Contractile function (i.e., torque-frequency relationship) of the left anterior crural muscles was measured in vivo immediately before and after, as well as 3, 7, and 14 days after, a single bout of 150 eccentric contractions, as previously described (11–14, 19, 36). After mice were anesthetized (0.3 mg/kg fentanyl citrate, 16.7 mg/kg droperidol, 5.0 mg/kg diazepam), the left hindlimb was aseptically prepared, the mouse was placed on a heated platform with the left knee clamped, and the left foot was secured to an aluminium “shoe” that is attached to the shaft of an Aurora Scientific 300B servomotor. Sterilized needles were inserted through the skin for stimulation of the left common peroneal nerve. Stimulation voltage and needle electrode placement were optimized with 5–15 isometric contractions (200-ms train of 0.1-ms pulses at 300 Hz). Contractile function of the anterior crural muscles was assessed by measuring peak isometric torque as a function of stimulation frequency (20–400 Hz). Injury to the anterior crural muscles was induced by the performance of 150 eccentric contractions (38° angular movement at 2,000°/s, starting from a 19° dorsiflexed position). Peak torque was measured for every eccentric contraction in the injury protocol and was saved and totaled (termed total eccentric torque) for every 10th contraction.
In Vitro Analysis of EDL Muscle
EDL muscles from FKBP12-deficient and WT mice were dissected free and studied at 35°C using an in vitro preparation, as previously described (11, 13–15, 30). EDL muscles were mounted in a chamber containing a Krebs-Ringer bicarbonate buffer (pH 7.4) with (in mM) 144 Na+, 126.5 Cl−, 6 K+, 1 Mg2+, 1 SO42−, 1 PO43−, 25 HCO3−, 1.25 Ca2+, 0.17 leucine, 0.10 isoleucine, 0.20 valine, 10 glucose, 10 μg/ml gentamicin sulfate, and 0.10 U/ml insulin (the buffer was equilibrated with 95% O2-5% CO2 gas). The distal tendon was attached by silk suture and cyanoacrylate adhesive to a fixed support, and the proximal tendon was attached to the lever arm of a servomotor system (Aurora Scientific 300B). Optimal physiological muscle length in the chamber was set with a series of twitch contractions (0.2-ms pulse at 150 V). Next, peak isometric force as a function of stimulation frequency (10–300 Hz) was measured during isometric contractions (200-ms trains of 0.2-ms pulses), with 3 min between contractions. Caffeine sensitivity was assessed by measuring changes in baseline force during exposure to increasing caffeine concentrations (1, 2, 4, 8, 16, and 50 mM) and twitch contractions at a rate of 0.2 Hz. Force produced by the EDL muscle was normalized to physiological cross-sectional area (N/cm2), as described previously (19).
Histology
Cryosections (10 μm) were obtained from three levels (proximal, middle, and distal) of the TA muscle and stained with hematoxylin and eosin to estimate the extent of muscle damage caused by exercise. All myofibers were counted in the muscle cross sections from the three levels using BioQuant Nova Prime (V6.90.10) software and averaged to estimate total muscle myofiber number. Discolored myofibers containing three or more internal nuclei were classified as active degenerating myofibers (i..e., injured), whereas myofibers containing one or two internal nuclei with normal staining were used as a marker of previous damage and/or regeneration. Our laboratory has previously used similar methods to estimate the extent of muscle damage after injury (19).
Statistical Analyses
Individual factorial ANOVAs and ANOVAs with repeated measures were used to determine statistical differences in isometric and eccentric strength, body weight (BW), skeletal muscle wet weight, and myofiber number, damage, and regeneration. In the event of a significant interaction, simple main-effects analysis and Bonferroni's post hoc tests were conducted. An α-level of 0.05 was used for all analyses. Except for the descriptive data in Table 1, values presented in results are means ± SE.
Table 1.
Initial BW, uninjured TA and EDL muscle wet weights, muscle wet weights normalized to BW, and peak torques produced during isometric and eccentric contractions
| WT |
FKBP12 Deficient | |||||
|---|---|---|---|---|---|---|
| Female | Male | WT Total | Female | Male | FKBP12 Deficient Total | |
| n | 12 | 12 | 24 | 12 | 12 | 24 |
| BW, g | 27.77±2.20 | 32.78±2.37 | 30.38±3.40 | 24.44±2.74 | 28.03±2.17 | 26.24±3.03 |
| TA muscle wet weight, mg | 46.20±4.07 | 56.75±5.40 | 52.24±7.29 | 43.84±6.12 | 49.71±6.30 | 46.76±6.89 |
| EDL muscle wet weight, mg | 8.05±0.69 | 10.10±0.85 | 9.20±1.29 | 7.62±0.82 | 8.33±1.35 | 7.99±1.17 |
| TA/BW ratio, mg/g | 1.69±0.12 | 1.76±0.14 | 1.73±0.14 | 1.79±0.14 | 1.78±0.17 | 1.79±0.15 |
| EDL/BW ratio, mg/g | 0.29±0.01 | 0.31±0.03 | 0.30±0.02 | 0.31±0.03 | 0.30±0.04 | 0.31±0.03 |
| Peak eccentric torque, N·mm | 5.49±0.50 | 6.96±0.82 | 6.28±1.01 | 4.73±0.64 | 6.29±0.64 | 5.51±1.00 |
| Peak eccentric torque, N·mm·kg−1 | 198.06±14.69 | 213.44±22.30 | 206.34±20.36 | 193.65±16.56 | 224.08±12.32 | 208.87±21.10 |
| Peak isometric tetanic torque, N·mm | 2.78±0.30 | 3.49±0.40 | 3.17±0.50 | 2.38±0.33 | 3.18±0.38 | 2.78±0.54 |
| Peak isometric tetanic torque, N·mm·kg−1 | 100.48±7.69 | 107.07±9.33 | 104.17±9.11 | 97.42±10.21 | 113.23±9.21 | 105.32±12.47 |
| Peak isometric twitch torque, N·mm | 0.70±0.12 | 0.89±0.16 | 0.80±0.17 | 0.52±0.15 | 0.68±0.12 | 0.60±0.16 |
| Peak isometric twitch torque, N·mm·kg−1 | 25.08±3.27 | 27.02±4.96 | 26.13±4.30 | 21.94±5.35 | 24.33±4.43 | 23.35±4.95 |
Values are means ± SD; n, no. of animals. WT, wild type; FKBP12, 12-kDa FK506 binding protein; BW, body weight; TA, tibialis anterior; EDL, extensor digitorum longus. Total refers to the combined values of male and female mice from the respective genotype.
RESULTS
Study 1
Basic morphological characteristics of FKBP12-deficient mice.
Compared with WT mice, FKBP12-deficient mice were 13.6% smaller (P < 0.05) at time of injury induction (Table 1). However, there was no significant difference in absolute EDL muscle wet weight between female FKBP12-deficient and WT mice, whereas there was an 18% difference (P < 0.05) in EDL muscle wet weight between male FKBP12-deficient and WT mice. There was a trend (P = 0.11) for the same effects in the TA muscle. Because BW is reduced 12–15% in female and male FKBP12-deficient mice, it appears that female FKBP12-deficient mice have less change in EDL muscle wet weight than male FKBP12-deficient mice. However, normalizing uninjured anterior crural muscle wet weights to BW eliminated statistical differences between FKBP12-deficient and WT mice (Table 1).
In vivo muscle injury induction.
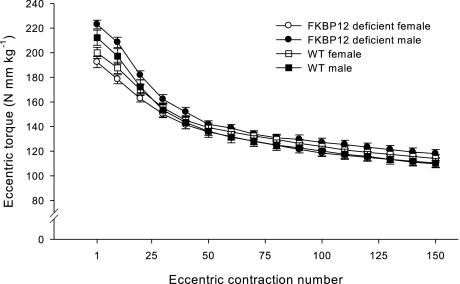
Anterior crural muscles from FKBP12-deficient mice produced 12.3% less (P < 0.05) peak absolute torque during the first eccentric contraction of the injury protocol than from WT mice (Table 1). Similarly, FKBP12-deficient mice produced 11.6% lower (P < 0.05) total torque during the 150 eccentric contractions than WT mice (59.2 ± 1.8 vs. 67.0 ± 2.0 N·mm). These strength differences appear to stem from differences in BW between the genotypes; normalizing peak eccentric torque and total eccentric torque to BW abolished the statistical differences between genotypes (Fig. 1 and Table 1).
Fig. 1.
In vivo peak torque (means ± SE) produced by anterior crural muscles from wild-type (WT) and 12-kDa FK506 binding protein (FKBP12) knockout mice during a single bout of eccentric contractions. Torques are normalized to body weight in kilograms (kg).
Because strength deficits associated with the performance of eccentric contractions depend, in large part, on peak force (32), the stimulus for inducing injury appears to be the same between FKBP12-deficient and WT mice when peak and total eccentric contraction torques are scaled to BW. The percent decline in eccentric torque over the 150 contractions is the same between FKBP12-deficient (44.9 ± 1.1%) and WT (45.5 ± 1.1%) mice.
In vivo isometric torque.
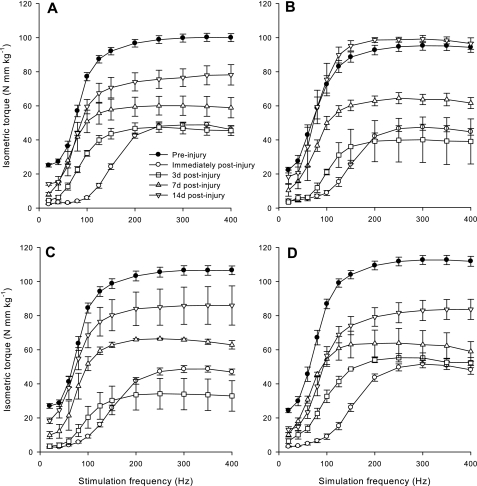
Absolute peak isometric tetanic torque is 12.3% less (P < 0.05) in uninjured anterior crural muscles from FKBP12-deficient mice compared with WT mice (Table 1). However, when isometric torque is normalized to BW, there are no significant differences between uninjured WT and FKBP12-deficient mice, except at 20 Hz (Fig. 2 and Table 1). Peak twitch torque at 20-Hz stimulation is 10.6% lower in FKBP12-deficient mice compared with WT mice (Table 1).
Fig. 2.
In vivo peak isometric torque (means ± SE) as a function of stimulation frequency produced by anterior crural muscle before and after a single bout of eccentric contractions in WT female (A) and male (C) mice, and in FKBP12-deficient female (B) and male (D) mice. Torques are normalized to body weight in kilograms (kg). d, Day.
The performance of 150 eccentric contractions resulted in immediate reductions (P < 0.05) in isometric torque as a function of stimulation frequency in both WT (52.7–92.1%) and FKBP12-deficient (52.8–89.1%) mice (Fig. 2). Our laboratory has shown previously that the decrease in isometric contraction torque immediately after the eccentric contraction protocol reflects primarily anterior crural muscle injury and not fatigue (36). Compared with WT mice, FKBP12-deficient mice exhibited less (P < 0.05) strength loss at 20 (84.5 vs. 90.0%), 40 (84.2 vs. 89.4%), and 80 Hz (89.1 vs. 92.2%) immediately after injury induction. However, strength deficits at stimulation frequencies >100-Hz contractions were virtually identical between genotypes (P = 0.57–0.96) at this time.
In general, few differences were apparent between WT and FKBP12-deficient mice in strength deficits observed during recovery from injury (i.e., 3–14 days). However, on closer inspection of the data, we observed, rather unexpectedly, differences in strength loss and recovery between groups based on sex. Three days after injury, male FKBP12-deficient mice produced significantly higher isometric torque than male WT mice at intermediate-to-high stimulation frequencies (100–400 Hz), whereas FKBP12-deficient and WT female mice were not significantly different (Fig. 2). For both WT and FKBP12-deficient mice, there was no significant recovery in isometric torque 3 days after injury at low (20–60 Hz) and high stimulation frequencies (200–400 Hz).
There were no differences between WT and FKBP12-deficient mice in the recovery of isometric strength 7 days after injury (Fig. 2). At this time, all mice displayed recovery of isometric torque at low and intermediate stimulation frequencies (20–200 Hz), but not in torque produced at relatively high frequencies (350–400 Hz) (Fig. 2).
All mice exhibited some recovery in isometric torque (20–400 Hz) by 14 days after injury induction (Fig. 2). However, only the female FKBP12-deficient mice displayed recovery back to preinjury strength values at intermediate and high stimulation frequencies (100–400 Hz) (Fig. 2). Moreover, only the female FKBP12-deficient mice displayed significant recovery of isometric torque from 7 to 14 days at moderate and high stimulation frequencies (100–400 Hz). In general, isometric torque produced by male and female WT mice, as well as male FKBP12-deficient mice, recovered little from 7 to 14 days at stimulation frequencies from 40 to 400 Hz.
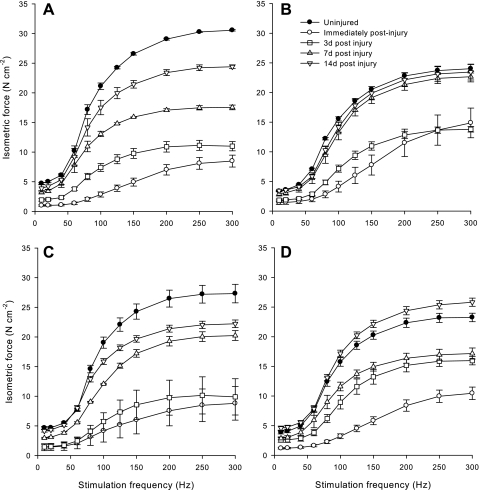
In vitro EDL muscle force.
The finding that isometric-specific force production by uninjured EDL muscles from FKBP12-deficient mice is less (P < 0.05) than WT muscle at all stimulation frequencies except 60 Hz (Fig. 3) is similar to what our laboratory has previously observed (30). The performance of 150 eccentric contractions in vivo resulted in immediate deficits in isometric-specific force in vitro immediately after injury in both WT and FKBP12-deficient mice (Fig. 3). There were no differences between WT and FKBP12-deficient mice in isometric-specific force at low to intermediate stimulation frequencies (i.e., 10–125 Hz). However, female FKBP12-deficient mice produced greater (P < 0.05) isometric-specific force than all other mice immediately after injury at high stimulation frequencies (150–300 Hz). There was no recovery in isometric-specific force 3 days after injury in male and female WT and female FKBP12-deficient mice at relatively low (10–40 Hz) and high (100–300 Hz) stimulation frequencies. However, male FKBP12-deficient mice demonstrated significant recovery in isometric-specific force 3 days after injury at all stimulation frequencies. By 7 days after injury, male and female WT and female FKBP12-deficient mice exhibited significant recovery in force at all stimulation frequencies; however, no recovery occurred between 3 and 7 days in male FKBP12-deficient mice at low (10 and 20 Hz) and high (100–300 Hz) stimulation frequencies. Female FKBP12-deficient mice were completely recovered by 7 days, and male FKBP12-deficient mice were completely recovered by 14 days postinjury. In contrast, although some further recovery in strength occurred at 14 days in male and female WT mice, isometric-specific force was still not completely recovered at relatively high stimulation frequencies (125–300 Hz). Force produced at relatively low stimulation frequencies (10–100 Hz) was completely recovered by 14 days after injury in male and female WT mice.
Fig. 3.
In vitro isometric-specific force (means ± SE) as a function of stimulation frequency in uninjured and injured extensor digitorum longus (EDL) muscles from WT female (A) and male (C) mice, and in FKBP12-deficient female (B) and male (D) mice after a single bout of eccentric contractions. The numbers of female and male WT muscles used, respectively, were as follows: uninjured (5 and 3), immediately postinjury (3 and 3), 3 days (3 and 3), 7 days (2 and 3), and 14 days (2 and 4). The number of female and male FKBP12-deficient muscles used, respectively, were as follows: uninjured (5 and 3), immediately postinjury (3 and 3), 3 days (3 and 3), 7 days (3 and 3), and 14 days (3 and 3).
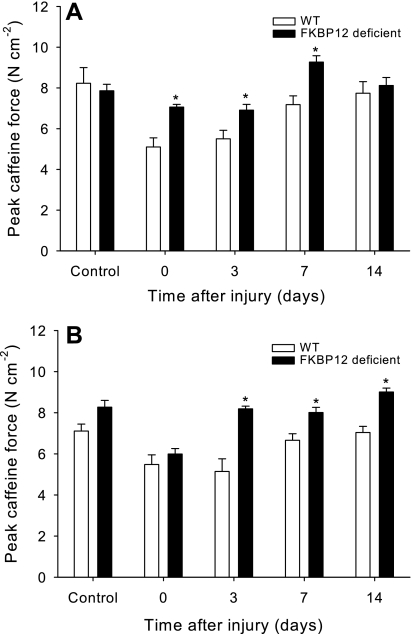
Although our laboratory has previously reported (30) that FKBP12 deficiency does not alter the sensitivity of uninjured skeletal muscle to caffeine, injured EDL muscle from FKBP12-deficient mice exhibited significantly greater (11.0–31.1%) caffeine-induced force than WT muscle at all caffeine concentrations (1–50 mM) (Fig. 4).
Fig. 4.
Peak caffeine (50 mM) contracture force (means ± SE) produced by uninjured and injured EDL muscles from female (A) and male (B) FKBP12-deficient and WT mice after a single bout of eccentric contractions. *FKBP12-deficient mice produce greater contracture force than WT mice (P < 0.05).
BW and muscle weights and muscle damage after injury.
The percent change in BW after injury induction was not significantly (P ≥ 0.32) different between FKBP12-deficient and WT mice. Compared with BWs at the time of injury induction, all mice lost 3.6 ± 1.0% of their BW 3 days after injury. However, the BWs of all mice at 7 and 14 days were not significantly different than the preinjury BW.
The wet weight of the EDL muscle responded differently after injury between WT and FKBP12-deficient mice. In female WT mice, the EDL muscle wet weight was significantly lower 7 days after injury (6.6 ± 0.5 mg) compared with immediately (9.2 ± 0.7 mg), 3 days (8.8 ± 0.2 mg), and 14 days (8.8 ± 0.1 mg) after injury. In contrast, there were no significant differences in wet weight among these time points in female FKBP12-deficient mice: immediately (7.9 ± 1.4 mg), 3 days (8.5 ± 0.4 mg), 7 days (7.6 ± 0.7 mg), and 14 days (8.6 ± 0.1 mg) after injury. In male WT mice, the EDL muscle wet weight was significantly higher 3 days after injury (12.3 ± 0.3 mg) compared with 14 days (10.7 ± 0.4 mg) after injury, but were not different immediately (11.8 ± 1.3 mg), 7 days (11.3 ± 0.6 mg), and 14 days after injury. In male FKBP12-deficient mice, the EDL muscle wet weight was significantly lower 3 (8.9 ± 0.6 mg) and 7 days (7.6 ± 0.7 mg) after injury compared with immediately (11.3 ± 0.9 mg) and 14 days (9.9 ± 0.2 mg) after injury.
There were few differences between FKBP12-deficient and WT mice in the pattern of change in TA muscle wet weight after injury. In FKBP12-deficient mice, TA muscle wet weight was significantly reduced 7 days (42.0 ± 2.7 mg) compared with immediately (51.6 ± 3.4 mg), 3 days (49.7 ± 3.2 mg), and 14 days (49.0 ± 2.0 mg) after injury. In WT mice, TA muscle wet weight was significantly reduced 7 (49.9 ± 4.0 mg) and 14 days (49.3 ± 2.3 mg) compared with immediately (54.8 ± 4.0 mg) and 3 days (57.0 ± 3.7 mg) after injury.
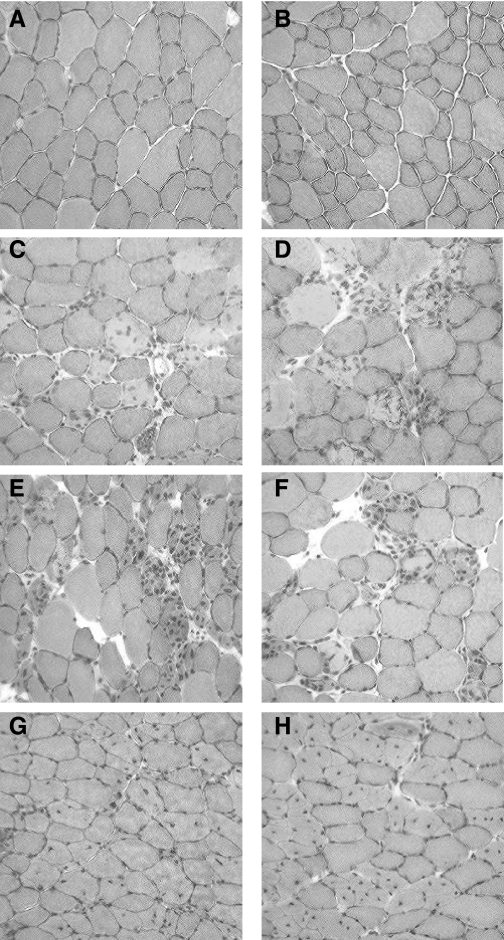
The eccentric contraction protocol did induce significant myofiber damage based on the hematoxylin and eosin stain (Fig. 5). There was no difference between FKBP12-deficient and WT mice in the relative degree of active myofiber degeneration and regeneration. The percentage of muscle fibers actively degenerating increased (P < 0.05) from 0.19 ± 0.04% in noninjured muscle to 8.4 ± 1.4, 10.2 ± 2.3, and 5.8 ± 1.0% at 3, 7, and 14 days after injury, respectively. The percentage of regenerating muscle fibers (i.e., presence of central nuclei) was 0.6 ± 0.2, 3.1 ± 0.3, 2.4 ± 0.6, and 8.9 ± 1.8% in noninjured muscle, and at 3, 7, and 14 days after injury, respectively. Only muscle from 14 days postinjury exhibited a significant increase in regeneration. Estimates of total fiber number were not different between WT and FKBP12-deficient mice and were not significantly different among the time points: uninjured (2,028 ± 84, n = 14), 3 days (1,911 ± 235, n = 7), 7 days (1,784 ± 233, n = 8), and 14 days (2,125 ± 206, n = 7) after injury.
Fig. 5.
Tibialis anterior muscle cryosections stained with hematoxylin and eosin after a single bout of eccentric contractions. Uninjured myofibers are from WT (A) and FKBP12-deficient mice (B), and tibialis anterior myofibers are from WT mice at 3 (C), 7 (E), and 14 days (G) after injury, and from FKBP12-deficient mice at 3 (D), 7 (F), and 14 days (H) after injury. Hematoxylin- and eosin-stained myofibers that were discolored and containing ≥3 internal nuclei were classified as active degenerating myofibers (e.g., C–F), whereas myofibers containing 1 or 2 internal nuclei with normal staining were classified as regenerating myofibers (G and H).
Study 2
In vivo eccentric contractile torque.
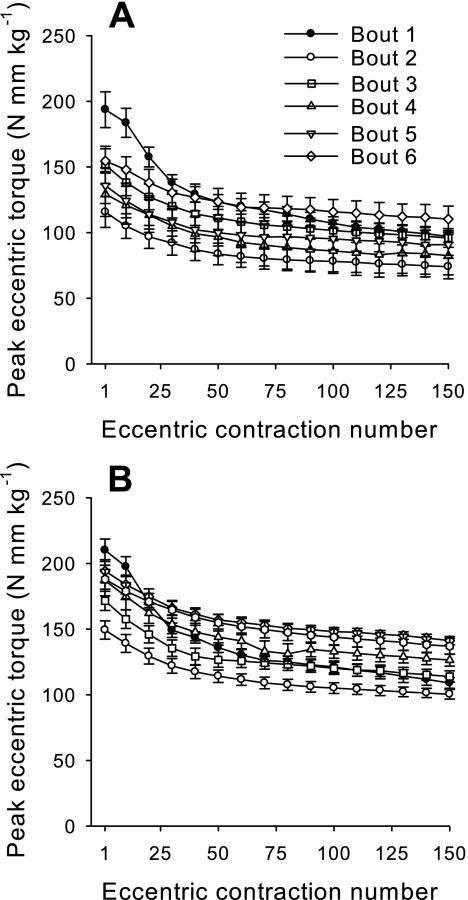
There was no significant difference in peak eccentric torque produced on the first contraction of the first bout between WT (193.6 ± 13.6 N·mm·kg−1) and FKBP12-deficient (210.04 ± 8.5 N·mm·kg−1) mice (Fig. 6). There was also no difference in the percent decrease in eccentric contraction torque over the first injury bout between WT (−47.2 ± 1.8%) and FKBP12-deficient (−48.0 ± 1.1%) mice. FKBP12-deficient mice adapted better during the weekly bouts of 150 eccentric contractions. This enhanced adaptation of anterior crural muscle strength after injury in FKBP12-deficient mice is reflected by higher (P < 0.05) peak eccentric torques on the first contraction across all bouts than the WT mice (183.3 ± 4.3 vs. 146.5 ± 6.7 N·mm·kg−1). For both groups, peak eccentric torque on the first contraction of bouts 2–6 was significantly less than that of bout 1. Given that full strength recovery takes ∼4 wk in this injury model, it is not surprising that anterior crural muscle torque was least recovered at the beginning of bout 2, with peak eccentric torques of both groups significantly different than all other bouts. Like the first eccentric contraction bout, there were no significant differences between groups in the percent decline in eccentric torque for all injury bouts. The average decrease in peak eccentric contraction torque for bouts 2–6 was 33.8 ± 1.4, 34.5 ± 1.9, 31.2 ± 1.3, 28.5 ± 1.6, and 27.7 ± 2.0%, respectively. The relative decrease in torque on the first bout (−47.7 ± 0.9%) was significantly different than all of the rest, whereas the percent decreases were not different among bouts 2–6.
Fig. 6.
In vivo peak eccentric torque (means ± SE) produced by mouse anterior crural muscles by WT (A) and FKBP12-deficient (B) mice during weekly injury bouts.
In vivo isometric contractile torque.
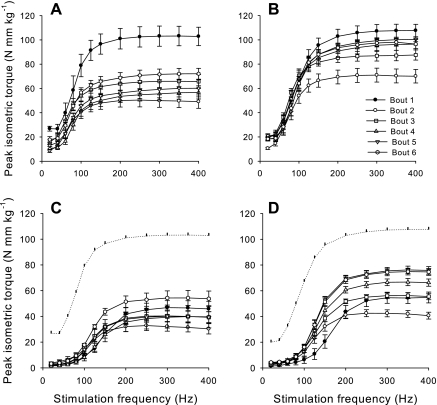
There were no significant differences in normalized isometric torque between WT and FKBP12-deficient mice before the first injury bout (Fig. 7). There were no significant differences between WT and FKBP12-deficient mice in the postinjury isometric torque (i.e., normalized to BW) at stimulation frequencies <150 Hz across all six injury bouts. Moreover, there was little adaptation in the strength deficits observed at relatively low frequencies across all injury bouts. It was not until the muscle was stimulated at higher frequencies (>100 Hz) that postinjury isometric torques were greater (P < 0.05) than that of the first bout in subsequent sessions. However, there were also significant genotype differences at these relatively high stimulation frequencies (>125 Hz). Although there were no differences between groups in postinjury isometric torque after the first bout, anterior crural muscles from FKBP12-deficient mice had greater (P < 0.05) isometric torque than WT mice at stimulation frequencies >125 Hz after either the first (150, 250, 300, 350 Hz), second (200 Hz), or third (400 Hz) bouts. The ability of skeletal muscles from WT mice to adapt to weekly injury sessions and produce higher postinjury isometric torques than previous sessions was markedly (P < 0.05) less than in FKBP12-deficient mice. There were no significant differences in postinjury isometric torque between the first and last injury bouts at higher stimulation frequencies (200–400 Hz) in WT mice. In contrast, FKBP12-deficient mice produced greater (P < 0.05) postinjury isometric torques at higher stimulation frequencies (200–400 Hz) in bouts 4–6 than in the first injury bout.
Fig. 7.
In vivo isometric torque (means ± SE) as a function of stimulation frequency before (A and B) and immediately after (C and D) weekly eccentric contraction training in WT (A and C) and FKBP12-deficient (B and D) mice. Dotted lines in C and D represent preinjury torque values.
Recovery of isometric contraction torque as a function of stimulation frequency before each injury bout reflects the ability of mouse anterior crural muscle to adapt to weekly sessions of eccentric contraction-induced injury (Fig. 7, A and B). There were significant differences between WT and FKBP12-deficient mice across injury bouts at 20 and 125- to 400-Hz stimulation frequencies. Specifically, FKBP12-deficient mice produced greater (P < 0.05) isometric contraction torques than WT mice during bouts 2–6 at stimulation frequencies >100 Hz. Isometric torque was significantly less at the second bout compared with all other bouts for FKBP12-deficient mice at stimulation frequencies of 20 and 125–400 Hz. Anterior crural muscles from FKBP12-deficient mice produce torque similar to uninjured values at low frequencies (i.e., 20–100 Hz) by the third bout and high frequencies (125–400 Hz) by the fifth injury bout. Although anterior crural muscles from WT mice also produce torque similar to uninjured values at low frequencies (i.e., 40–80 Hz) by the third bout, torque produced at relatively high stimulation frequencies (125–400 Hz) was still significantly lower than preinjury values by the sixth injury bout.
FKBP12-deficient mice exhibited greater (P < 0.05) isometric torques than WT mice at relatively high stimulation frequencies (125–400 Hz) 3 days after the last injury bout. However, there were no differences between WT and FKBP12-deficient mice in isometric torques at low-to-moderate stimulation frequencies (20–100 Hz) 3 days after the last eccentric contraction bout. Both groups of mice exhibited significant recovery in isometric torques at these stimulation frequencies 3 days after the last injury bout (data not shown).
In vitro EDL muscle-specific force and caffeine sensitivity.
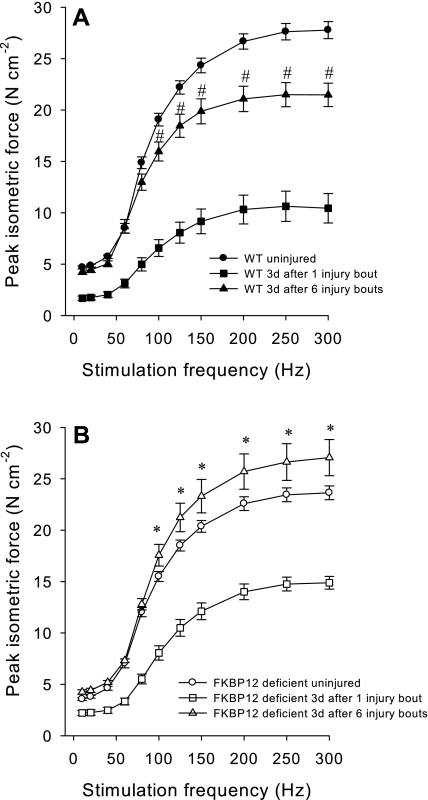
The EDL muscle of both groups of mice appeared to have adapted to the weekly training based on the degree of recovery in specific force 3 days after the last injury induction compared with specific force values 3 days after one injury bout (Fig. 8). However, FKBP12-deficient mice produced greater isometric-specific force than WT mice at high stimulation frequencies (200–300 Hz) 3 days after the last injury bout (Fig. 8). In addition, FKBP12-deficient mice appear to recover force-producing capacity with eccentric contraction training, as isometric force produced at moderate-to-high stimulation frequencies (100–300 Hz) was greater (P < 0.05) 3 days after the sixth injury bout compared with before injury. In contrast, WT mice had isometric-specific force values significantly lower 3 days after the last injury bout compared with preinjury values at relatively moderate-to-high stimulation frequencies (Fig. 8).
Fig. 8.
In vitro isometric-specific force (means ± SE) as a function of stimulation frequency in EDL muscles from WT (A) and FKBP12-deficient (B) mice 3 days after the performance of the sixth bout of 150 eccentric contractions. Isometric-specific force values from EDL muscles that are uninjured and 3 days after a single injury bout are shown for reference. All force values after the sixth injury bout are significantly greater than force values 3 days after the single injury bout. #Significant differences between 3 days post-sixth injury bout and uninjured (P < 0.05). *Significant differences between 3 days post-sixth injury bout and uninjured (P < 0.05).
EDL muscles from FKBP12-deficient mice exhibited significantly more sensitivity to caffeine than from WT mice 3 days following the last injury bout. EDL muscles from FKBP12-deficient mice produced greater (P < 0.05) caffeine-induced forces than WT mice at 2 (23%), 4 (33%), and 50 mM (27%) caffeine concentrations. Caffeine forces at 8 and 16 mM concentrations also tended (P ≤ 0.09) to be greater in FKBP12-deficient mice than WT mice.
Muscle weights and muscle damage.
The BW of WT mice (30.2 ± 2.3 g) was significantly greater than that of FKBP12-deficient mice (24.6 ± 1.1 g) 3 days after the last injury bout. Despite the 19% smaller BW of the FKBP12-deficient mice, there was no significant difference in injured TA muscle wet weight in WT (47.3 ± 4.5 mg) and FKBP12-deficient (49.3 ± 1.7 mg) mice. Moreover, the injured TA muscle wet weight normalized to BW from FKBP12-deficient mice was significantly larger than injured TA muscle from WT mice. The injured TA muscle from FKBP12-deficient mice was significantly larger than its contralateral control muscle, whereas the injured and contralateral control TA muscles from WT mice were not different. There was no significant (P = 0.07) difference in EDL wet weight between WT (10.3 ± 0.5 mg) and FKBP12-deficient (9.1 ± 0.3 mg) mice, nor (P = 0.14) in normalized wet weight (0.33 ± 0.02 vs. 0.37 ± 0.01 mg/g).
Estimates of total myofibers from injured TA muscles 3 days after the last injury bout were greater (P < 0.05) than uninjured contralateral control TA muscles in both WT (2,678 ± 176 vs. 2,481 ± 42; n = 3) and FKBP12-deficient (2,592 ± 115 vs. 2,248 ± 118; n = 7) mice. Both WT and FKBP12-deficient mice still exhibited significant active degeneration of TA myofibers 3 days after the last injury bout compared with contralateral control muscles. However, TA muscle from FKBP12-deficient mice exhibited significantly less myofiber degeneration than that of WT mice (3.9 ± 0.6 vs. 7.0 ± 1.4%). The percentage of myofibers displaying regeneration 3 days after the last injury bout was not different between WT (15.0 ± 2.9%) and FKBP12-deficient (10.3 ± 2.1%) mice.
DISCUSSION
We have shown that FKBP12 is critical to life and regulation of the E-C coupling in skeletal muscle. Specifically, when the gene encoding the FKBP12 protein is completely removed in mice, most animals die in utero due to severe cardiac defects (25). Using a skeletal muscle-specific FKBP12 knockout mouse model, we have demonstrated that myotubes from these mice display reduced voltage-gated SR Ca2+ release and enhanced L-type Ca2+ influx and caffeine sensitivity (30). EDL muscles from these mice have a reduced isometric force-producing capability and normal peak caffeine contracture force, consistent with the idea of alterations in E-C coupling.
The present study sought to test the hypothesis that skeletal muscle-specific FKBP12 deficiency would reduce strength deficits and muscle damage observed following eccentric contraction-induced muscle injury. Compared with WT mice, we found that anterior crural muscles from FKBP12-deficient mice exhibited less initial isometric strength deficits, faster recovery after both single and repeated bouts of eccentric contractions, and less damage to the TA muscle after repeated injury bouts. Specifically, anterior crural muscles from male and female FKBP12-deficient mice produced 42–130% greater isometric torque at low stimulation frequencies than WT mice immediately after injury. In addition, EDL muscle from female FKBP12-deficient mice produced 29–75% greater isometric-specific force at high stimulation frequencies than all other mice immediately after injury. Isometric torque produced by the anterior crural muscle from female FKBP12-deficient mice recovered significantly faster than that in all other mice. In addition, EDL muscles from male and female FKBP12-deficient mice recovered significantly faster than their WT counterparts. However, as with the anterior crural muscle (i.e., TA muscle) recovery from a single injury bout, EDL muscles from female FKBP12-deficient mice recovered significantly faster than that in all other mice.
The reason anterior crural muscle from FKBP12-deficient mice experience less initial strength deficit and faster recovery from injury than WT mice is unknown. Less apparent injury in TA muscles from FKBP12-deficient mice does not appear to stem from a weaker injury stimulus. The magnitude of strength loss associated with the performance of eccentric contractions is known to be affected by peak force (8, 22, 32). Anterior crural muscles from FKBP12-deficient and WT mice produced similar peak eccentric torque when normalized to BW, and the relative decrease in torque over the eccentric contraction protocol was nearly identical (Fig. 1). Moreover, despite producing greater relative eccentric torques during repeated injury bouts (Fig. 6), anterior crural muscles from FKBP12-deficient mice adapted significantly faster and exhibited markedly less damage to the TA muscle. Unlike the TA muscle, it is possible that less strength deficits observed in the EDL muscle after injury stem from a weaker injury stimulus, given the decrease in intrinsic isometric force output (Fig. 3). Previously, our laboratory noted that FKBP12 deficiency reduced intrinsic contractile force in the EDL muscle, but not in the soleus and diaphragm muscles (30).
Our laboratory (13) and others (3) have shown that short-term (i.e., immediately to 3 days after) strength loss associated with eccentric contraction-induced injury results primarily from the inability of the RyR1 to release Ca2+ (i.e., E-C uncoupling). When the normal excitation pathway in these injured muscles is circumvented by caffeine-like compounds acting directly on the RyR1, SR Ca2+ release and force output are restored (3, 13, 14, 37). If SR Ca2+ release is better maintained immediately after injury, then this could also explain the improved EDL and TA muscle force output from FKBP12-deficient mice. Apparent differences in E-C coupling between WT and FKBP12-deficient mice (30) could explain possible improvements in SR Ca2+ release after injury. Altered E-C coupling and increased Ca2+ influx and caffeine sensitivity observed in FKBP12-deficient myotubes (30) may be mediated by a gain of function by FKBP12.6. FKBP12.6 is the predominant isoform bound to the RyR in cardiac muscle and likely plays a role in mediating Ca2+-induced Ca2+ release. Therefore, it is possible that, if Ca2+-induced Ca2+ release contributes to E-C coupling in anterior crural muscles from FKBP12-deficient mice, then these muscles might not be as vulnerable to stress-induced disruptions in the E-C coupling mechanism normally present in skeletal muscle. Consistent with this idea, the ability of caffeine to trigger SR Ca2+ release and force production was not impaired in female FKBP12-deficient mice and only minimally affected in male FKBP12-deficient mice compared with WT mice (Fig. 4).
Our laboratory has previously reported that long-term (i.e., 2–4 wk) recovery of strength deficits depend on the resolution of inflammation, which entails myofiber degeneration and regeneration processes (11, 19, 35). Long-term, but not short-term, recovery of injured mouse anterior crural muscle depends on satellite cell recruitment in this injury model (24). Faster recovery of strength in FKBP12-deficient mice could result from less initial damage to the skeletal muscle, faster regeneration of the muscle, or both. Although the TA muscle in FKBP12-deficient mice is damaged less than in WT mice after repeated bouts of injury, this is not the case with a single injury bout. Therefore, it appears that recovery of anterior crural muscle function after a single injury bout stems from a more efficient regeneration process. Accelerated recovery from eccentric contraction-induced muscle injury in FKBP12 mice may be related to altered activity of transcriptional factors that are mediated by Ca2+ influx, although this does not appear to be related to E-C uncoupling per se. We have reported that FKBP12 deficiency results in increased Ca2+ influx through the L-type Ca2+ channel and increased phosphorylation of cAMP response element binding protein and calcineurin content (30). Calcineurin-mediated dephosphorylation of nuclear factor of activated T cell-2 may then play a role in the fusion of satellite cells (23) in the injured myofibers.
One surprising finding in the present study was that female FKBP12-deficient mice recovered strength deficits markedly faster than all other mice. Because final recovery of strength in this injury model depends on satellite cell recruitment (24), it appears that the removal of FKBP12 expedites this recovery process in female mice. FKBP12 is known to interact with both mammalian target of rapamycin (mTOR) (10) and the transforming growth factor (TGF)-1β receptor (31), signaling pathways known to regulate cell cycle (1, 10). It is possible that the mTOR and/or TGF-1β signaling in skeletal muscle from female mice is sensitive to the removal of FKBP12. Recent studies indicate that TGF-β, which can inhibit satellite cell activity (18), localizes around injured myofibers after eccentric contractions (27), and that activation of the Akt-mTOR signaling pathway is important in suppressing TGF-β signaling (28). Although estrogen may enhance recovery of skeletal muscle mass by affecting extracellular matrix remodeling and satellite cell activity (16, 21, 26), we did not observe this in female WT mice. How estrogen interacts with these pathways to expedite recovery of muscle strength after injury is unknown and awaits further investigation.
Anterior crural muscles from FKBP12-deficient mice exhibited better recovery of eccentric (Fig. 6) and isometric (Fig. 7) strength in the weekly exercise bouts than those from the WT mice.
Despite producing greater relative eccentric torque, and thus greater stimulus for injury, FKBP12-deficient mice also exhibited less isometric strength deficits immediately postinjury at high stimulation frequencies (150–400 Hz) after the third bout (Fig. 7). Our laboratory has previously suggested that the small attenuation in strength deficits immediately after eccentric contractions stems from the removal of stress-susceptible elements within the skeletal muscle, whereas the remaining strength deficits stem from E-C uncoupling (15). Attenuation in immediate peak strength deficits and faster recovery of weekly strength deficits could be explained, in part, by less myofiber damage and degeneration. In support of this notion, we observed significantly less myofiber degeneration 3 days after the last injury bout in FKBP12-deficient mice compared with WT mice.
All female mice exhibited greater eccentric torques than male mice in injury bouts 2–6. Moreover, female mice tended to produce greater isometric torque before and after injury bouts 2–6. Whether sex hormones amplify the mechanisms of enhanced recovery associated with FKBP12 deficiency (e.g., calcineurin) or act in an additive manner (e.g., inhibiting TGF-β, activating mTOR) after a single injury bout, and/or after multiple injury bouts, is unknown and warrants further investigation. In addition, accelerated recovery from eccentric contraction-induced injury may stem from events that are related to either the loss of FKBP12 or the upregulation of proteins (e.g., FKBP12.6) that are compensating for the loss of FKBP12.
In conclusion, skeletal muscle-specific FKBP12 deficiency results in a smaller and weaker phenotype. Consistent with our hypothesis, FKBP12-deficient mice exhibit less initial strength deficits, faster recovery from single and multiple bouts of eccentric contraction-induced injury, and less TA muscle damage after multiple injury bouts than WT mice. Moreover, anterior crural muscles from female FKBP12-deficient mice recover faster from injury than those of other mice. Future studies should address the role of FKBP12.6 in mediating force production in FKBP12-deficient mice and whether FKBP12-sensitive signaling pathways related to satellite cell activity contribute to the enhanced recovery of anterior crural muscle strength from eccentric contraction-induced injury.
GRANTS
This work is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-41802 (to S. L. Hamilton).
Acknowledgments
We thank Dr. Wei Tang and Talal Nofal for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aghdasi B, Ye K, Resnick A, Huang A, Ha HC, Guo X, Dawson TM, Dawson VL, Snyder SH. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci USA 98: 2425–2430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahern GP, Junankar PR, Dulhunty AF. Subconductance states in single-channel activity of skeletal muscle ryanodine receptors after removal of FKBP12. Biophys J 72: 146–162, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol 488: 25–36, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77: 513–523, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chelu MG, Danila CI, Gilman CP, Hamilton SL. Regulation of ryanodine receptors by FK506 binding proteins. Trends Cardiovasc Med 14: 227–234, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Duan C, Delp MD, Hayes DA, Delp PD, Armstrong RB. Rat skeletal muscle mitochondrial [Ca2+] and injury from downhill walking. J Appl Physiol 68: 1241–1251, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Dulhunty AF Excitation-contraction coupling from the 1950s into the new millennium. Clin Exp Pharmacol Physiol 33: 763–772, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Hentzen ER, Lahey M, Peters D, Mathew L, Barash IA, Friden J, Lieber RL. Stress-dependent and -independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. J Physiol 570: 157–167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesselink MKC, Kuipers H, Geurten P, Van Straaten H. Structural muscle damage and muscle strength after incremental number of isometric and forced lengthening contractions. J Muscle Res Cell Motil 17: 335–341, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle 5: 1391–1396, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Ingalls CP, Warren GL, Armstrong RB. Dissociation of force production from MHC and actin contents in muscles injured by eccentric contractions. J Muscle Res Cell Motil 19: 215–224, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Ingalls CP, Warren GL, Lowe DA, Boorstein DB, Armstrong RB. Differential effects of anesthetics on contractile function of mouse ankle dorsiflexor muscles in vivo. J Appl Physiol 80: 332–340, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Ingalls CP, Warren GL, Williams JH, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol 85: 58–67, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Ingalls CP, Warren GL, Zhang J, Hamilton SL, Armstrong RB. Dihydropyridine and ryanodine receptor binding after eccentric contractions in mouse skeletal muscle. J Appl Physiol 96: 1619–1625, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Ingalls CP, Wenke J, Nofal TS, Armstrong RB. Adaptation to lengthening contraction-induced injury in mouse muscle. J Appl Physiol 97: 1067–1076, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Levin ER Invited Review: Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol 91: 1860–1867, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Lieber RL, Thornell LE, Fridén J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol 80: 278–284, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15: 2950–2966, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe DA, Warren GL, Ingalls CP, Boorstein DB, Armstrong RB. Muscle function and protein metabolism after initiation of eccentric contraction-induced injury. J Appl Physiol 79: 1260–1270, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium 22: 373–383, 1997. [DOI] [PubMed] [Google Scholar]
- 21.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol 100: 2012–2023, 2006. [DOI] [PubMed] [Google Scholar]
- 22.McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol 61: 293–299, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Pavlath GK, Horsley V. Cell fusion in skeletal muscle: central role of NFATC2 in regulating muscle cell size. Cell Cycle 2: 420–423, 2003. [PubMed] [Google Scholar]
- 24.Rathbone CR, Wenke JC, Warren GL, Armstrong RB. Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 285: R1490–R1495, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, Mathews LM, Schneider MD, Hamilton SL, Matzuk MM. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature 391: 489–492, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol 100: 286–293, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Smith CA, Stauber F, Waters C, Alway SE, Stauber WT. Transforming growth factor-beta following skeletal muscle strain injury in rats. J Appl Physiol 102: 755–761, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. EMBO J 25: 58–69, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol 533: 571–583, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang W, Ingalls CP, Durham WJ, Snider J, Reid MB, Wu G, Matzuk MM, Hamilton SL. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. FASEB J 18: 1597–1599, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Donahoe PK, Zervos AS. Specific interaction of type I receptors of the TGF-beta family with the immunophilin FKBP-12. Science 265: 674–676, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Warren GL, Hayes DA, Lowe DA, Armstrong RB. Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J Physiol 464: 457–475, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren GL, Ingalls CP, Armstrong RB. A stimulating nerve cuff for chronic in vivo measurements of torque produced about the ankle in the mouse. J Appl Physiol 84: 2171–2176, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Warren GL, Ingalls CP, Armstrong RB. Temperature dependency of force loss and Ca2+ homeostasis in mouse EDL muscle after eccentric contractions. Am J Physiol Regul Integr Comp Physiol 282: R1122–R1132, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exerc Sport Sci Rev 29: 82–87, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Warren GL, Ingalls CP, Shaw S, Armstrong RB. Uncoupling of in vivo torque production from EMG in muscles injured by eccentric contractions. J Physiol 515: 609–619, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol 468: 487–499, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren GL, Williams JH, Ward CW, Matoba H, Ingalls CP, Hermann KM, Armstrong RB. Decreased contraction economy in mouse EDL muscle injured by eccentric contractions. J Appl Physiol 81: 2555–2564, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Yeung EW, Balnave CD, Ballard HJ, Bourreau JP, Allen DG. Development of T-tubular vacuoles in eccentrically damaged mouse muscle fibres. J Physiol 540: 581–592, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]