Abstract
Intermittent hypoxia (IH) has been found to protect brain from ischemic injury. We investigated whether IH mitigates brain oxidative stress and behavioral deficits in rats subjected to ethanol intoxication and abrupt ethanol withdrawal (EW). The effects of IH on overt EW behavioral signs, superoxide generation, protein oxidation, and mitochondrial permeability transition pore (PTP) opening were examined. Male rats consumed dextrin or 6.5% (wt/vol) ethanol for 35 days. During the last 20 days, rats were treated with repetitive (5–8 per day), brief (5–10 min) cycles of hypoxia (9.5–10% inspired O2) separated by 4-min normoxia exposures. Cerebellum, cortex, and hippocampus were biopsied on day 35 of the diet or at 24 h of EW. Superoxide and protein carbonyl contents in tissue homogenates and absorbance decline at 540 nm in mitochondrial suspensions served as indicators of oxidative stress, protein oxidation, and PTP opening, respectively. Although IH altered neither ethanol consumption nor blood ethanol concentration, it sharply lowered the severity of EW signs including tremor, tail rigidity, and startle response. Compared with dextrin and ethanol per se, in the three brain regions, EW increased superoxide and protein carbonyl contents and accelerated PTP opening in a manner ameliorated by IH. Administration of antioxidant N-acetylcysteine throughout the IH program abrogated the reductions in EW signs and superoxide content, implicating IH-induced ROS as mediators of the salutary adaptations. We conclude that IH conditioning during chronic ethanol consumption attenuates oxidative damage to the brain and mitigates behavioral abnormalities during subsequent EW. IH-induced ROS may evoke this powerful protection.
Keywords: mitochondrial permeability transition, superoxide, hippocampus, reactive oxygen species
hypoxia, the reduction of O2 supply below physiological levels, is generally considered harmful, especially to tissues with high O2 requirements (13). Hypoxia occurs naturally at high altitude, during strenuous exercise, and with various respiratory diseases. The fact that tissues survive such hypoxic stresses suggests that adaptive responses are mobilized to compensate for limitations in O2 supply. Indeed, recent research demonstrates that cyclic exposure to moderate hypoxia with intervening periods of normoxia bolsters cellular resistance to ischemic stress in several organs (6, 7, 9, 25, 26, 31, 48), including brain (3, 33, 40). These studies suggest that intermittent hypoxia (IH) may stimulate endogenous defense mechanisms, affording significant cytoprotection.
Brain mitochondria appear to be the principal targets of oxidative stress engendered by ethanol intoxication and withdrawal (20). The inner mitochondrial membrane's highly selective permeability to electrolytes, nucleotides, and metabolic substrates is essential for mitochondrial ATP production. The opening of nonselective mitochondrial permeability transition pores (PTP) in the inner membrane disrupts ATP production by collapsing the electrochemical gradient for H+, the driving force for oxidative phosphorylation. During ethanol withdrawal (EW), excessive glutamate-induced neuronal excitation increases intracellular concentrations of Ca2+ and reactive oxygen species (ROS), factors that provoke PTP opening (5, 11, 14, 15, 35, 43, 48). These pores permit nonselective passage of solutes and water, leading to mitochondrial swelling and possible rupture and to decreased efficiency of mitochondrial respiration (15, 48). Zhu et al. (49) reported that IH protected against Ca2+-induced PTP opening in rat ventricular myocardium.
Meerson et al. (27, 28) reported that exposure of rats to high altitude-induced IH attenuated various comorbidities associated with EW, such as pain, liver damage, and cardiac arrhythmias. We previously reported that EW caused oxidative stress in brain areas vulnerable to ethanol, such as cerebellum and cortex (19, 34). Accordingly, the current study was conducted to determine whether IH can prevent brain oxidative stress and behavioral impairment during EW. First, we tested whether IH indeed protects against overt behavioral signs of EW and whether such effects of IH are due to mechanisms activated during the antecedent alcohol consumption regimen. At the cellular level, we tested whether IH protects against EW's pro-oxidant effects and mitochondrial PTP opening. The cerebellum, cortex, and hippocampus were specifically examined because of the known susceptibility of these brain regions to ethanol and EW insult and to oxidative stress (12, 19, 38).
METHODS
Chemicals.
Analytic grade reagents were purchased from Sigma Aldrich (St. Louis, MO) or Calbiochem (San Diego, CA). Diet ingredients were obtained from Research Organics (Cleveland, OH) or MP Biomedicals (Irvine, CA).
Animals.
Male Sprague-Dawley rats (Charles River, Wilmington, MA), 3.5 mo old at the beginning of the study, were housed individually at controlled temperature (22–25°C) and humidity (55%) with ad libitum access to water. A 12:12-h light-dark cycle was maintained with lights on between 7:00 AM and 7:00 PM. Each rat's weight was recorded daily until death. All animal experimentation was conducted in accordance with the Guide to the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 85-23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205] and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Chronic ethanol administration.
Experimental procedures were conducted according to the protocol depicted in Fig. 1. Rats were divided into six groups (5–7 rats/group) according to diet (ethanol vs. control dextrin), treatment (IH vs. non-IH sham), and time of death (ethanol exposure vs. EW). The groups were dextrin sham, dextrin/IH, ethanol sham, ethanol/IH, EW sham, and EW/IH. Ethanol and EW rats were administered a liquid diet containing 6.5% (wt/vol) ethanol (8). Control animals were fed a liquid diet with dextrin isocalorically substituted for ethanol. Each liter of these diets contains 42 g of pulverized casein, 0.6 g of l-methionine, 2.1 g of vitamin mixture, 7.3 g of mineral mixture, 25 g of sucrose, 3 g of xanthum gum, 0.4 g of choline bitartrate, 1 g of Celufil cellulose, 10.5 g of corn oil, and either ethanol or dextrin in aqueous suspension. One hundred milliliters of diet were placed in each cage daily for 5 wk. These diets were abruptly terminated after 35 days and replaced with conventional chow diet to initiate withdrawal.
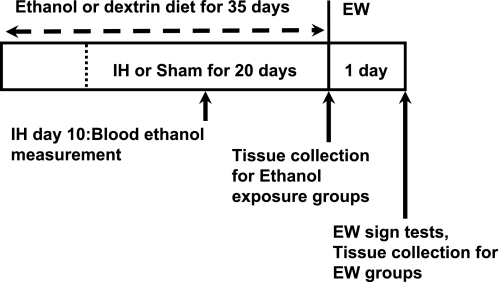
Fig. 1.
Ethanol intoxication-withdrawal protocol. Rats were administered a liquid ethanol or dextrin diet for 5 wk and treated with a daily intermittent hypoxia (IH) session for the last 20 days of the diet. At day 10 of IH, blood was collected from rats that received the ethanol diet with or without IH. Rats were killed and tissues harvested at the end of the 5-wk diet or at 24 h of ethanol withdrawal (EW).
Evaluation of EW signs.
Behavioral deficit was assessed 24 h after withdrawing ethanol or dextrin control diet by scoring seven criteria as previously described (19): 1) vocalization, urination, and defecation on handling (score: 0–3); 2) tail rigidity when the tail was drawn between the rater's fingers (0–3); 3) frequency and severity of tremor during handling (0–3); 4) startle (twitching, jumping, or freezing) observed for 15 s after auditory stimulus (0–3); 5) convulsion during and after handling (0 or 1); 6) spontaneous seizure (0 or 2); and 7) death (0 or 10). The sum of the seven scores equaled each animal's total score. Scoring was done by an evaluator blinded to each rat's diet and IH vs. sham program. All tests were conducted in a designated room in the vivarium to limit auditory stimulation. Rats were killed immediately after the sign test (19), and tissues were harvested for assessment of oxidative stress and mitochondrial resilience.
IH conditioning.
Rats were hypoxia- or sham-conditioned in custom-made 267-liter acrylic chambers. These chambers are large enough to accommodate multiple rat cages and to allow the atmosphere within the chamber to be adjusted quickly and precisely using compressed gas. The IH program (Table 1; Ref. 50) was administered each morning during the last 20 days of the 35-day ethanol diet but not during EW. The program consisted of brief (5–10 min) hypoxic exposures (5–8 per day) with intervening 4-min periods of reoxygenation. Percent O2 in the chamber was monitored with a precision O2 sensor (Alpha Omega Instruments model 2000). Compressed N2 was introduced into the chamber to lower percent O2 to the prescribed value (Table 1) within 90 s. Abrupt reoxygenation was achieved by opening the top and ends of the chamber. Non-IH groups underwent sham conditioning protocols in which compressed air instead of N2 was introduced to maintain the fractional inspired O2 (FiO2) at 21%. The rats exhibited no discomfort or distress during the hypoxia or sham sessions. To examine the role of oxyradicals in IH-induced adaptations, we administered an additional four ethanol-consuming rats the antioxidant N-acetylcysteine (100 mg/kg ip) 2 h before each IH session throughout the 20-day program.
Table 1.
IH conditioning program
| Session | FiO2, % | Hypoxia, min | Normoxia, min | Cycles | Total Hypoxia, min |
|---|---|---|---|---|---|
| 1 | 10 | 5 | 4 | 5 | 25 |
| 2 | 10 | 5 | 4 | 6 | 30 |
| 3 | 10 | 5 | 4 | 7 | 35 |
| 4 | 10 | 5 | 4 | 8 | 40 |
| 5 | 10 | 5 | 4 | 8 | 40 |
| 6 | 9.5 | 6 | 4 | 7 | 42 |
| 7 | 9.5 | 6 | 4 | 8 | 48 |
| 8 | 9.5 | 6 | 4 | 8 | 48 |
| 9 | 9.5 | 7 | 4 | 7 | 49 |
| 10 | 9.5 | 8 | 4 | 7 | 56 |
| 11–20 | 9.5 | 10 | 4 | 7 | 70 |
Intermittent hypoxia (IH) conditioning was administered during the last 20 days of the 35-day ethanol diet and then discontinued during ethanol withdrawal. Non-IH rats were sham conditioned by introduction of compressed air (21% O2) instead of N2 into the chamber. FiO2, fractional inspired O2.
Blood ethanol concentrations.
Blood ethanol concentrations were measured in additional (n = 3) dextrin, ethanol, and ethanol/IH rats. After completion of the day 10 IH conditioning session, i.e., 3 h after placement of fresh diet bottles, the rats were secured in a Plexiglas restraint device and a syringe fitted with a 25-gauge needle was inserted at a 45° angle toward the vein. Blood (200 μl) was withdrawn and immediately mixed with 90 μl of ice-cold 0.55 M HClO4. Samples were centrifuged at 1,500 g for 10 min to sediment protein precipitate. Supernatants were adjusted to ∼pH 5 with 200 μl of a solution containing 0.6 M KOH and 50 mM acetic acid and then centrifuged to sediment KClO4 precipitate. Ethanol in the supernatant was measured by colorimetric assay (42) in which NAD+ reduction to NADH is coupled to ethanol oxidation by alcohol dehydrogenase (extinction coefficient ɛ = 6.2 mM−1·cm−1) in a Beckman DU 640 spectrophotometer.
Assessment of oxidative markers.
Superoxide (O2•−) content in brain homogenates was measured as the superoxide dismutase-inhibitable reduction of acetylated ferricytochrome c (1). Ferricytochrome c reduction was monitored at 550 nm in a Beckman DU 640 spectrophotometer after addition of 7.5 mM succinate in the absence and presence of 100 U/ml superoxide dismutase. Ferricytochrome c concentration was obtained by applying an extinction coefficient of 27.7 mM−1·cm−1 to the absorbance values (1).
Protein carbonyls were measured (23) using 2, 4-dinitrophenylhydrazine (DNPH), which combines with aldehyde or ketone moieties in proteins to form DNP-protein adducts. The rats were anesthetized with xylazine (20 mg/kg ip) and ketamine (100 mg/kg ip) and decapitated. Cerebellum, cerebral cortex, and hippocampus were homogenized in 50 mM HEPES buffer (pH 7.2) containing 10 mM KCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail (Calbiochem, San Diego, CA). To 1 ml of homogenate, we added 0.2 ml of 10 mM DNPH in 2 N HCl, and 0.2 ml of 2 N HCl was added to another 1 ml of homogenate to provide a blank. Mixtures were incubated for 60 min at room temperature. The protein was precipitated with an equal volume of 20% trichloroacetic acid and was washed three times with ethanol-ethyl acetate (1:1 vol/vol). The final precipitate was dissolved in 2 ml of 6 M guanidine hydrochloride (pH 2.3), and insoluble debris was removed by centrifugation. Absorbance of DNP-protein adducts was measured at 360 nm (ɛ = 22 mM−1·cm−1; Ref. 23) in a Beckman DU 640 spectrophotometer. Carbonyl contents were expressed as nanomoles of carbonyl per milligram of protein.
Assessment of mitochondrial membrane swelling.
Mitochondria were isolated from brain homogenates by differential centrifugation (41). Tissue biopsies were dissected, rinsed, and homogenized in ice-cold isolation buffer (320 mM sucrose, 1 mM K2EDTA, 10 mM Tris·HCl). Homogenates were centrifuged at 1,330 g for 5 min at 4°C. The pellet was resuspended in 0.5 volume of isolation buffer and recentrifuged. The two supernatants were combined and centrifuged at 21,200 g for 5 min. The pellet was resuspended in 12% Percoll solution and centrifuged at 6,900 g for 10 min, yielding a soft pellet that was washed once with mitochondrial isolation buffer and centrifuged again at 6,900 g for 10 min. The resulting mitochondrial pellet was used for the assay of mitochondrial swelling and rupture as a marker of PTP opening (48).
Mitochondrial membrane swelling was assessed by suspending mitochondria in medium containing phosphate, which induces swelling and rupture more rapidly in vulnerable mitochondrial membranes than healthy membranes (29). Mitochondria (0.25 mg protein) were suspended in medium containing 250 mM sucrose, 10 mM Tris-MOPS, 0.05 mM EGTA, 5 mM pyruvate, 5 mM malate, and 1 mM phosphate (pH 7.4), and absorbance by this suspension was measured at 540 nm in a Beckman DU 640 spectrophotometer. Intact mitochondria scatter light at 540-nm wavelength; mitochondrial swelling and rupture due to prolonged or excessive mitochondrial PTP opening reduces mitochondrial light scattering and, thus, absorbance (48). The time required for absorbance to fall to the midpoint between initial and final values was determined to assess how readily PTP open in different treatment groups.
Statistical analysis.
Measurements were done in duplicate or triplicate, and mean values were computed for each rat. Values are means ± SE. Single-factor analyses of variance (ANOVA) were performed to identify group differences among EW sign scores, blood ethanol concentrations, or ethanol consumption. Two-factor ANOVA were performed on O2•− and carbonyl contents and mitochondrial membrane swelling rates to determine group differences due to two factors, i.e., treatment and brain region, and to identify interactions between these two factors. When significant differences were detected by ANOVA, post hoc Tukey's tests were performed to identify the specific differences. P values <0.05 were taken to indicate statistical significance.
For correlation analyses, data from all 6 groups were pooled, yielding 31 data points for each brain region. Bivariate Pearson correlation analyses were conducted to assess correlations among EW sign scores, protein carbonyl contents, O2•− contents, and 50% PTP opening times. Results of these analyses were reported as Pearson correlation coefficients between all pairwise combinations. The greater the displacement of the correlation coefficient from zero, the more robust the correlation between the variables.
RESULTS
At the end of the diet regimen, there were no significant differences in body weights between the dextrin (320 ± 9 g), ethanol (311 ± 11 g), and ethanol/IH (321 ± 3 g) groups. The rats that received IH during ethanol diet appeared to be more active and cleaner than the non-IH ethanol rats. No abnormal behaviors or signs were observed during the IH sessions.
Ethanol consumption and blood ethanol concentrations.
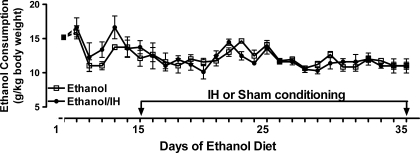
Figure 2 presents the daily ethanol intake in non-IH (ethanol group) or IH-treated rats (ethanol/IH group). The ethanol and ethanol/IH rats drank 57 ± 1.2 and 58 ± 1.3 ml of liquid ethanol diet (6.5% wt/vol) per day, respectively. When normalized to body weights, the daily ethanol intakes averaged 12.0 ± 0.2 g/kg in ethanol rats and 12.6 ± 0.3 g/kg in ethanol/IH rats. There were no significant differences between the two groups, indicating that IH did not alter ethanol consumption.
Fig. 2.
Ethanol consumption. Rats (14 rats/group) received 6.5% (wt/vol) ethanol diet for 35 days, with sham (ethanol group) or IH treatment (ethanol/IH group) for the last 20 days, the period bracketed by the arrows. Ethanol consumption in the 2 groups did not differ on any day (P > 0.05).
Blood ethanol concentration was measured at day 10, i.e., the midpoint of the IH or sham program, to test whether IH alters circulating ethanol concentrations. As expected, blood ethanol was undetectable in the dextrin group. Ethanol concentrations were similar in ethanol-consuming rats completing sham (1.00 ± 0.06 mg/ml) and IH (0.96 ± 0.04 mg/ml) protocols, indicating that IH did not influence ethanol exposure.
Behavioral signs of EW.
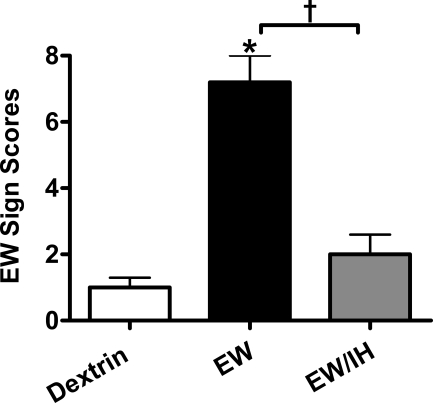
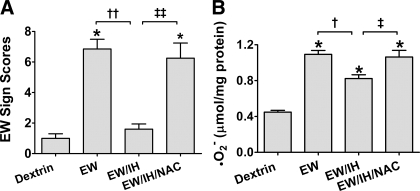
Figure 3 presents the severity of overt behavioral signs of EW, measured 24 h after withdrawal of ethanol or dextrin diets. ANOVA detected statistically significant differences in EW sign scores among dextrin rats, EW rats with IH, and EW rats without IH treatment [F(2, 14) = 37, P < 0.001]. A post hoc Tukey comparison revealed that, as expected, the EW group had higher sign scores (7.2 ± 0.8) than the dextrin group (1.0 ± 0.3, P < 0.001). Sign scores in the EW/IH group were sharply lower (2.0 ± 0.6) than those in the EW group (P < 0.001) and did not significantly differ from those in the dextrin group, demonstrating that IH ameliorated EW-induced behavioral signs.
Fig. 3.
Behavioral signs of ethanol withdrawal. These data and those depicted in Figs. 4–7 are from rats (5–7 rats/group) that received dextrin or 6.5% (wt/vol) ethanol diet for 35 days, with sham (dextrin and EW groups) or IH treatment (EW/IH group) for the last 20 days of the diet. The ethanol diet was then abruptly terminated to produce EW, and the IH program was also stopped. Rats were tested for behavioral signs at 24 h of EW. Antecedent IH treatment sharply attenuated the severity of the EW signs. *P < 0.001 vs. dextrin. †P < 0.01 vs. EW.
O2•− generation during EW.
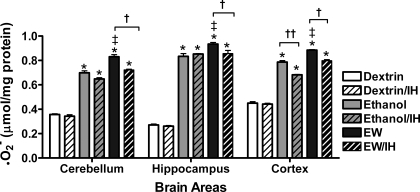
The finding that IH markedly attenuated EW behavioral signs raised the possibility that IH might have dampened oxidative stress imposed by EW. Superoxide contents were measured to index ROS formation in brain. ANOVA revealed significant treatment effects on O2•− contents in cerebellum [F(5, 23) = 277, P < 0.001], hippocampus [F(5, 23) = 384, P < 0.001], and cortex [F(5, 23) = 451, P < 0.001]. Ethanol exposure sharply increased O2•− contents in all three regions compared with dextrin controls (Fig. 4; P < 0.001). In non-IH rats, O2•− increased further during EW in all three regions (P < 0.05). During ethanol exposure, IH only reduced O2•− accumulation in cortex. During EW, IH treatment attenuated O2•− in all three brain regions (P < 0.001).
Fig. 4.
O2•− generation during EW. Rats (5–7 rats/group) received either dextrin or an ethanol diet (6.5% wt/vol, 5 wk) with sham or IH treatment for the last 20 days of the diet. Rats were killed at the end of the ethanol diet (ethanol groups) or at 24 h of EW (EW groups). In all 3 brain areas, EW groups had higher O2•− contents than the respective dextrin or ethanol groups. IH treatment during ethanol diet decreased subsequent O2•− accumulation during EW. *P < 0.001 vs. dextrin. †P < 0.01 vs. EW/IH. ‡P < 0.01 vs. ethanol exposure.
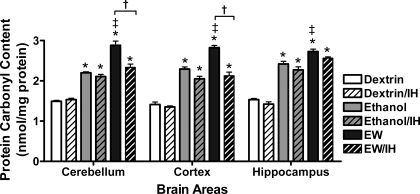
Protein carbonyl content.
The effects of ethanol exposure, EW, and IH on protein oxidation were assessed from protein carbonyl contents in brain homogenates. In general, carbonyl contents (Fig. 5) paralleled O2•− contents (see Fig. 4). ANOVA revealed significant treatment effects on carbonyl contents in cerebellum [F(5, 25) = 74, P < 0.001], cortex [F(5, 25) = 76, P < 0.001], and hippocampus [F(5, 25) = 71, P < 0.001]. The ethanol diet sharply increased carbonyl contents in all three brain areas (P < 0.01). During EW in the non-IH rats, carbonyl content increased even further (P < 0.01 vs. ethanol exposure), indicating that EW inflicted appreciable oxidative stress on brain proteins. IH protection against carbonyl production was observed during EW (P < 0.01) but not during ethanol exposure, indicating more effective IH protection against the protein oxidation inflicted by EW than that caused by ethanol per se.
Fig. 5.
Protein carbonyl contents. Measurements were conducted in the same rats as in Fig. 4. Ethanol diet increased carbonyl contents to similar extents in the IH- and sham-treated rats, but IH prevented further increases in cerebellar and cortical carbonyl content during EW. *P < 0.001 vs. dextrin. †P < 0.01 vs. EW/IH. ‡P < 0.01 vs. ethanol exposure.
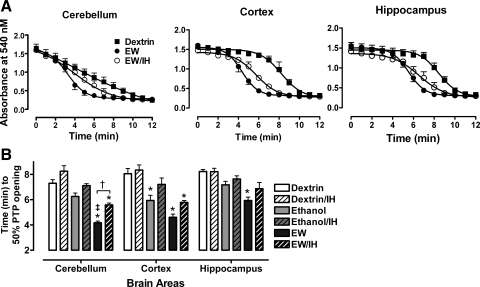
Mitochondrial membrane swelling.
The effects of ethanol exposure, EW, and IH on mitochondrial membrane swelling, an indication of PTP opening, were assessed by measuring light scattering by mitochondrial membranes. In general, light scattering declined most rapidly in mitochondria from brains of non-IH EW rats (Fig. 6A). Two-factor ANOVA revealed significant treatment-dependent [F(5, 10) = 179, P < 0.001] and brain region-dependent [F(2, 10) = 7.8, P = 0.001] differences in time to 50% absorbance change. In addition, there was a significant interaction between treatment and brain region [F(10, 75) = 12, P = 0.001], suggesting that the different brain regions are not equally vulnerable to PTP opening under specific treatment conditions. Indeed, a post hoc Tukey test revealed that cerebellar mitochondria were more vulnerable to swelling and rupture during EW than were cortical and hippocampal mitochondria. In cortical mitochondria, rates of swelling and rupture during ethanol exposure and EW did not differ significantly. Hippocampal mitochondria were the least affected by ethanol exposure or EW (Fig. 6B). IH produced significant protection only in cerebellar mitochondria during EW (P = 0.01); however, there was a tendency to protection in the cortical and hippocampal regions. Collectively, these results indicate that EW increases vulnerability of brain mitochondrial membranes and that IH minimizes this effect of EW.
Fig. 6.
Mitochondrial membrane swelling as a marker of permeability transition pore (PTP) opening. Mitochondria were isolated from the same brain regions as in Figs. 4 and 5 and were suspended in medium containing 1 mM phosphate to induce membrane swelling and rupture. A: decline in absorbance at 540 nm reflects mitochondrial PTP opening. Mitochondrial membranes broken by sonication established minimum absorbance. B: EW without IH treatment took less time to 50% PTP opening than dextrin (*P < 0.001) or ethanol exposure (‡P < 0.001). IH treatment slowed PTP opening, primarily during EW and especially in cerebellar mitochondria (†P < 0.01).
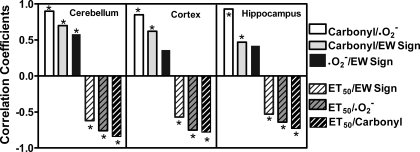
Bivariate correlations.
Bivariate Pearson analyses were performed to test correlations between EW-induced behavioral signs and oxidative injury (Fig. 7). Positive correlations (P < 0.001) were detected among EW sign scores, O2•− contents, and carbonyl contents, all of which were negatively correlated (P < 0.001) with mitochondrial membrane resilience (time to 50% absorbance decrease). The correlations between EW signs and oxidative markers were less robust than those between oxidative markers per se. Indeed, EW sign scores did not significantly correlate with O2•− contents in cortex or hippocampus. These analyses indicate that O2•− contents, carbonyl contents, and mitochondrial PTP collectively determine EW damage and/or IH neuroprotection.
Fig. 7.
Bivariate correlations. EW sign tests, O2•− and carbonyl contents, and time to 50% PTP opening (ET50) measured in the same 31 rats as in Figs. 4–6 were subjected to Pearson bivariate correlation coefficient analyses. With the exception of O2•− and EW signs in cortex and hippocampus (P > 0.05), there were significant positive correlations among EW signs, O2•− contents, and carbonyl contents, all of which negatively correlated with time to 50% PTP opening (*P < 0.01).
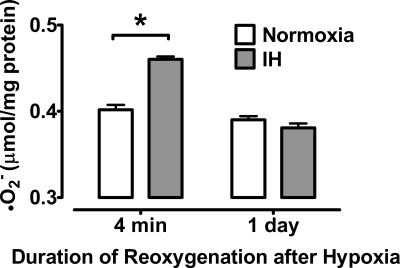
IH induction of moderate, brief O2•− formation.
IH may protect brain from EW by inducing moderate, brief bursts of oxyradical formation that evoke beneficial adaptations. To test this possibility, we measured cerebellar O2•− contents 4 min or 24 h after the final cycle of hypoxia or sham conditioning in rats consuming dextrin diet. IH did indeed increase cerebellar O2•− content at 4 min of reoxygenation, but O2•− content returned to baseline by 24 h (Fig. 8). Thus IH evoked moderate but transient oxyradical formation in cerebellum.
Fig. 8.
IH-induced O2•− formation in cerebellum. Cerebellar O2•− content was measured in dextrin diet rats 4 min and 24 h after completion of the IH or normoxic sham conditioning programs. Data are means ± SE from 4 rats per group. *P < 0.001 vs. sham.
Antioxidant blockade of IH-induced neuroprotection.
To investigate further the salutary role of IH-induced oxyradicals, we injected the broad-spectrum sulfhydryl antioxidant N-acetylcysteine (NAC) intraperitoneally 2 h before each IH conditioning session throughout the 20-day program. EW sign scores and cerebellar O2•− content were measured at 24 h of EW. NAC abrogated IH protection against EW signs (Fig. 9A). Moreover, the antioxidant treatment paradoxically increased cerebellar O2•− content at 24 h of EW, 1 day after the final NAC injection and IH session (Fig. 9B). Thus antioxidant treatment during IH blunted development of protection against EW-induced brain oxidative stress, implicating IH-induced ROS as pivotal elements of the antioxidative mechanism.
Fig. 9.
Antioxidant N-acetylcysteine (NAC) abrogates development of IH-induced resistance against EW-induced behavioral abnormalities and oxidative stress. Behavioral signs (A) and cerebellar O2•− content (B) were measured at 24 h of EW and in ethanol-free dextrin control rats. Measurements in untreated EW rats were compared with those in EW rats that completed 20 days of IH conditioning with (EW/IH/NAC) or without (EW/IH) daily intraperitoneal NAC injections. Data are means ± SE from 4–6 rats per group. *P < 0.001 vs. dextrin. †P < 0.01; ††P < 0.001 vs. EW. ‡P < 0.01; ‡‡P < 0.001 vs. EW/IH.
DISCUSSION
This study examined for the first time whether normobaric IH could evoke protection against behavioral deficit, cellular oxidative stress, and mitochondrial membrane fragility in rat brain during EW. The main findings are that daily administration of a program of brief, intermittent bouts of hypoxia and reoxygenation to rats consuming ethanol 1) prevents overt behavioral signs during subsequent EW, 2) decreases brain contents of the oxidative stress markers O2•− and protein carbonyls, and 3) preserves the resilience of mitochondrial membranes in the face of EW. Daily antioxidant (NAC) administration throughout the IH program abrogated IH protection against EW behavioral signs and oxidative stress. Collectively, these results demonstrate that the IH program afforded significant protection against EW stress. These findings extend to the brain the studies of Meerson et al. (27, 28), who reported that adaptation to hypobaric IH attenuated hepatic lipid peroxidation and cardiac dysfunction in rats during EW.
Because the IH treatment prevented the overt behavioral signs of EW, we tested whether such protection was due to reduced ethanol consumption and/or blood ethanol concentration. If IH decreased ethanol intake or accelerated clearance of blood ethanol, IH conditioned rats would likely be less addicted to ethanol and, thus, suffer less EW stress. However, neither intake nor blood concentration of ethanol significantly differed between IH and non-IH rats. These results argue against the possibility that the reduction in EW signs was due to altered ethanol intake or clearance in IH-treated rats. These results seem to contradict the findings of Meerson et al. (27), where hypobaric hypoxia exposures of several hours per day decreased ethanol consumption. Conceivably, IH cycles longer than the 5–10 min of this study, IH programs longer than 20 days, or different modes of IH administration such as hypobaric vs. normobaric may account for the different outcomes.
Our previous findings that EW induces oxidative stress in the rat brain (18, 34) prompted us to test whether IH protects against EW-induced free radicals and protein oxidation. In the current study, EW consistently produced higher O2•− and protein carbonyl contents than ethanol exposure per se, and IH treatment dampened these markers of oxidative stress. Rats experiencing EW suffer acute distress due to an abrupt transition from ethanol-induced suppression of glutamate-mediated excitation to withdrawal-induced neuronal hyperexcitability (10, 16). Also, oxidative stress is more intense during EW than during antecedent ethanol exposure (18, 34, 47). The pro-oxidant nature of ethanol intoxication-withdrawal also has been demonstrated in a clinical study where cerebrospinal fluid of withdrawn alcoholics contained higher concentrations of O2•− and excitatory amino acids than control subjects (46).
How IH protects against EW remains uncertain. We (24, 37) recently found that the protection against cardiac ischemia-reperfusion injury in dogs conditioned by the current IH regimen was abrogated by antioxidant NAC treatment during the IH program, suggesting that ROS play a pivotal role in producing the protection. Excess ROS cause neurological damage (46), but moderate levels of ROS such as those produced during IH may augment cellular antioxidant mechanisms. In this study, IH transiently increased cerebellar O2•− content. Moreover, administration of NAC to scavenge ROS during the IH sessions abrogated protection against neurological impairment and O2•− accumulation during subsequent EW. At physiological concentrations, ROS function as signaling molecules that activate transcription and may eventually increase synthesis of cytoprotective proteins (26). Indeed, Fiskum et al. (11) reported that an increase in basal ROS production evokes expression of antioxidant enzymes, bolstering resistance to oxidative stress in brain.
The possibility that IH protection is expressed at the mitochondrial level is crucial, because oxidative phosphorylation requires O2, and thus, alteration in O2 concentration during IH directly impacts mitochondrial ATP production. Furthermore, we have demonstrated that EW inactivates the key respiratory complex cytochrome c oxidase (17) and accelerates swelling and rupture of brain mitochondria from ovariectomized female rats experiencing EW (20). Moreover, the identical IH regimen attenuated EW signs and oxidative stress in female rats (18). The current study demonstrated that EW increased mitochondrial membrane fragility in male rats, too, and extended this finding to IH protection against mitochondrial fragility during EW. Consistent with these results, a moderate IH regimen suppressed mitochondrial permeability transition in ischemic rat myocardium (49). In contrast, reintroduction of O2 following anoxia (i.e., extreme hypoxia) elicited a burst of excess O2•− formation that provoked mitochondrial permeability transition (30). IH may directly or indirectly regulate intracellular Ca2+, which in excess opens PTPs (14, 43, 48, 49). Alternatively, IH may increase synthesis of cytoprotective proteins, such as heat shock proteins, that are produced under stressful conditions (4, 21).
Finally, bivariate correlation analyses revealed robust correlations between EW sign scores and amounts of oxidative markers. This observation is consistent with a previous report of a strong association between ROS and EW-induced seizure activity in rats (47). Nevertheless, the magnitude of IH protection against EW signs is much greater than its protection against oxidative stress or mitochondrial fragility. EW signs are a global manifestation of hyperexcitability mediated by multiple factors, although the contribution of an individual factor to EW signs or IH protection may be modest. Indeed, Pearson correlation coefficients between EW signs and oxidative markers were less in value than those between oxidative markers per se. Moreover, our data suggest that the degree of association between the oxidative markers in response to EW and IH varies among different brain regions. In this study, cerebellar mitochondria were more vulnerable to EW than hippocampal or cerebrocortical mitochondria. Cerebellum normally contains large amounts of parvalbumin, a Ca2+ binding protein that is depleted during EW (34). Because Ca2+ is directly associated with mitochondrial permeability transition (14, 35, 43), the depletion of Ca2+ binding proteins by EW might have permitted excess cytosolic Ca2+, resulting in Ca2+ entry into cerebellar mitochondria.
The duration of the IH regimen and the length and intensity of the hypoxia bouts are important determinants of whether IH is protective or harmful. Detrimental hypoxia programs, such as those that model sleep apnea or brain ischemia, typically utilize multiple, very brief cycles (e.g., 30–60 s) of severe hypoxia (FiO2 2–5%) (13, 36, 39, 49) or sustained hypoxia without intervening normoxia (26, 44, 45). On the other hand, more moderate hypoxia is reported to evoke adaptations that protect the brain from subsequent ischemic insults (2, 22, 40). Thus, depending on the regimen, hypoxia can either harm or protect the brain. Protection is reliably achieved when hypoxia is moderately severe, divided by intermittent reoxygenation into multiple, brief (several minutes) bouts and of a cumulative duration much shorter than that of normoxia, e.g., 1 h/day (24, 26). The current IH regimen included all of these essential features. Behavioral and antioxidative protection during EW by this IH regimen may provide new insights into endogenous defense mechanisms against EW.
GRANTS
This work was supported by National Institutes of Health Grants AA013864, AA015982, and AT003598.
Acknowledgments
We thank Daniel Metzger for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Azzi A, Montecucco C, Richter C. The use of acetylated cytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun 65: 597–603, 1975. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol 48: 285–296, 2000. [PubMed] [Google Scholar]
- 3.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab 22: 393–403, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2: 645–652, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem 80: 207–218, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol 290: C1221–C1229, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Ding HL, Zhu HF, Dong JW, Zhu WZ, Zhou ZN. Intermittent hypoxia protects the rat heart against ischemia/reperfusion injury by activating protein kinase C. Life Sci 75: 2587–2603, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dodd H, Shorey-Kutschke RL. Ethanol lowers heart carnitine in the methionine and choline deficient rat. Alcohol 4: 395–399, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res 13: 385–391, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Evans MS, Li Y, Faingold C. Inferior colliculus intracellular response abnormalities in vitro associated with susceptibility to ethanol withdrawal seizures. Alcohol Clin Exp Res 24: 1180–1186, 2000. [PubMed] [Google Scholar]
- 11.Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr 36: 347–352, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA 93: 4765–4769, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halestrap AP Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans 34: 232–237, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem 10: 1507–1525, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman PL Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis 10: 73–79, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Jung ME, Agarwal R, Simpkins JW. Ethanol withdrawal posttranslationally decreases the activity of cytochrome c oxidase in an estrogen reversible manner. Neurosci Lett 416: 160–164, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung ME, Downey HF, Confessore A, Wilson A, Simpkins JW, Mallet RT. Intermittent hypoxia conditioning protects against mitochondrial damage induced by ethanol withdrawal in female rats (Abstract). FASEB J 21: A1175, 2007. [Google Scholar]
- 19.Jung ME, Rewal M, Perez E, Wen Y, Simpkins JW. Estrogen protects against brain lipid peroxidation in ethanol-withdrawn rats. Pharmacol Biochem Behav 79: 573–586, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Jung ME, Yan LJ, Forster MJ, Simpkins JW. Ethanol withdrawal provokes mitochondrial injury in an estrogen preventable manner. J Bioenerg Biomembr 40: 35–44, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Lai Y, Du L, Dunsmore KE, Jenkins LW, Wong HR, Clark RS. Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stress and excitotoxicity. J Neurochem 94: 360–366, 2005. [DOI] [PubMed] [Google Scholar]
- 22.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol 207: 3163–3169, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186: 464–478, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Mallet RT, Ryou MG, Manukhina EB, Downey HF. Intermittent hypoxia conditioning of canine myocardium: robust protection against ischemia-reperfusion injury. In: Adaptation Biology and Medicine (Vol 5: Health Potentials), edited by Lukyanova L, Takeda N, and Singal PW. New Delhi: Narossa, 2008, p. 59–78.
- 25.Mallet RT, Ryou MG, Williams AG Jr, Howard L, Downey HF. β1-Adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res Cardiol 101: 436–446, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med (Maywood) 231: 343–365, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Meerson FZ, Arkhipenko I, Rozhitskaia II, Didenko VV, Sazontova TG. [Opposite effects on antioxidant enzymes of adaptation to continuous and intermittent hypoxia]. Biull Eksp Biol Med 114: 14–15, 1992. [PubMed] [Google Scholar]
- 28.Meerson FZ, Krasikov SI, Chavkin II, Bikbulatov MS, Tverdokhlib VP. [Adaptation to periodic hypoxia decreases ethanol consumption and abstinence-related damages to the internal organs during withdrawal in chronically alcoholized animals]. Biull Eksp Biol Med 114: 574–578, 1999. [PubMed] [Google Scholar]
- 29.Menze MA, Hutchinson K, Laborde SM, Hand SC. Mitochondrial permeability transition in the crustacean Artemia franciscana: absence of a calcium-regulated pore in the face of profound calcium storage. Am J Physiol Regul Integr Comp Physiol 289: R68–R76, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Navet R, Mouithys-Mickalad A, Douette P, Sluse-Goffart CM, Jarmuszkiewicz W, Sluse FE. Proton leak induced by reactive oxygen species produced during in vitro anoxia/reoxygenation in rat skeletal muscle mitochondria. J Bioenerg Biomembr 38: 23–32, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Neubauer JA Physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol 90: 1593–1599, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther 309: 594–599, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke 34: 1981–1986, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Rewal M, Jung ME, Simpkins JW. Role of the GABA-A system in estrogen-induced protection against brain lipid peroxidation in ethanol-withdrawn rats. Alcohol Clin Exp Res 28: 1907–1915, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds IJ Mitochondrial membrane potential and the permeability transition in excitotoxicity. Ann NY Acad Sci 893: 33–41, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167:1548–1553, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Ryou MG, Flaherty DC, Williams AG Jr, Manukhina EB, Downey HF, Mallet RT. Reactive oxygen species (ROS) mediate intermittent hypoxia conditioning (IHC)-induced cardioprotection (Abstract). FASEB J 21: A823, 2007. [Google Scholar]
- 38.Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res 25: 924–934, 2001. [PubMed] [Google Scholar]
- 39.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med (Maywood) 233: 627–650, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, Ardizzone T, Bernaudin M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx 1: 26–35, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sims NR Selective impairment of respiration in mitochondria isolated from brain subregions following transient forebrain ischemia in the rat. J Neurochem 56: 1836–1844, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Smolen A, Marks MJ, Smolen TN, Collins AC. Dose and route of administration alter the relative elimination of ethanol by long-sleep and short-sleep mice. Alcohol Clin Exp Res 10: 198–204, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium 36: 257–264, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Tai KK, Truong DD. NMDA receptor-mediated excitotoxicity contributes to the cerebral hypoxic injury of a rat model of posthypoxic myoclonus. Brain Res 1133: 209–215, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Titus AD, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, Chattarji S, Raju TR. Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145: 265–278, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry 155:726–732, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Vallett M, Tabatabaie T, Briscoe RJ, Baird TJ, Beatty WW, Floyd RA, Gauvin DV. Free radical production during ethanol intoxication, dependence, and withdrawal. Alcohol Clin Exp Res 21: 275–285, 1997. [PubMed] [Google Scholar]
- 48.Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J 21: 5164–5172, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu WZ, Xie Y, Chen L, Yang HT, Zhou ZN. Intermittent high altitude hypoxia inhibits opening of mitochondrial permeability transition pores against reperfusion injury. J Mol Cell Cardiol 40: 96–106, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med (Maywood) 229: 806–812, 2004. [DOI] [PubMed] [Google Scholar]