Abstract
Over-expression of EGFR, as in most cases of ovarian cancer, is associated with advanced-stage disease and poor prognosis. Activation of EGFR signaling pathway is involved in increased cell proliferation, angiogenesis, metastasis, and decreased apoptosis. Tyrosine kinase activity is essential for signal transduction and receptor downregulation. However, we found in this study that tyrosine kinase activity is not necessary in ligand-induced EGFR downregulation in ovarian cancer cell line CaOV3 cells. EGFR tyrosine kinase inhibitors, such as PD153035, AG1478, as well as non-specific tyrosine kinase inhibitor PP2 can not reverse EGF-induced downregulation of EGFR. These findings thus permit us to develop the following exciting but unconventional strategy to sensitize cancer cells, namely, by priming ovarian cancer cells with EGF and EGFR inhibitor PD153035, before chemotherapy. This priming procedure down-regulates EGFR without induction of mitogenic signals such as ERK and PI3K/AKT. EGF plus EGFR inhibitor-primed ovarian cancer cells display increased sensitivity to taxol-induced cell death, resistant to EGF-induced cell migration and cell proliferation as well as ERK and PI3K/AKT activation. Further studies showed that PD153035, which does not reverse ligand-induced EGFR down-regulation, blocks EGF-induced EGFR activation as well as EGFR’s binding to c-cbl and Grb2. Taken together, we contend that priming with EGFR inhibitors plus EGF inhibits cell signaling pathways leading to cell proliferation and survival, while down-regulating EGFR. This priming approach sensitizes ovarian cancer cells and would ultimately result in better chemotherapeutical outcome.
Keywords: EGF, EGFR inhibitor, down-regulation, tyrosine kinase, ovarian cancer
1. Introduction
EGFR has recently received considerable attention in ovarian cancer research, since this 170kDa glycosylated membrane-spanning protein receptor is over-expressed in up to 75% of primary ovarian cancers [1]. Over-expression of EGFR is associated with advanced-stage disease and poor prognosis [2]. Furthermore, activation of EGFR signaling pathway in cancer cells is involved in increased cell proliferation, angiogenesis, metastasis, and decreased apoptosis. Thus, EGFR is considered as a critical molecular target for therapy in advanced ovarian cancer [3].
EGF stimulates proliferation of both normal ovarian epithelial cells and ovarian cancer cells via autocrine and paracrine mechanisms [4, 5]. To initiate cellular signal transduction, tyrosine kinase growth factor receptors typically dimerize with a result of autophosphorylation of both intracellular domains. The dimerization initiates a signaling cascade via ras/mitogen-activated protein kinase (MAPK), PI3K/AKT and other pathways leading to transcriptional activation of a number of proteins that are important for cell proliferation, survival and migration [6, 7].
Studies involving EGFR mutants lacking the intrinsic protein kinase activity and selective EGFR tyrosine kinase inhibitors such as ZD1839 have shown that tyrosine kinase activity is essential for signal transduction and cancer progression [8–10]. A truncated EGFR deficient in the cytoplasmic tyrosine kinase domain inhibits pancreatic cancer cell growth [11]. This defective EGFR is designed to function as a dominant-negative receptor (EGFR-DNR) to suppress cancer cell growth and potentially have therapeutic implications toward the treatment of cancers.
Activation of EGFR by a variety of extracellular stimuli is generally followed by transient refractoriness to the same stimulus [12]. One of the better characterized mechanisms of homologous desensitization involves a rapid decrease in the number of receptors through accelerated endocytosis. There are several endocytotic pathways that mediate the internalization and down-regulation of EGFR. The best-studied pathway is clathrin-dependent endocytosis or CDE, requiring clathrin which is the major component of the endocytic vesicle coat [13].
Recent studies have also shown that there are multiple clathrin-independent endocytosis or CIE pathways, such as caveolae dependent internalization and down-regulation, that generally depend on cholesterol-rich membrane domains [14]. Abundant data indicate that CDE pathway is cytoplasmic tyrosine kinase phosphorylation dependent, which is involved in EGFR Y1045 phosphorylation, c-cbl binding [15] and Grb2 adaptor [13, 16, 17]. However, the question whether the CIE pathway also depends upon tyrosine kinase has not been well addressed.
In this study, we found that EGFR tyrosine kinase inhibitors can not reverse ligand-induced EGFR down-regulation in cultured ovarian cancer cell line CaOV3 cells. We primed ovarian cancer cells with EGF and EGFR inhibitor PD153035, which down-regulates EGFR without activation of tyrosine kinase and downstream mitogenic signals such as ERK and PI3K/AKT. Primed ovarian cancer cells display delayed cell migration, slower cell proliferation and less mitogenic signals activation, and increased sensitivity to taxol-induced cell death.
2. Materials and methods
2.1. Chemicals and reagents
PD153035, AG1478, LY294002, PD98059 and PP2 were from CalbioChem (San Diego, CA). EGFR (1005) antibody, caveolin-1, goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal mouse anti-β-actin was obtained from Sigma (St. Louis, MO). phospho-AKT (Ser473), phospho-EGFR (Tyr1068), phosphor-c-myc (Thr58/Ser62), cyclin D1, MMP9 and AKT antibody were from Cell Signaling Technology (Bevery, MA).
2.2. Cell culture
Cultured ovarian cancer cells (CaOV3 cell line), EGFR wild type and EGFR knockout MEFs (Mouse Embryonic Fibroblasts) [18, 19] were maintained in a DMEM medium (Sigma, St. Louis, MO) supplemented with a 10% fetal bovine serum (Invitrogen, Carlsbad, CA), Penicillin/Streptomycin (1:100, Sigma, St. Louis, MO) and 4 mM L-glutamine (Sigma, St. Louis, MO), in a CO2 incubator at 37°C.
2. 3. Western blot analysis
As reported previously [20, 21], cultured cells with and without treatments were washed with cold PBS and harvested by scraping into 150 µl of RIPA buffer with protease inhibitors. 20 µg of proteins were separated by SDS-PAGE and transferred onto PVDF membrane (Millipore, Bedford, MA). After blocking with 10% milk, membranes were incubated with specific antibodies in dilution buffer (2% BSA in TBS) overnight at 4°C, followed by horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG at appropriate dilutions and incubated at room temperature for 1 h. Antibody binding was detected using enhanced chemiluminescence (ECL) detection system from GE Biosciences (Piscataway, NJ) following manufacturer’s instructions and visualized by fluorography with Hyperfilm.
2.4. Immunoprecipitation
As described previously [22], cultured cells with or without treatments for indicated time are lysed in a lysis buffer (160 mM NaCl, 20 mM Tris–HCl, pH 7.4, 0.1% Triton X-100, 10% glycerol, 1 mM EDTA, 20 mM-glycerol phosphate, 0.2 mM Na3VO4 and protease inhibitor cocktails (Roche Diagnostics, IN). Endogenous EGFR was immunoprecipitated by overnight incubation with anti-EGFR (2 µg/mg of lysates) and 20 µl of protein A/G Sepharose (beads) (Santa Cruz Biotechnology, Santa Cruz, CA). Immune complexes were washed four to five times with lysis buffer, boiled and subjected to 10% SDS–PAGE.
2.5. Cell viability assay (MTT dye assay)
Cell viability was measured by the 3-[4,5-dimethylthylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) method [20]. Briefly, cells were collected and seeded in 96-well plates at a density of 105 cells/cm2. Different seeding densities were optimized at the beginning of the experiments. After incubation for 24 h, cells were exposed to fresh medium containing various concentrations of reagents at 37°C. After incubation for up to 24 h, 20 µl of MTT tetrazolium (Sigma, St. Louis, MO) salt dissolved in Hank’s balanced salt solution at a final concentration of 5 mg/ml was added to each well and incubated in CO2 incubator for 4 h. Finally, the medium was aspirated from each well and 150 µl of DMSO (Sigma, St. Louis, MO) was added to dissolve formazan crystals and the absorbance of each well was obtained using a Dynatech MR5000 plate counter at a test wavelength of 490 nm with a reference wavelength of 630 nm.
2.6. Assessment of the percentage of apoptotic cells
To detect apoptotic cells, cells were stained with DNA binding dye Hoechst 33342 (Sigma, St. Louis, MO) [20]. After the cells were treated with or without test compounds for the allotted time periods, they were fixed with 4% formaldehyde in PBS for 10 min at 4°C, and then washed with PBS. To stain the nuclei, cells were incubated for 20 min with 20 µg/ml of Hoechst 33342. After washing with PBS, the cells were observed under a fluorescence microscope (Zeiss Axiophoto 2, Carl Zeiss, Germany). Cells exhibiting condensed chromatin and fragmented nuclei were scored as apoptotic cells. A minimum of 200 cells was scored from each sample.
2.7. Phagokinetic track motility assay
As described previously [21], 12-well plates were coated with coating medium of 20 µg/ml fibronectin (Sigma, St Louis, MO) in PBS, and placed in CO2 incubator at 37°C for at least 2 hours. After removing the coating medium gently with a Pasteur pipette, the wells were washed with PBS and 2.4 ml of microsphere suspension (86 µl stock microbeads solution in 30 ml PBS) was added to each well. The plates were then centrifuged at 1,200 rpm at 4°C for 20 minutes and carefully transferred to CO2 incubator and incubated at 37°C for at least one hour. 1.8 ml of supernatant was removed from each well and finally 1500 freshly trypsinized cells in 2 ml assay-medium (DMEM supplemented with a 0.05% fetal bovine serum) were seeded per well. Cells with or without treatment were cultured for 24 hours and photographed in microscope.
2.8. RNA interference (RNAi) experiments
As described previously [21], RNAi duplexes for EGFR were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), CaOV3 cells were cultured in complete medium that did not contain antibiotics for 4 days [21]. 50×104 cells were seeded into a 6-well plate 1 day prior to transfection and cultured to 60–70% confluence the following day. For RNAi experiments, 6.25 µl of Lipofectamine™ LTX together 2.5 µl PLUS™ Reagent (Invitrogen, Carlsbad, CA) was diluted in 90 µl of DMEM for 5 min in room temperature. Then, 8 µl EGFR siRNA was mixed with DMEM containing Lipofectamine together with PLUS reagent and incubated for 30 minutes at room temperature for complex formation. Finally, the complex was added to the well containing 2 ml medium with the final EFGR siRNA concentration of 100 nM. EGFR protein expression was determined by Western blot 48 hours after treatment.
2.9. Cell migration assay or ("Scratch") assay
As described previously [21], twelve-well plates were precoated with polylysine (30 µg/ml), followed by further BSA blocking. A sufficient number of serum-starved CaOV3 cells were plated, so that they became confluent in the wells right after attachment (~1–2 h). Same area of each well is then displaced by scratching a line through the layer with a needle. Floating cells were removed by PBS washing. Media containing 0.2% FBS without or with indicated treatment were added to the wells and incubated for additional 24 h. Mitomycin C (10 µg/ml) was always included in the media to prevent cell proliferation. Five representative images of the scratched areas under each condition were photographed. To estimate the relative migration of the cells, the unclosed cell-free areas from five prints under each condition were excised and weighed on a scale (Mettler AE50). We used "average gap" (average gap, %) to quantify the data. The polylysine alone at 0 h was considered 100% average gap.
2.10. Immunofluorescence
As previously described [20, 21], cultured CaOV3 cells after treatment were fixed in cold acetone for 10 min at 4°C. Fixed cells were blocked with 10% goat serum in TBST for 5 min at room temperature and then incubated with 1:200 rabbit anti-EGFR for 1h, followed by FITC-anti-rabbit secondary antibody (Chemicon, Temecula, CA) at 1:100 for 30 minutes and immunofluorescence was observed in Zeiss fluorescence microscope and digitized.
2. 11. In vitro BrdU labeling
Assays were performed using BrdU labelling. CaOV3 cells with or without treatment were exposed to 10 µmol/l of BrdU for 6 h at 37°C and fixed in 70% ethanol for 20 min at −20°C. Anti-BrdU antibody was applied for 30 min at 37°C and the fluorescein-conjugated secondary antibody for 30 min at 37°C. Coverslips were mounted and slides were observed under fluorescence microscope.
2.12. Statistical analysis
The values in the figures are expressed as the means ± standard error (SE). The figures in this study were representative of more than 3 different experiments. Statistical analysis of the data between the control and treated groups was performed by a student t test. Values of p < 0.05 were considered as statistically significant.
3. Results
3.1. EGFR tyrosine kinase inhibitors can not reverse EGF-induced EGFR down-regulation cultured ovarian cancer cells
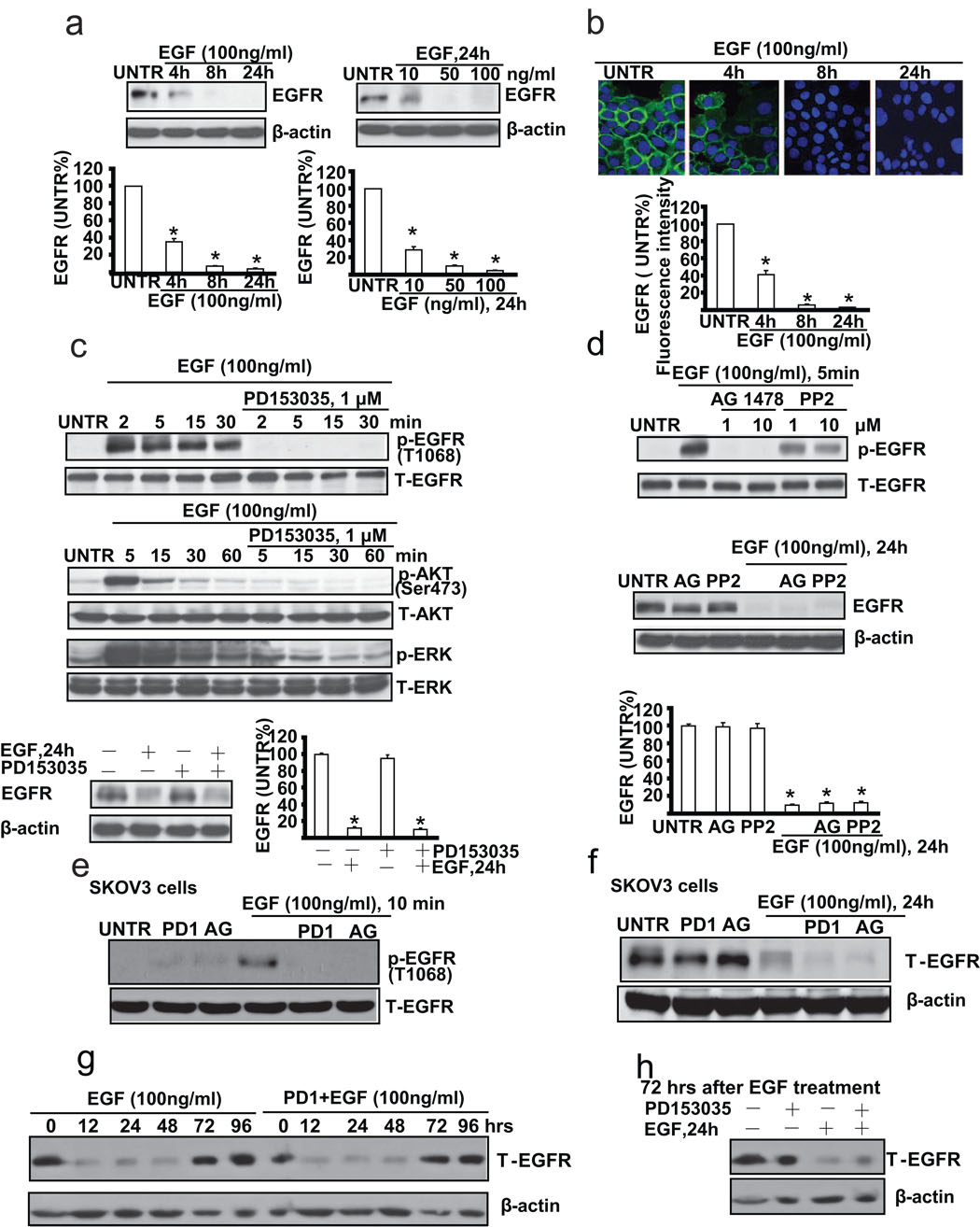
Upon ligand binding, EGFR, through autophosphorylation, internalization and eventually degradation, plays an important role in cancer cell proliferation and cell survival in a number of cell models. First, we tested whether EGF down-regulates EGFR in cultured ovarian cancer cells (CaOV3 cells). Western blot analysis results showed that EGF (100 ng/ml) down-regulates EGFR in a time dependent manner. EGFR begins to decrease as early as at 4 hours and almost disappears at 24 hours after EGF treatment. EGF also down-regulates EGFR in a dose dependent manner, and EGFR begins to decrease at the EGF dose of 10 ng/ml and almost disappears at EGF dose of 50 ng/ml at 24 hours after treatment (Fig. 1a). Immunofluorescence microscopic data further confirmed that EGF induces EGFR down-regulation in CaOV3 cells (Fig. 1b). Since previous studies have demonstrated a key role of EGFR tyrosine kinase activation in ligand-induced EGFR down-regulation [23–26], we next tested the effects of several tyrosine kinase inhibitors on EGFR down-regulation in response to EGF stimulation in CaOV3 cells. Western blot analysis data indicated that specific EGFR tyrosine kinase inhibitor PD153035 inhibits EGF-induced EGFR, and downstream AKT/ERK activation. However, surprisingly, PD153035 can not reverse EGF-induced EGFR down-regulation (Fig. 1c). Furthermore, neither another inhibitor AG1478, nor non-specific tyrosine kinase inhibitor PP2, can reverse EGF-induced EGFR down-regulation (Fig. 1d). To further confirm our hypothesis, another ovarian cancer cell line SKOV3 cells was used. As demonstrated in Fig. 1e and 1f, PD153035 and AG1478, which blocked EGF-induced EGFR activation (phosphorylation at Tyr 1068), had no effect on EGF-induced EGFR down-regulation in SKOV3 cells. We also test the duration of this EGF or PD1+EGF-induced EGFR down-regulation. As shown in Fig. 1g, EGFR was not returned basal level until 72–96 hours after both EGF and PD1+EGF treatment. Furthermore, EGF or PD1+EGF still down-regulated EGFR after 72 hours of original EGF treatment (Fig. 1h) As expected, all those inhibitors alone or together with EGF have no apparent effect on cell viability as detected by MTT assay (data not shown).
Fig. 1. EGFR tyrosine kinase inhibitor does not reverse EGF-induced EGFR down-regulation in cultured ovarian cancer cell line CaOV3 cells.
(a) CaOV3 cells were treated with EGF (100 ng/ml), harvested at different time points (4, 8 and 24 h) or treated with various doses of EGF (10, 50, and 100 ng/ml) and harvested at 24 hours. EGFR expression was analyzed by Western blot and its expression was quantified as normalized to beta actin. (b) CaOV3 cells were treated with 100 ng/ml of EGF and EGFR expression detected by immunofluorescence method as described above was observed at different time points (4, 8 and 24 h). (c) CaOV3 cells were pretreated with EGFR inhibitor PD153035 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for different time as indicated. p-EGFR (Tyr1068), T-EGFR, p-AKT (Ser473), T-AKT, p-ERK, T-ERK were detected by Western blot. CaOV3 cells were also pretreated with PD153035 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 24 hours. T-EGFR was detected by Western blot. EGFR expression was quantified as normalized to beta actin. (d) CaOV3 cells were pretreated with AG1478 (1 and 10 µM) or PP2 (1 and 10 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 5 minutes. p-EGFR (T-1068) and T-EGFR were detected by Western blot. CaOV3 cells were pretreated with AG1478 (AG, 1 µM) or PP2 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 24 hours. T-EGFR and β-actin were detected by Western blot as normalized to beta actin. (e) SKOV3 cells were pretreated with PD153035 (PD1, 1 µM) or AG 1478 (AG, 1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 10 minutes, p-EGFR (Tyr1068), T-EGFR were detected by Western blot. (f) SKOV3 cells were pretreated with PD153035 (PD1, 1 µM) or AG 1478 (AG, 1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 24 hours, T-EGFR and β-actin were detected by Western blot. (g) CaOV3 cells were pretreated with or without PD 153035 (PD1, 1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for indicated time, T-EGFR and β-actin were detected by Western blot. (h) After 72 hours of original EGF treatment, CaOV3 were pretreated with or without PD153035 (PD, 1 µM) for 1 hour, followed by secondary EGF (100 ng/ml) treatment for additional 24 hours, T-EGFR and β-actin were detected by Western blot. The data represent mean ± SE of triplicate experiments. * P < 0.05 versus UNTR groups. For immnofluorescence experiment, a minimum of six random fields and 200 cells per group were selected and average intensity for each group was quantified. Magnification: (b) 1: 400.
3.2. Cytoplasmic tyrosine kinase activity is not necessary for ligand-induced EGFR down-regulation
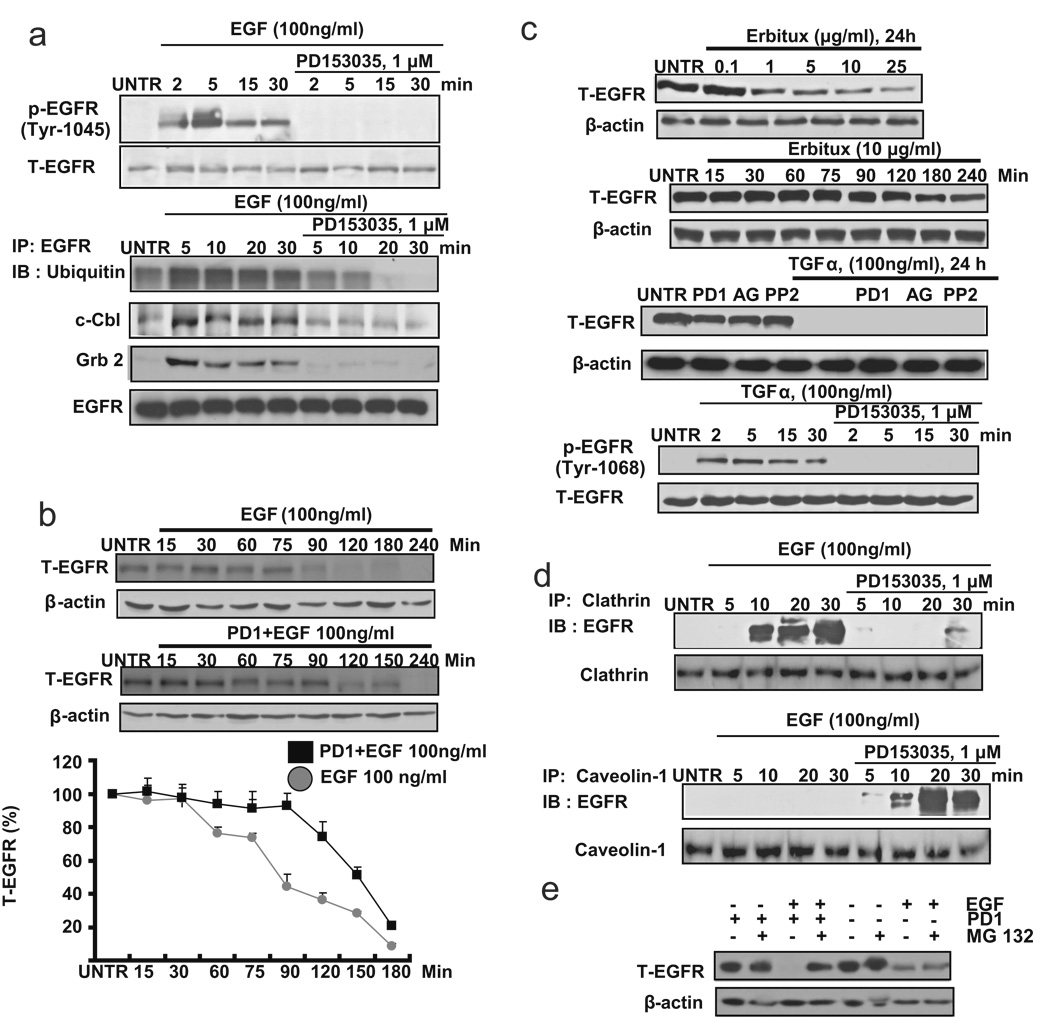
Published data have suggested that the robustness of EGF-induced down-regulation is attributed to c-Cbl/Grb2-mediated conjugation of ubiquitin to EGFR into clathrin-coated pits [13, 15, 17]. c-cbl is recruited to the activated EGFR directed by Tyr1045 or Grb2 [15, 17, 27]. We next tested the activation of Tyr1045 and recruitment of c-cbl and Grb2 in EGFR inhibitor PD153035 and EGF-treated CaOV3 cells. Western blot analysis data showed that EGFR inhibitor PD153035, which has been shown not to reverse ligand-induced down-regulation of EGFR, inhibits Tyr1045 activation as well as recruitment of ubiquitin, c-cbl and Grb2 to EGFR induced by EGF (Fig. 2a). Furthermore, PD153035-pretreated CaOV3 cells display delayed down-regulation of EGFR (Fig. 2b). These data suggest that cytoplasmic domains of EGFR, such as tyrosine kinase domains, are not necessarily involved in EGF-induced down-regulation of EGFR. To further confirm this notion, EGFR mAb Erbitux, which also induces EGFR down-regulation without the need of the cytoplasmic domain of the receptor [28], was utilized. The results showed that Erbitux induces EGFR down-regulation in a weaker and slower manner, compared to ligand-induced EGFR down-regulation (Fig. 2c). TGFα, another known EGFR ligand, induces EGFR down-regulation, which is not reversed by EGFR inhibitors PD153035, or AG 1478 or PP2 (Fig. 2c). Since membrane components such as caveolae might also be involved in EGFR down-regulation, we next tested the interaction between EGFR and caveolae upon PD1+EGF treatment. As demonstrated in Fig. 2d, there is more EGFR localized with caveolin-1 and less EGFR localized with clathrin in PD1+EGF treated CaOV3 cells. To confirm our hypotheses, proteasome inhibitor MG132 was used. As demonstrated in Fig. 2e, MG132, which has little effects on EGF-induced EGFR down-regulaton, inhibited PD1+EGF-induced EGFR down-regulation, and the similar result were also seen in another proteasome inhibitor lactacystin (data not shown). These results suggest that proteasome-mediated pathways might be involved in this PD1+EGF-induced EGFR down-regulation.
Fig. 2. Cytoplasmic tyrosine kinase is not necessary for ligand-induced EGFR down- regulation.
(a) CaOV3 cells were pretreated with PD153035 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for indicated time points. P-EGFR (Tyr1045) and T-EGFR was detected by Western blot. CaOV3 cells were also pretreated with PD153035 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 2, 5, 15 and 30 minutes. 200 µg of proteins from cell lysates was incubated with EGFR antibody and 20 µl of protein A/G beads at 4°C overnight. Beads were washed four times with lysis buffer, boiled, loaded onto a SDS–PAGE and transferred onto a PVDF membrane followed by an IB assay to detect ubiquitin, c-Cbl and Grb2. (b) CaOV3 cells pretreated with or without PD153035 (1 µM) for 1 hour were treated with EGF (100 ng/ml) for 15, 30, 60, 75, 90, 120, 180 and 240 minutes. T-EGFR was detected by Western blot and quantified as normalized to beta actin. (c) CaOV3 cells were treated with different doses of Erbutix (1, 2, 5, 10, 15 and 25 µg/ml) for 24 hours. T-EGFR was detected by Western blot. CaOV3 cells were also treated with Erbutix (10 µg/ml) for 15, 30, 60, 75, 90, 120, 180 and 240 minutes (up). CaOV3 cells were pretreated with PD153035 (PD1, 1 µM), AG1478 (AG, 1 µM) or PP2 (1 µM) for 1 hour, followed by treatment with TGF-α (100 ng/ml) for 24 hours. T-EGFR was detected by Western blot. CaOV3 cells were also pretreated with PD153035 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for for 2, 5, 15 and 30 minutes. P-EGFR (Tyr 1068) and T-EGFR were detected by Western blot (down). (d) CaOV3 cells were pretreated with PD153035 (1 µM) for 1 hour, followed by EGF (100 ng/ml) treatment for 2, 5, 15 and 30 minutes. 200 µg of proteins from cell lysates was incubated with clathrin or caveolin-1 antibody and 20 µl of protein A/G beads at 4°C overnight. Beads were washed four times with lysis buffer, boiled, loaded onto a SDS–PAGE and transferred onto a PVDF membrane, followed by an IB assay to detect EGFR. (e) CaOV3 cells were pre-treated with MG132 (2 µM) for 1 hour, followed by EGF or PD1/EGF treatment for 24 hours, T-EGFR and β-actin were detected by Western blot. The data represent mean ± SE of triplicate experiments.
3.3. EGFR inhibitor PD153035 plus EGF priming desensitizes CaOV3 cells to respond to EGF re-stimulation
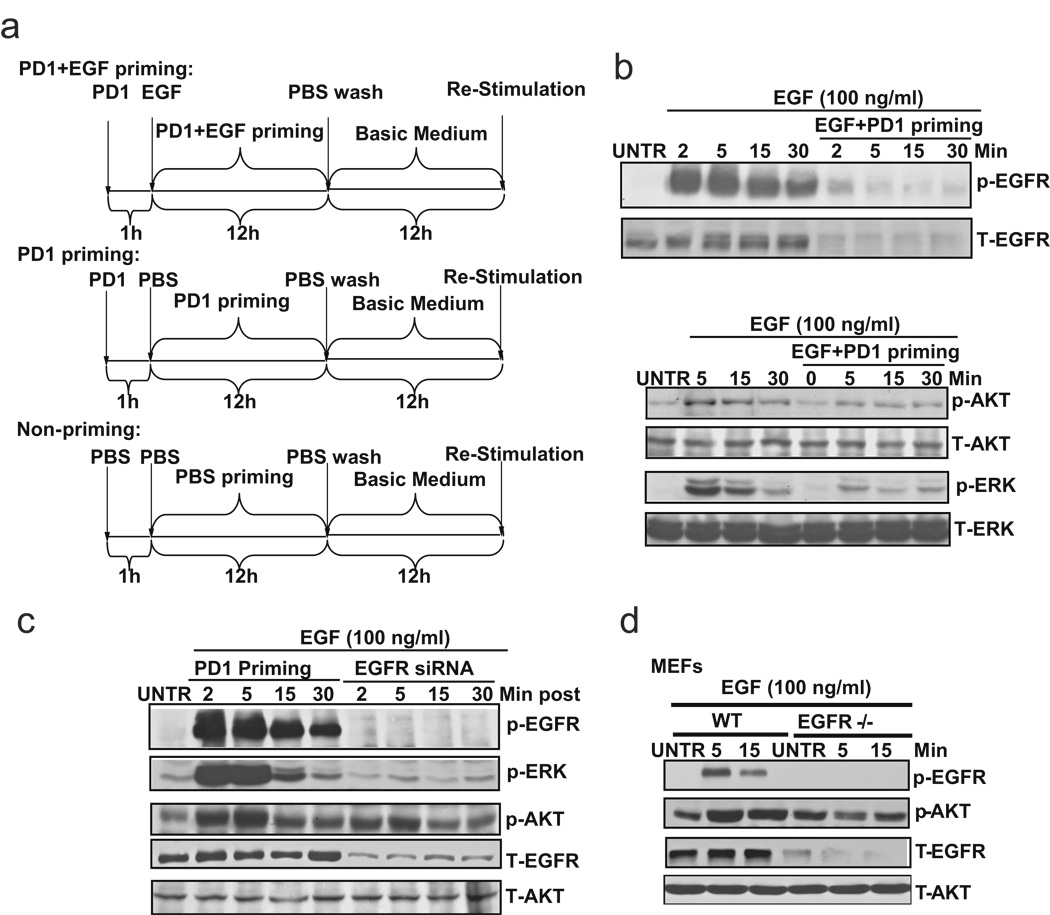
The data above have demonstrated that EGFR tyrosine kinase inhibitors, such as PD153035, do not reverse ligand-induced EGFR down-regulation. We next established a scheme to prime the cells with EGFR inhibitor PD153035 and EGF to inhibit EGFR kinase activity but maintain EGFR down-regulation, to eventually desensitize ovarian cancer cells. As shown in Fig. 3a, we primed CaOV3 cells with EGF and EGFR inhibitor PD153035 to down-regulate EGFR without activating the tyrosine kinase and downstream mitogenic and cell survival signals. CaOV3 cells were pretreated with 1 µM of PD153035 for 1 h, followed by 100 ng/ml of EGF and cultured for 12 hours. The primed cells were then washed with PBS for three times, and changed back to basic medium (0.5% FBS in DMEM) and cultured for another 12 hours. For PD153035 priming control, CaOV3 cells were treated with 1 µM of PD153035 for 12 hours, followed by washing with three times of PBS and cultured in basic medium for another 12 hours. For non-priming control, CaOV3 cells were treated with PBS for 12 hours. The cells were then washed with PBS for three times and changed to basic medium for another 12 hours. The results showed that EGFR inhibitor PD153035 plus EGF-primed cells display down-regulation of EGFR, with an absence of activation of tyrosine kinase activity and downstream signals such as AKT and ERK (Fig. 3b). EGFR inhibitor PD153035 priming alone has no effect on either EGFR expression or tyrosine kinase activation (Fig. 3c, left). Furthermore, EGFR siRNA treated cells (Fig. 3c, right) or EGFR knockout MEFs (Fig. 3d) also demonstrate the resistance to EGF-induced tyrosine kinase and downstream signal activation. Taken together, our data suggest that EGFR inhibitor PD153035 plus EGF priming desensitizes CaOV3 cells to respond to EGF re-stimulation, namely EGFR phosphorylation and downstream AKT and ERK activation.
Fig. 3. PD 153035 plus EGF (PD1+EGF) primed cells (CaOV3) lose the ability of activation of tyrosine kinase and downstream mitogenic signals upon EGF restimulation.
(a) Scheme of EGFR inhibitor PD153035 plus EGF priming to treat ovarian cancer cell line CaOV3 cells. For P D153035+EGF priming, CaOV3 cells were pretreated with 1 µM of PD153035 for 1 h followed by 100 ng/ml of EGF and cultured for 12 hours. After incubation, the primed cells were washed with PBS for three times and returned to basic medium (0.5% FBS in DMEM) and cultured for another 12 hours before re-stimulation. For PD153035 priming control, CaOV3 cells were treated with 1 µM PD153035 for 12 hours, followed by washing three times with PBS and cultured in basic medium for another 12 hours before restimulation. For non-priming control, CaOV3 cells were treated with PBS for 12 hours. After that, the cells were washed with PBS for three times and cultured in basic medium for another 12 hours before re-stimulation. (b) Unprimed or PD 153035 plus EGF primed CaOV3 (As described above) were treated with EGF (100 ng/ml) for indicated time point, p-EGFR (Tyr 1068), T-EGF p-AKT (Ser 473), p-ERK (Thr202/Tyr204), T-AKT and T-ERK was detected by Western blot. (c) PD153035 control primed CaOV3 (as described above) and EGFR siRNA knockdown cells were treated with EGF (100 ng/ml) for 2, 5, 15 and 30 minutes, p-EGFR (Tyr 1068), p-EGFR (Tyr 1068), p-AKT (Ser 473), p-ERK (Thr202/Tyr204), T-AKT and T-EGF were detected by Western bolt. (d) Wild type and EGFR knock out MEFs were treat with EGF (100 ng/ml) for 5 and 15 minutes, p-EGFR (Tyr 1068), p-AKT (Ser 473), T-AKT and T-EGF were detected by Western blot. The data represent mean ± SE of triplicate experiments.
3.4. EGFR inhibitor PD153035 plus EGF-primed CaOV3 cells display reduced cell migration upon EGF re-stimulation
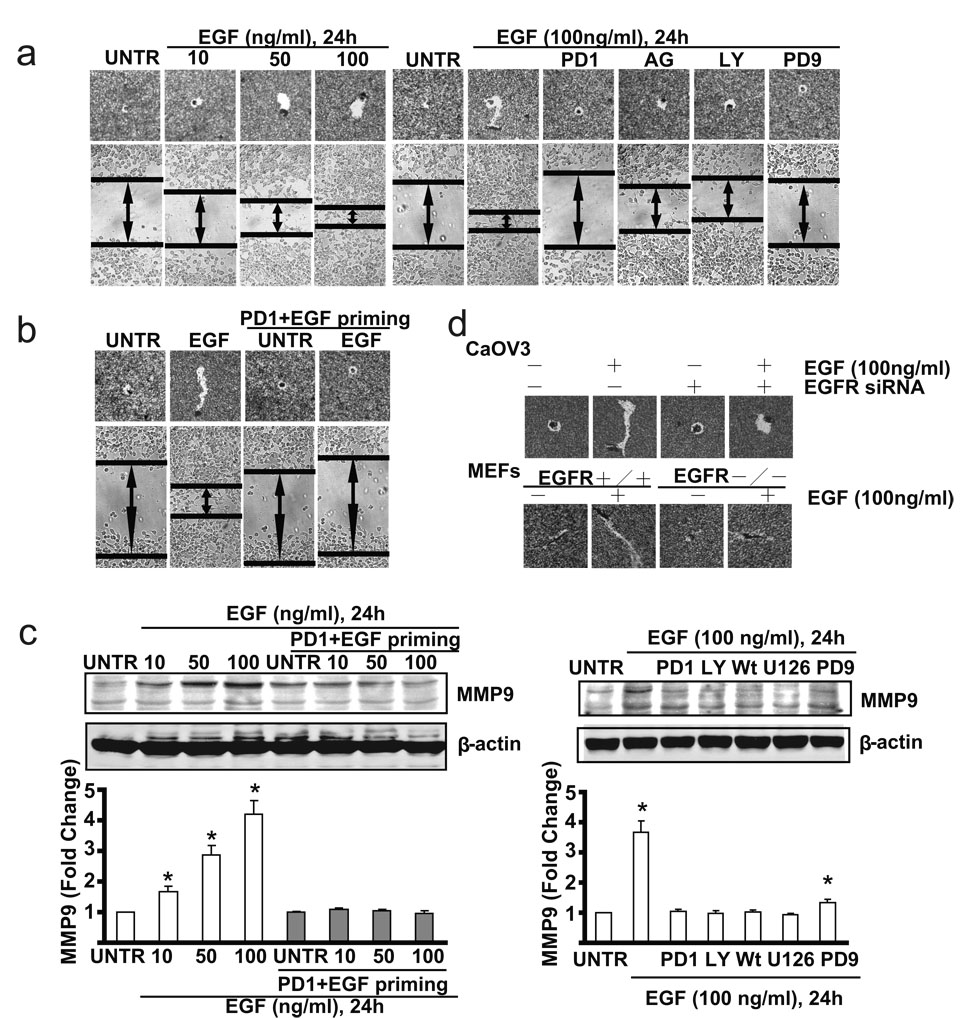
Given the important role of EGFR in ovarian cancer metastasis, we next tested whether priming affects CaOV3 cell migration using the same priming method as shown in Fig. 4. As expected, the results showed that in non-primed cells, EGF induces cell migration in a dose dependent manner. EGFR inhibitors PD153035 and AG1478, PI3K inhibitor LY294002, and MEK/ERK inhibitor PD98059 inhibit EGF-induced cell migration (Fig. 4a). However, EGF treatment fails to induce cell migration in EGFR inhibitor PD153035 plus EGF-primed CaOV3 cells (Fig. 4b), while PD153035 priming control or PBS control display almost the same cell migration as non-primed cells (data not shown). EGFR siRNA-treated CaOV3 cells and EGFR knockout MEFs also display reduced cell migration in response to EGF stimulation (Fig. 4c). Furthermore, the induction of cell migration associated proteins such as MMP9 is observed in unprimed CaOV3 cells but not in PD153035+EGF-primed CaOV3 cells (Fig. 4c). As expected, EGFR inhibitor PD153035, MEK/ERK inhibitor U0126 and PD98059, PI3K inhibitor LY294002 and Wortmannin inhibit EGF-induced expression of MMP9 (Fig. 4d).
Fig. 4. PD1+EGF primed ovarian cancer cells (CaOV3 cells) display delayed cell migration upon EGF restimulation.
(a) CaOV3 cell were treated with 10, 50 or 100 ng/ml EGF for 24 hours, in vitro cell migration were detected by “Phagokinetic track motility assay” and “scratch” assay. CaOV3 cells were also pre-treated with PD 153035 (PD1, 1 µM), AG 1478(AG, 1 µM), LY 294002 (LY, 10 µM) or PD 98059 (PD9, 1 µM) for 1 hour followed by EGF (100 ng/ml) for 24 hours. In vitro cell migration was detected. (b) PD153035+EGF primed or unprimed control CaOV3 cells were treated with or without EGF (100 ng/ml) for 24 hours, in vitro cell migration was detected. (b) Wild type CaOV3 cells and EGFR knockdown CaOV3 cells as well as wild type and EGFR knockout MEFs were pre-treated with PD153035(1 µM) for 1 hour, followed by EGF (100 ng/ml) for 24 hours, in vitro cell migration were detected by “Phagokinetic track motility assay”. (c) PD153035+EGF primed or unprimed control CaOV3 cells were treated with or without EGF (100 ng/ml) for 24 hours, MMP9 expression was detected by Western blot. (d) CaOV3 cell were pre-treated with PD153035 (PD1, 1µM), LY 294002 (LY, 10 µM), Wortmannin (Wt, 1 µM), U 0126 (U126, 1 µM) or PD98059 (PD9, 1 µM) for 1 hour followed by EGF (100 ng/ml) for 24 hours. MMP9 expression was detected by Western blot. The data represent mean ± SE of triplicate experiments. * P < 0.05 versus UNTR groups (lane 1). For microscope experiment, figure represents a minimum of ten random fields for each group. Magnification: 1: 100 for “scratch” assay. Magnification: 1: 200 for “Phagokinetic track motility assay” assay.
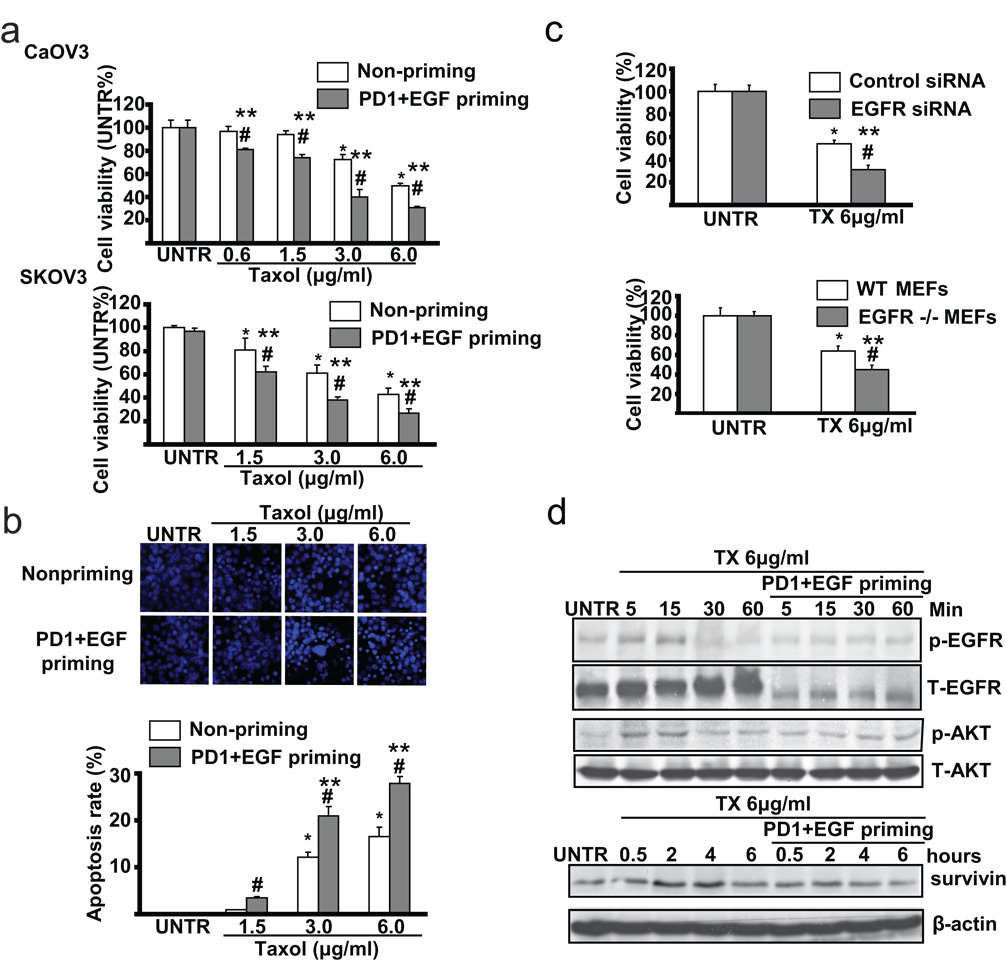
3.5. EGFR inhibitor PD153035 plus EGF-primed CaOV3 cells are more sensitive to taxol-induced cell death
Accumulated studies have shown that over-expression of EGFR is attributed to ovarian cancer cells’ resistance to taxol-induced cell death [18, 19]. We next tested the sensitivity of EGFR inhibitor PD153035 plus EGF-primed CaOV3 cells to taxol treatment. Using cell viability assay or MTT assay as well as apoptosis assay or Hoechst 33342 assay, we found that PD153035+EGF-primed cells are more sensitive to taxol-induced cell death in both CaOV3 cells and SKOV3 cells (Fig. 5a), while PD153035 priming or PBS priming control show similar sensitivity as the non-primed cells (data not shown). The results in Fig. 5b also indicate that there is increased apoptotic cell death in PD153035+EGF primed cells after taxol treatment. Furthermore, CaOV3 cells with EGFR knockdown using specific siRNA or EGFR knockout MEFs are also more sensitive to taxol-induced cell death (Fig. 5C). Since our previous studies have demonstrated that taxol not only triggers apoptotic signals, but also transiently activates EGFR and downstream cell survival signals such as AKT and surviving [18, 19], we next tested these survival signals in PD153035+EGF-primed CaOV3 cells. As demonstrated in Fig. 5d, PD153035+EGF-priming abrogates taxol-induced EGFR/AKT activation and expression of survivin in CaOV3 cells. Taken together, these data support our conclusion that primed cells are more sensitive to taxol-induced cell death.
Fig. 5. PD1+EGF primed CaOV3 cells are more sensitive to taxol-induced cell death.
(a) Non-primed and PD 153035 plus EGF (PD1+EGF) primed CaOV3 and SKOV3 cells were treated with 0.6, 1.5, 3.0 and 6.0 µg/ml Taxol for 24 hours, cell viability was detected by MTT method. (b) Non-primed and PD1+EGF primed CaOV3 cells were treated with 1.5, 3.0 and 6.0 µg/ml Taxol for 24 hours, apoptotic cell death was detected by Hoechst 333342 method. (c) CaOV3 cells treated with EGFR siRNA or transfection control were treated with 6.0 µg/ml Taxol for 24 hours, cell viability was detected by MTT method. Wild type and EGFR knockout MEFs were treated with 6.0 µg/ml Taxol for 24 hours, cell viability was detected by MTT method. (d) Non-primed and PD1+EGF primed CaOV3 cells were treated with 6.0 µg/ml taxol for indicated time points, survivin expression, p-EGFR (Tyr 1068), p-AKT (Ser 473), T-EGFR and T-AKT were detected by Western blot. The data represent mean ± SE of triplicate experiments. * P < 0.05 versus UNTR groups for non-primed group. ** P < 0.05 versus UNTR groups for primed group. # P < 0.05 primed groups versus un-primed groups. For the Hoechst experiment, a minimum of ten random fields and 500 cells were counted for apoptotic death rate. Magnification: 1: 200 for the Hoechst assay.
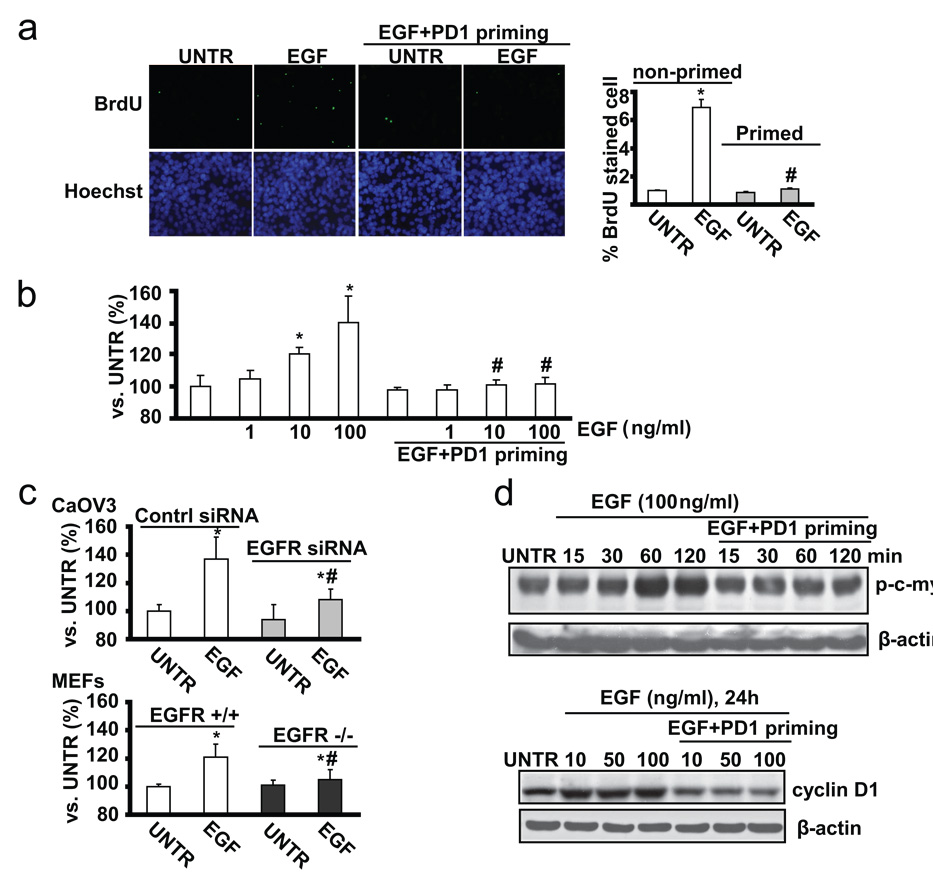
3.6. EGFR inhibitor PD153035 plus EGF-primed CaOV3 cells display less cell proliferation upon EGF re-stimulation
Existing data have indicated that over-expression of functional EGFR and over-activated EGFR signaling pathway are involved in increased cancer cell proliferation and angiogenesis[29]. We next compared the cell proliferation between non-primed and primed CaOV3 cells. Using BrdU stain and MTT assay, we found that PD153035+EGF-primed CaOV3 cells are resistant to EGF-induced cell proliferation (Fig. 6a, and b), while PD153035 or PBS priming controls show similar cell proliferation as the non-primed cell (data not shown). CaOV3 cells with EGFR knockdown or EGFR knockout MEFs are also more resistant to EGF-induced cell proliferation, compared to control or EGFR wild type (Fig. 6c). Furthermore, the induction of proliferation associated proteins such as cyclin D1, or signal molecules such as c-myc, were observed in non-primed control but not in primed CaOV3 cells (Fig. 6d). Taken together, the data indicate that EGFR inhibitor PD153035 plus EGF priming inhibits cell proliferation induced by EGF.
Fig. 6. PD1+EGF primed ovarian cancer cells (CaOV3 cell) display less cell proliferation upon EGF restimulation.
(a) The proliferation of non-primed and PD 153035 plus EGF (PD1+EGF) primed CaOV3 cells was detected by in vitro BrdU labeling and was quantified. (b) Non-primed and PD 153035 plus EGF (PD1+EGF) primed CaOV3 cells were treated with EGF (100ng/ml) for 36 hours, cell proliferation was detected by MTT assay. (c) Control siRNA and EGFR siRNA treated CaOV3 cells were treated with EGF (100ng/ml) for 36 hours, cell proliferation was detected by MTT assay. Wild type and EGFR knockout MEFs were treated with EGF (100 ng/ml) for 36 hours, cell proliferation was detected by MTT assay. (d) Non-primed and PD1+EGF primed CaOV3 cells were treated with 10, 50 or 100 ng/ml for indicated time points, cyclin D1, p-c-myc and β-actin were detected by Western blot. The data represent mean ± SE of triplicate experiments. * P < 0.05 versus UNTR groups for non-primed group. # P < 0.05 primed groups versus un-primed groups. For the BrdU assay, a minimum of ten random fields and 500 cells were counted for average growth rate. Magnification: 1: 200 for the BrdU assay.
4. Discussion
In this study, we found that EGFR inhibitor PD153035 alone-pretreated CaOV3 cells display reduced EGF-induced tyrosine kinase activation, and yet undergo nearly normal EGFR down-regulation in response to EGF (Fig. 1). The robustness of EGF-induced down-regulation is attributed to c-Cbl-mediated conjugation of ubiquitin to EGFR into clathrin-coated pits [13, 15, 17]. c-Cbl is recruited to activated EGFR either by directly binding to their TKB domain at the phosphotyrosine residue 1045 in EGFR [15] or by an indirect mechanism mediated by Grb2- the SH3 domains of Grb2 bind the proline-rich region of the Cbl proteins and the SH2 domain of Grb2 binds the phosphorylated EGFR [15, 17, 27]. The RING finger domain of the Cbl proteins allows them to function as ubiquitin ligases (E3s) and to target the EGFR signaling complex for internalization in clathrin-coated pits [13] and subsequent degradation in the lysosome. Other internalization and down-regulation mechanisms, however, were also recently reported to be involved in endocytosis of the activated EGFR via membrane components such as caveolae [30]. The question whether this internalization and down-regulation mechanism is EGFR tyrosine kinase dependent has not been addressed.
Unexpectedly, our results (Fig. 1 and 2) demonstrate that EGFR inhibitor PD153035, which can not reverse ligand-induced EGFR down-regulation, does inhibit ligand-induced Tyr1045 activation and recruitment of c-cb1 and Grb2 to EGFR which are critical to clathrin-induced internalization and down-regulation [13, 17, 24](Fig. 2). Furthermore, unlike EGF-treated cells, cells primed with PD153035+EGF display weaker, if any, ubiquitylation of EGFR. And PD153035+EGF-induced down-regulation of EGFR is slower relative to EGF treatment only (Fig. 2b). These data suggest that PD153035+EGF-induced EGFR down-regulation require no tyrosine kinase activity or cytoplasmic domain of the receptor and this down-regulation may therefore be caveolae dependent (Fig. 2d). This notion is supported by recent studies which demonstrated that EGFR mAb also induces slower down-regulation, in a caveolae dependent manner, involving only weak ubiquitylation and no cytoplasmic receptor's determinants [28]. Furthermore, reports have shown that EGFR down-regulation induced by UVA [31] or oxidative stress [32] does not require receptor kinase activity, but rather depends on caveolae, to which EGFR localization requires no cytoplamic motifs [28, 33]. However, the detailed signals involved in this process need further investigation.
Ligands such as EGF or TGFα binding to EGFR triggers the activation of signaling cascades that connect the activated receptor at the cell surface with the nucleus, including signals transduced by the MAP kinases, PI3K/AKT, and members of the STAT and other family of transcription factors, which specifically result in oncogenic signaling, transformation, and tumorigenicity. Deregulation of EGFR receptor family members contributes to the etiology and progression of epithelial ovarian cancer. In this study, we found that PD153035+EGF-primed CaOV3 cells, with significantly reduced EGFR expression, display much less EGFR tyrosine kinase activity and downstream mitogenic signal activation upon ligand re-stimulation (Fig. 3). The results further confirm the key role of EGFR on ligand-induced oncogenic signal transduction. And this priming approach effectively inhibits ligand-induced oncogenic signal transduction at least in ovarian cancer cell line CaOV3 cells.
The mechanism by which EGFR-induced cell surface signals lead to malignant progression is partially understood. Recent experimental evidence showed a link between EGFR-induced matrix metalloproteinases, or MMPs up-regulation, and cell migration and MMPs have been shown to be associated with invasion and metastasis of cancer cells [34, 35]. Degradation of the extracellular matrix by producing imbalanced or inducing production of MMP9 is an essential step for tumor cells to invade and metastasize [36]. MMP9 is important in creating and maintaining an environment that supports the initiation and maintenance in growth of primary and metastatic tumors [37]. In this study, we observed that EGF induces MMP9 expression, in EGFR/AKT/ERK dependent manner, as well as cell migration in non-primed cells but not in PD153035+EGF-primed CaOV3 cells (Fig. 4). These results further support the notion that EGFR plays a critical role in cancer cell migration and the priming approach may lead to the development of a new clinical therapy against ovarian cancer.
As well as triggering cell death signals such as G2/M arrest, paclitaxel or taxol, as reported by our and other groups, transiently transactivates EGFR, leading to activation of cell survival factors, such as ERK and AKT, and expression of survivin, which are all inclusively accountable for ovarian cancer cell resistance to paclitaxel treatment [18, 19, 38]. In this study, we also found that taxol induces survival signal in an EGFR dependent manner. Furthermore, PD153035+EGF-primed cells, where EGFR is down-regulated, display more sensitivity to taxol-induced cell death (Fig. 5). These results provide evidence to further support the notion of the involvement of EGFR in taxol resistance in cancer cells. The priming of EGFR inhibitor such as PD153035 with EGF will enhance the potency of chemotherapeutical drugs such as taxol.
Another important characteristic of cancer cells is overgrowth. Recently, genetic and biochemical investigations of the molecular mechanisms governing the G1 to S progression in mammalian cells have demonstrated an important role of cyclin D and their cyclin-dependent kinases (cdks) [39–41]. EGF affects cyclin D1 protein expression through several pathways. Among these pathways are activation of STAT3, activation of mitogen-activated protein kinase (MAPK), and direct induction of cyclin D1 transcription by binding and activation of cyclin D1 promoter. In this study, we found that EGF induces cyclin D1 expression as well as c-myc signal activation in non-primed but not in EGFR inhibitor PD153035+EGF primed CaOV3 cells. This may partially explain why primed cells display less proliferation as demonstrated in Fig. 6. The results further confirm the key involvement of EGFR in ligand-induced cancer cell proliferation. The priming approach strongly inhibits ovarian cancer cell proliferation.
In conclusion, we found for the first time that EGFR tyrosine kinase inhibitors can not reverse ligand-induced down-regulation in cultured ovarian cancer cells (CaOV3 cells). Priming of CaOV3 cells with EGF and EGFR inhibitor PD153035 down-regulates EGFR without activation of tyrosine kinase and downstream mitogenic signals. EGF plus EGFR inhibitor-primed ovarian cancer cells display delayed cell migration, slower cell proliferation and less mitogenic signals activation, and increased sensitivity to taxol-induced cell death. Further studies have shown that cytoplasmic tyrosine kinase is not necessary for this priming-induced EGFR down-regulation, suggesting that clathrin-independent endocytosis, such as caveolae, is involved this priming-induced EGFR down-regulation. Our studies further suggest that EGF and EGFR tyrosine kinase inhibition priming may yield better outcome of chemotherapies.
Acknowledgement
This research was supported in part by a grant from NIH (P20 RR016457 from INBRE Program of the National Center for Research Resources), a grant for biomedical research from Rhode Island Foundation, a CAFR grant from Providence College, and a grant from Slater Center for Environmental Biotechnology.
Abbreviations
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- PI3K
phosphoinositide-3 kinase
- AKT
active human protein kinase
- MAPK
mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan JK, Pham H, You XJ, Cloven NG, Burger RA, Rose GS, Van Nostrand K, Korc M, Disaia PJ, Fan H. Suppression of ovarian cancer cell tumorigenicity and evasion of Cisplatin resistance using a truncated epidermal growth factor receptor in a rat model. Cancer Res. 2005;65:3243–3248. doi: 10.1158/0008-5472.CAN-03-3013. [DOI] [PubMed] [Google Scholar]
- 2.Berchuck A, Rodriguez GC, Kamel A, Dodge RK, Soper JT, Clarke-Pearson DL, Bast RC., Jr Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am. J. Obstet. Gynecol. 1991;164:669–674. doi: 10.1016/s0002-9378(11)80044-x. [DOI] [PubMed] [Google Scholar]
- 3.Maihle NJ, Baron AT, Barrette BA, Boardman CH, Christensen TA, Cora EM, Faupel-Badger JM, Greenwood T, Juneja SC, Lafky JM, Lee H, Reiter JL, Podratz KC. EGF/ErbB receptor family in ovarian cancer. Cancer Treat. Res. 2002;107:247–258. doi: 10.1007/978-1-4757-3587-1_11. [DOI] [PubMed] [Google Scholar]
- 4.Morishige K, Kurachi H, Amemiya K, Fujita Y, Yamamoto T, Miyake A, Tanizawa O. Evidence for the involvement of transforming growth factor alpha and epidermal growth factor receptor autocrine growth mechanism in primary human ovarian cancers in vitro. Cancer Res. 1991;51:5322–5328. [PubMed] [Google Scholar]
- 5.Ottensmeier C, Swanson L, Strobel T, Druker B, Niloff J, Cannistra SA. Absence of constitutive EGF receptor activation in ovarian cancer cell lines. Br. J. Cancer. 1996;74:446–452. doi: 10.1038/bjc.1996.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriai T, Kobrin MS, Hope C, Speck L, Korc M. A variant epidermal growth factor receptor exhibits altered type alpha transforming growth factor binding and transmembrane signaling. Proc. Natl. Acad. Sci. U S A. 1994;91:10217–10221. doi: 10.1073/pnas.91.21.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 8.Albanell J, Gascon P. Small molecules with EGFR-TK inhibitor activity. Curr. Drug Targets. 2005;6:259–274. doi: 10.2174/1389450053765888. [DOI] [PubMed] [Google Scholar]
- 9.Honegger AM, Dull TJ, Felder S, Van Obberghen E, Bellot F, Szapary D, Schmidt A, Ullrich A, Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- 10.Eldredge ER, Korf GM, Christensen TA, Connolly DC, Getz MJ, Maihle NJ. Activation of c-fos gene expression by a kinase-deficient epidermal growth factor receptor. Mol. Cell. Biol. 1994;14:7527–7534. doi: 10.1128/mcb.14.11.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M. Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res. 2002;62:5611–5617. [PubMed] [Google Scholar]
- 12.Sweeney C, Carraway KL., 3rd Negative regulation of ErbB family receptor tyrosine kinases. Br. J. Cancer. 2004;90:289–293. doi: 10.1038/sj.bjc.6601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, Stenmark H, Madshus IH. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol. Biol. Cell. 2004;15:3591–3604. doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar RC, Wendland B. Endocytosis of membrane receptors: two pathways are better than one. Proc. Natl. Acad. Sci.U S A. 2005;102:2679–2680. doi: 10.1073/pnas.0500213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Huang F, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol. Biol. Cell. 2003;14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. Embo J. 2002;21:303–313. doi: 10.1093/emboj/21.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu L, Wang Q, Di W, Jiang Q, Schefeller E, Derby S, Wanebo H, Yan B, Wan Y. Transient activation of EGFR/AKT cell survival pathway and expression of survivin contribute to reduced sensitivity of human melanoma cells to betulinic acid. Int. J. Oncol. 2005;27:823–830. [PubMed] [Google Scholar]
- 19.Qiu L, Di W, Jiang Q, Scheffler E, Derby S, Yang J, Kouttab N, Wanebo H, Yan B, Wan Y. Targeted inhibition of transient activation of the EGFR-mediated cell survival pathway enhances paclitaxel-induced ovarian cancer cell death. Int J Oncol. 2005;27:1441–1448. [PubMed] [Google Scholar]
- 20.Cao C, Healey S, Amaral A, Lee-Couture A, Wan S, Kouttab N, Chu W, Wan Y. ATP-sensitive potassium channel: A novel target for protection against UV-induced human skin cell damage. J Cell Physiol. 2007;212:252–263. doi: 10.1002/jcp.21026. [DOI] [PubMed] [Google Scholar]
- 21.Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S, Kouttab N, Chu W, Wan Y. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J. 2006;400:225–234. doi: 10.1042/BJ20060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dragoi AM, Fu X, Ivanov S, Zhang P, Sheng L, Wu D, Li GC, Chu WM. DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA. Embo J. 2005;24:779–789. doi: 10.1038/sj.emboj.7600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravid T, Heidinger JM, Gee P, Khan EM, Goldkorn T. c-Cbl-mediated ubiquitinylation is required for epidermal growth factor receptor exit from the early endosomes. J. Biol. Chem. 2004;279:37153–37162. doi: 10.1074/jbc.M403210200. [DOI] [PubMed] [Google Scholar]
- 24.Han W, Zhang T, Yu H, Foulke JG, Tang CK. Hypophosphorylation of residue Y1045 leads to defective downregulation of EGFRvIII. Cancer Biol. Ther. 2006;5:1361–1368. doi: 10.4161/cbt.5.10.3226. [DOI] [PubMed] [Google Scholar]
- 25.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 26.Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16:551–559. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Grovdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp. Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Friedman LM, Rinon A, Schechter B, Lyass L, Lavi S, Bacus SS, Sela M, Yarden Y. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc. Natl. Acad. Sci. U S A. 2005;102:1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blank SV, Chang R, Muggia F. Epidermal growth factor receptor inhibitors for the treatment of epithelial ovarian cancer. Oncology. 2005;19:553–559. [PubMed] [Google Scholar]
- 30.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He YY, Huang JL, Gentry JB, Chignell CF. Epidermal growth factor receptor down-regulation induced by UVA in human keratinocytes does not require the receptor kinase activity. J. Biol. Chem. 2003;278:42457–42465. doi: 10.1074/jbc.M303376200. [DOI] [PubMed] [Google Scholar]
- 32.Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J. Biol. Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 33.Yamabhai M, Anderson RG. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J. Biol. Chem. 2002;277:24843–24846. doi: 10.1074/jbc.C200277200. [DOI] [PubMed] [Google Scholar]
- 34.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int. J. Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 35.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781–792. [PubMed] [Google Scholar]
- 36.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 37.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 38.Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J. Biol. Chem. 2004;279:15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 39.Kamb A. Cell-cycle regulators and cancer. Trends Genet. 1995;11:136–140. doi: 10.1016/s0168-9525(00)89027-7. [DOI] [PubMed] [Google Scholar]
- 40.Sherr CJ. D-type cyclins. Trends Biochem. Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 41.Strauss M, Lukas J, Bartek J. Unrestricted cell cycling and cancer. Nat. Med. 1995;1:1245–1246. doi: 10.1038/nm1295-1245. [DOI] [PubMed] [Google Scholar]