Abstract
A key feature of the aging process is that the mitochondrial respiratory capacity declines and production of reactive oxygen species increases in the later part of life span. In previous studies, cytochrome c oxidase (CcO), the terminal component of the mitochondrial electron transport chain, was found to be the only oxidoreductase exhibiting an age-related decrease in activity in Drosophila melanogaster. The present study tested the hypothesis that decreases in the abundance of catalytic subunits of CcO, encoded in mitochondrial DNA, could underlie the age-associated loss of enzyme activity. Protein amounts of subunits I, II and III, which form the catalytic core of CcO, were determined by immunoblot analysis in 15-, 25-, 35-, 47- and 60-day-old flies. Subunits II and III decreased with age by up to 43% and 75%, respectively, whereas the decrease in subunit I was only 15%. The results pinpoint specific changes in a component of the mitochondrial electron transport chain, which could underlie the age-related decrease in mitochondrial respiratory activity and an increase in oxidant production. Apparently, the stoichiometry of CcO holoprotein is dynamically altered during the aging process in Drosophila melanogaster.
Keywords: Mitochondria, aging, oxidative stress, cytochrome c oxidase, electron transport chain
INTRODUCTION
Deteriorative alterations in mitochondrial functions have been widely postulated to play a key role in the decline of physiological vigor of animals during the aging process (Harman, 1972; Sohal and Weindruch, 1996; Beckman and Ames, 1998). The two most commonly observed age-related changes in mitochondrial activity are: (i) an elevation in the rates of generation of superoxide anion radical and its stoichiometric product, hydrogen peroxide, which are progenitors for a variety of other intracellular reactive oxygen species (ROS) (Sohal and Weindruch, 1996; Halliwell and Gutteridge, 1999; Marnett et al., 2003), and (ii) a decline in the rate of state 3 (ADP-stimulated) or maximal (uncoupled) respiration (Chiu and Richardson, 1980; Trounce et al., 1989; Ferguson et al., 2005). The enhanced rate of ROS production accords with the oxidative stress hypothesis of aging, which proposes that the accumulation of macromolecular oxidative damage, inflicted by physiologically generated ROS and other oxidants, is the primary cause of the senescence-associated deleterious alterations (Beckman and Ames, 1998; Barja and Herrero, 2000; Sohal et al., 2002). This hypothesis is additionally supported by the findings that the rates of accrual of macromolecular oxidative damage increase with age in a wide variety of species, and tend to be inversely related to longevity of different species (Stadtman, 1992; Sohal et al., 1995; Barja and Herrero, 2000). The decrease in energy production and usage during aging, which is reflected by the accompanying decline in physical stamina and locomotion in different species, may be both a consequence of oxidative damage to components of the mitochondrial electron transport chain (ETC), and a cause of additional oxidative stress.
The hypothesis, that structural damage to components of the ETC could contribute to oxidative stress, emanates from the findings that in cells superoxide anion radical/hydrogen peroxide is generated largely by the components of the ETC during the process of respiration (Chance et al., 1979). The rate of superoxide anion radical generation is dependent upon several factors, but the mitochondrial membrane potential and redox state of the ETC have particularly critical effects under physiological conditions (Nicholls, 2002; Kadenbach, 2003). The activity of cytochrome c oxidase (CcO; EC 1.9.3.1; also known as ferrocytochrome c:oxygen oxidoreductase or complex IV) is of special importance to the membrane potential, and to the redox state of the ETC, because (i) CcO is the terminal oxidoreductase and a proton pump of the ETC in aerobes, where it is also subject to allosteric inhibition by ATP (Kadenbach, 2003), (ii) the reduction of oxygen by CcO is the only essentially irreversible step in the ETC (Poyton and McEwen, 1996), (iii) CcO activity constitutes a rate-limiting step in mitochondrial respiration in intact cells, and its activity does not significantly exceed the respiratory capacity in vivo (Poyton and McEwen, 1996; Villani et al., 1998; Villani and Attardi, 2000), and (iv) a decrease in CcO activity enhances the rate of mitochondrial production of superoxide anion radical/hydrogen peroxide due to elevation of the redox state of upstream ETC components. Thus, any loss of CcO activity would be predicted to create a bottleneck in the activity of the ETC as a whole (Capaldi, 1990), which would not only attenuate the respiratory rate and potential for oxidative phosphorylation, but also increase superoxide anion radical production. Within the ETC, a decrease in CcO activity is among the most consistently reported age-related alterations in both insect and mammalian mitochondria (Benzi et al., 1992; Bowling et al., 1993; Schwarze et al., 1998).
CcO exists as a homodimer, embedded in the inner mitochondrial membrane, and each monomer within the mammalian dimer consists of thirteen subunits (Capaldi, 1990). Ten subunits (IV, Va, Vb, VIa, VIb, VIc, VIIa, VIIb, VIIc and VIII) are encoded by the nuclear genome. They do not play a direct role in electron transfer or proton pumping, but are considered to be essential for the assembly and regulation of the holoenzyme (Khalimonchuk and Rodel, 2005). Three CcO subunits (I, II and III) are encoded by the mitochondrial DNA. They form the catalytic or functional core, which is fully competent for both electron transfer and proton pumping. In this context, the key question is whether or not the age-related decline in catalytic activity of CcO may be explained by a decrease in the abundance of catalytic subunits I, II and III, which could be quite vulnerable to oxidative damage, because both their mRNA as well as protein are in close proximity to the sites of ROS generation. Accordingly, the hypothesis, that there may be an age-related decrease in the expression of CcO subunits, encoded by mitochondrial DNA, was tested in D. melanogaster. The results indicate that there are indeed significant, specific age-related losses in the protein amounts of CcO subunits.
MATERIALS AND METHODS
Animals
A y w strain of Drosophila melanogaster in an Oregon R background was used for all experiments. Male flies were isolated 1–2 days after eclosion and maintained on a standard cornmeal-sucrose-yeast-agar medium, as described previously (Mockett et al., 2002).
Immunoblot analysis
Due to the absence of commercially available anti-Drosophila CcO monoclonal antibodies, mammalian antibodies were purchased from MitoSciences (Eugene, OR) or Molecular Probes (Carlsbad, CA), as indicated in Table 1.
Table 1.
Reactivity of various commercially available anti-cytochrome c oxidase monoclonal antibodies with mitochondrial proteins in different species
| CcO Subunit | Supplier | Immunogen | Interspecies immunoreactivity | |||
|---|---|---|---|---|---|---|
| Bovine | Mouse | Rat | Drosophila | |||
| I | MP | Human | + | + | + | + |
| II | MP/MS | Human | — | + | + | + |
| III | MS | Yeast | + | + | + | + |
| IV | MP | Human | + | — | — | — |
| Va | MS | Bovine | + | + | + | — |
| Vb | MP | Human | + | + | — | — |
| VIa(L) | MS | Bovine | + | + | + | — |
| VIb | MP | Bovine | + | + | — | + |
| VIc | MS | Bovine | + | ND | + | — |
| VIIa | MP | Bovine | + | + | + | — |
| VIIb | MS | Bovine | + | ND | + | — |
| Porin | MS | Human | + | + | + | + |
| Complex Vα | MS | Bovine | + | + | + | + |
Antibody cross-reactivity was assessed by immunoblot analysis, carried out as described in the legend of Fig. 1. Ox, mouse and rat mitochondria were isolated from the heart. Drosophila mitochondria were from the thoracic flight muscles. Specific epitope recognition information was either not available or was proprietary. MP, Molecular Probes; MS, MitoSciences; + = cross-reaction; — = no cross-reaction; ND = not determined.
Mitochondria were isolated from the flight muscles of two different populations of flies at 15, 25, 35, 47, and 60 days of age, as described previously (Ferguson et al., 2005). Mitochondrial proteins were resolved by one dimensional SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Briefly, proteins (10 µg) were electrophoresed in 1.0-mm gels, consisting of 4% stacking and 10% separating gels, using a Bio-Rad Miniprotean III gel apparatus. The electrophoretic separations were carried out at a constant current of 30 V for 40 min for the stacking gels, and 100 V for 90 min for separating gels. For each experiment, one gel was stained with Coomassie Blue, while the other was electrotransferred at 4°C to a PVDF membrane in a buffer containing 16 mM Tris base, 120 mM glycine and 10% (v/v) methanol, applying constant current of 120 V for 80 min. For immunodetection, PVDF membranes were incubated with blocking solution containing 5% dry milk and 0.1% Tween 20 in Tris buffered saline (10 mM Tris/HCl buffer, pH 7.5, and 150 mM NaCl ) for 45 min at 37°C, or overnight at 4°C. Membranes were then quickly rinsed in distilled water and incubated with the primary antibody at 37°C for 1 h at dilutions of 1: 5000 for anti-CcO subunits I, II, III and anti-complex Vα; and 1:1500 for anti-CcO subunit VI b. A horseradish peroxidase -conjugated goat anti-mouse IgG (H+L) secondary antibody (Pierce, Rockford, IL) was used at a dilution of 1:100,000 for 1 h at 37°C. Following ECL chemiluminescence detection (Amersham Biosciences, Piscataway, NJ), membranes were stripped and re-probed with mouse anti-complex Vα and anti-porin antibodies, used as internal loading controls. Protein identification was based on the specificity of the immunoreaction between primary antibodies and the bands at positions corresponding to the published molecular weights (57, 26, 30, and 10 kDa for CcO subunits I, II, III, and VIb, respectively; Capaldi, 1990). Protein abundance was quantified by densitometric analysis of equal loading amounts of samples from different age groups, using Lab Works 4.0 (UVP Inc., Upland, CA) and NIH ImageJ 1.37 software. Additional corroboration was provided by loading different amounts of the sample. This strategy was used to control for the nonlinearity of the relationship between the amount of protein and signal intensity.
RESULTS and DISCUSSION
Reactivity of anti-mouse antibodies
Commercially available mouse anti-CcO monoclonal antibodies were confirmed in this laboratory to be immunoreactive with bovine, rat, and mouse samples, which established their viability as well as specificity (Table 1). Cross-reactivity was observed for the Drosophila CcO subunits, I, II and III, encoded in mitochondrial DNA, as well as for porin and complex Vα, used as internal loading controls. The observation, that mouse anti-CcO antibodies exhibited cross-reactivity with all three mitochondrial DNA-encoded subunits, is consistent with the established view that these subunits are relatively well conserved in different species. In contrast, only one Drosophila nuclear DNA-encoded subunit, VIb, cross-reacted with the mouse antibody, which suggests relatively lower sequence conservation of subunits encoded by the nuclear genome (Table 1).
Age-associated changes in protein abundance of individual CcO subunits in Drosophila melanogaster
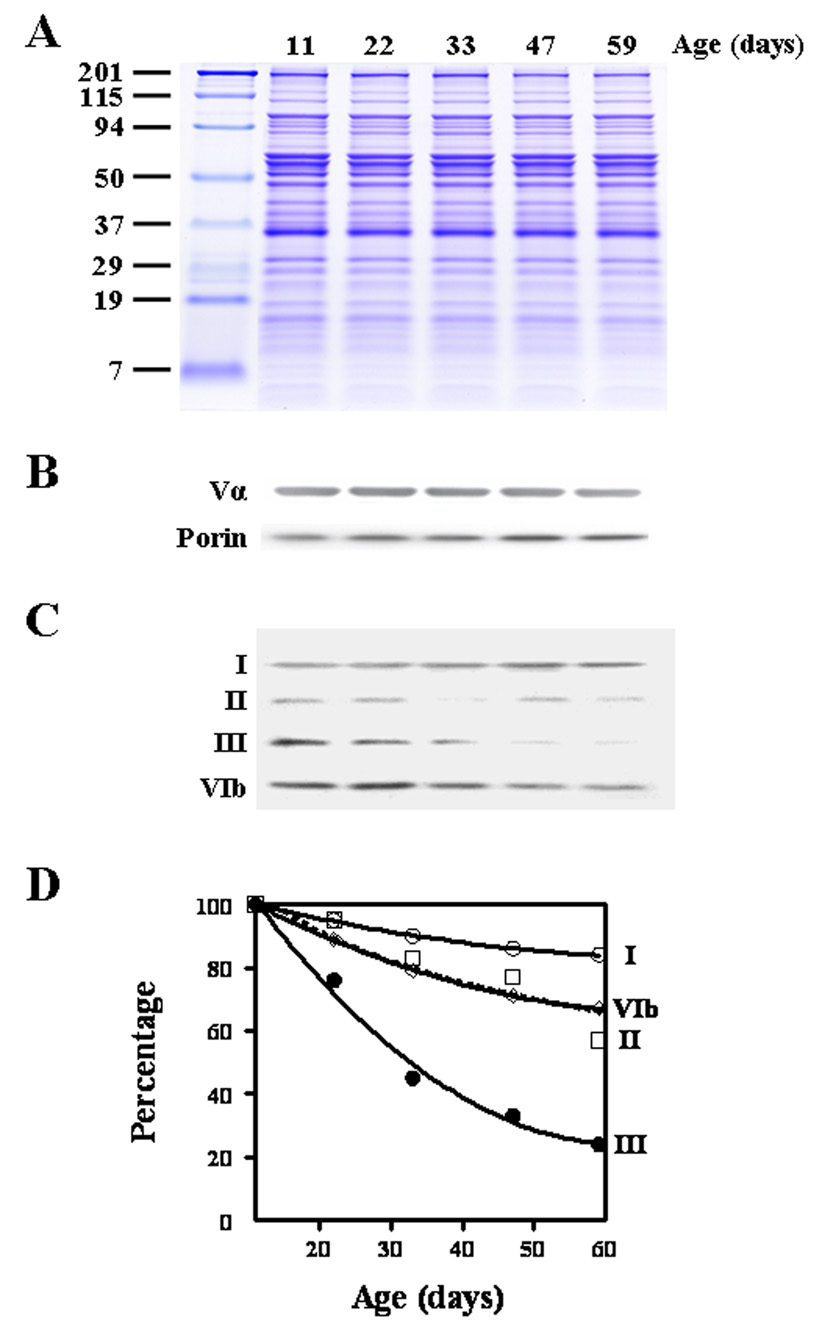
Initially, aliquots of mitochondrial proteins from 15-, 25-, 35-, 47- and 60-day-old flies were separated and stained with Coomassie Blue (Fig. 1A), in order to determine whether the amounts of different proteins, reflected by the densities of the various bands, showed alterations during aging. However, no discernable age-related differences in the densities of the readily visible bands were detected.
Figure 1.
Effect of age on the abundance of cytochrome c oxidase subunits in Drosophila melanogaster. Proteins (10 µg/lane) from flight muscle mitochondria were separated by discontinuous 10% SDS-PAGE and stained with Coomassie Blue (A), or probed with anti- complex Vα, anti-porin (B) and anti-CcO subunits I, II, III and VIb (C), Results presented in panel D are means of at least three samples, obtained from two independent mitochondrial preparations. For purposes of clarity, error bars are not shown (the coefficient of variation was < 20% for all points).
An extensive set of quantification experiments, with different dilutions of Drosophila mitochondrial proteins, was carried out for each specific antigen:antibody combination, and profiles of signal:antigen abundance were established by intra-and inter-gel comparisons of signal intensity. Between 15 to 60 days of age, the densities of the immunopositive bands reacting with antibodies against porin or complex Vα showed no notable differences at different ages (Fig. 1B), thereby corroborating the finding that mitochondrial protein amounts do not undergo a generalized loss/gain with age. In contrast, during this period, subunit III exhibited a dramatic, 75% decrease in immunoreactive content, and subunit II protein showed a decrease of 43% (Fig. 1C, D). Two-way ANOVA indicated that the effects of age as well as the differences between the subunits were significant (P=< 0.0001). The age related loss of subunit I was relatively minor (15%). The present study could not address the issue of the distribution or relative magnitude of the age-related attrition in nuclear DNA encoded CcO subunits, due to their poor immunoreactivity with anti-mouse antibodies, albeit the sole immunodetectable nuclear encoded subunit, VIb showed a 30% age-related decrease in immunodensity.
Subunits I, II and III constitute the catalytic functional core of the CcO holoenzyme. Subunit I contains the heme a3/CuB active site, where molecular oxygen is reduced to water. It also pumps protons from the mitochondrial matrix into the inter-membrane space (Khalimonchuk and Rodel, 2005). Subunit II incorporates the CuA center, and provides sites for docking and transfer of electrons from cytochrome c (Khalimonchuk and Rodel, 2005). It also transfers electrons to heme a in subunit I (Hosler et al., 2006). Subunit III, which showed the greatest attrition, modulates the rate of proton translocation and also facilitates a late step in the assembly of CcO. The depletion of subunit III has been shown to be associated with an irreversible loss of CcO activity, known as “suicide inactivation”, caused by a structural alteration at the CuB site (Hosler et al., 2006). It is thus apparent that due to the inter-dependent roles of CcO subunits, differential losses of the mitochondrial DNA-encoded subunits could result in a decrease in enzymatic activity, as has been observed during aging in Drosophila (Ferguson et al., 2005).
The differential losses in subunits I, II and III could theoretically be explained, at least in part, by mitochondrial DNA deletions, which affect some gene sequences more frequently than others, and which are known to accumulate as a function of age in Drosophila (Calleja et al., 1993; Yui et al., 2003). However, the frequency of such deletions occurring in vivo is much too low to be the primary reason for up to 75% loss in subunits in samples pooled from all of the mitochondria in the flight muscles. Nevertheless, the mitochondrial transcriptional activity has also been reported to decline during aging (Calleja et al., 1993) Thus, it seems plausible that the losses in CcO subunits may arise from a combination of factors, including decreased transcription, susceptibility of specific subunits to degradation, disassembly or inactivation by oxidants generated by upstream electron carriers.
Cytochrome c oxidase is the only mitochondrial respiratory complex which shows an age- related decline in activity in housefly and Drosophila, and this decline is accompanied by a decrease in ADP-stimulated respiration (state 3), and elevation of mitochondrial superoxide anion radical/hydrogen peroxide production (Sohal et al., 1995; Ferguson et al., 2005). Decrease in CcO activity (~30–50%) and increase in O2.- generation are also among the most consistent age-related alterations in mammalian tissues (Chen et al., 1972; Abu-Erreish and Sanadi, 1978; Benzi et al., 1992; Cooper et al., 1992; Desai et al., 1996; Martinez et al., 1996; Kwong and Sohal, 2000). The results of the present study suggest that a decrease in the abundance of specific CcO subunits may be responsible for the age-related changes in CcO activity, which may in turn affect the rates of mitochondrial respiration and ROS production. In particular, two of the three subunits of CcO, encoded in mitochondrial DNA, exhibit remarkable decreases in protein abundance, suggesting a structural basis for the age-related decline in COX activity and an increase in mitochondrial ROS production. Results also imply that the composition of the CcO holoprotein is progressively altered during aging. Notably the age-related expressions of the subunits are not coordinately down regulated.
Acknowledgment
This work was supported by grant RO1 AG7657 from the National Institute on Aging - National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu-Erreish GM, Sanadi DR. Age-related changes in cytochrome concentration of myocardial mitochondria. Mech. Ageing Dev. 1978;7:425–432. doi: 10.1016/0047-6374(78)90083-0. [DOI] [PubMed] [Google Scholar]
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Benzi G, Pastoris O, Marzatico F, Villa RF, Dagani F, Curti D. The mitochondrial electron transfer alteration as a factor involved in the brain aging. Neurobiol. Aging. 1992;13:361–368. doi: 10.1016/0197-4580(92)90109-b. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Mutisya EM, Walker LC, Price DL, Cork LC, Beal MF. Age-dependent impairment of mitochondrial function in primate brain. J. Neurochem. 1993;60:1964–1967. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- Calleja M, Pena P, Ugalde C, Ferreiro C, Marco R, Garesse R. Mitochondrial DNA remains intact during Drosophila aging, but the levels of mitochondrial transcripts are significantly reduced. J. Biol. Chem. 1993;268:18891–18897. [PubMed] [Google Scholar]
- Capaldi RA. Structure and function of cytochrome c oxidase. Annu Rev Biochem. 1990;59:569–596. doi: 10.1146/annurev.bi.59.070190.003033. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chen JC, Warshaw JB, Sanadi DR. Regulation of mitochondrial respiration in senescence. J. Cell Physiol. 1972;80:141–148. doi: 10.1002/jcp.1040800115. [DOI] [PubMed] [Google Scholar]
- Chiu YJ, Richardson A. Effect of age on the function of mitochondria isolated from brain and heart tissue. Exp. Gerontol. 1980;15:511–517. doi: 10.1016/0531-5565(80)90003-0. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J. Neurol. Sci. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- Desai VG, Weindruch R, Hart RW, Feuers RJ. Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch. Biochem. Biophys. 1996;333:145–151. doi: 10.1006/abbi.1996.0375. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Hosler JP, Ferguson-Miller S, Mills DA. Energy transduction: proton transfer through the respiratory complexes. Annu. Rev. Biochem. 2006;75:165–187. doi: 10.1146/annurev.biochem.75.062003.101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim. Biophys. Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- Khalimonchuk O, Rodel G. Biogenesis of cytochrome c oxidase. Mitochondrion. 2005;5:363–388. doi: 10.1016/j.mito.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Hernandez AI, Martinez N, Ferrandiz ML. Age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1996;731:246–248. doi: 10.1016/0006-8993(96)00708-1. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Orr WC, Sohal RS. Overexpression of Cu,ZnSOD and MnSOD in transgenic Drosophila. Methods Enzymol. 2002;349:213–220. doi: 10.1016/s0076-6879(02)49336-6. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int. J. Biochem. Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- Poyton RO, McEwen JE. Crosstalk between nuclear and mitochondrial genomes. Annu Rev Biochem. 1996;65:563–607. doi: 10.1146/annurev.bi.65.070196.003023. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic. Biol. Med. 1998;25:740–747. doi: 10.1016/s0891-5849(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal A, Agarwal S, Orr WC. Simultaneous overexpression of copper- and zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem. 1995;270:15671–15674. doi: 10.1074/jbc.270.26.15671. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic. Biol. Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in human cells. Free Radic Biol. Med. 2000;29:202–210. doi: 10.1016/s0891-5849(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Villani G, Greco M, Papa S, Attardi G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J. Biol. Chem. 1998;273:31829–31836. doi: 10.1074/jbc.273.48.31829. [DOI] [PubMed] [Google Scholar]
- Yui R, Ohno Y, Matsura ET. Accumulation of deleted mitochondrial DNA in aging Drosophila melanogaster. Genes Genet. Syst. 2003;78:245–251. doi: 10.1266/ggs.78.245. [DOI] [PubMed] [Google Scholar]