Abstract
Calcium and magnesium are essential for survival but it is unknown how animals detect and consume enough of these minerals to meet their needs. To investigate this, we exploited the PWK/PhJ (PWK) strain of mice, which, in contrast to the C57BL/6J (B6) and other inbred strains, displays strong preferences for calcium solutions. We found that the PWK strain also has strong preferences for MgCl2 and saccharin solutions but not representative salty, sour, bitter, or umami taste compounds. A genome scan of B6 × PWK F2 mice linked a component of the strain difference in calcium and magnesium preference to distal chromosome 4. The taste receptor gene, Tas1r3, was implicated by studies with 129.B6ByJ-Tas1r3 congenic and Tas1r3 knockout mice. Most notably, calcium and magnesium solutions that were avoided by wild-type B6 mice were preferred (relative to water) by B6 mice null for the Tas1r3 gene. Oral calcium elicited less electrophysiological activity in the chorda tympani nerve of Tas1r3 knockout than wild-type mice. Comparison of the sequence of Tas1r3 with calcium and saccharin preferences in inbred mouse strains found 1) an inverse correlation between calcium and saccharin preference scores across primarily domesticus strains, which was associated with an I60T substitution in T1R3, and 2) a V689A substitution in T1R3 that was unique to the PWK strain and thus may be responsible for its strong calcium and magnesium preference. Our results imply that, in addition to its established roles in the detection of sweet and umami compounds, T1R3 functions as a gustatory calcium-magnesium receptor.
Keywords: mineral appetite, quantitative trait locus, sweet, tas1r3, gustation
calcium and magnesium play crucial roles in many physiological processes so there is selective pressure on animals to detect these minerals in the event of scarcity and to monitor closely the amounts ingested (35, 40). The sense of taste provides a way to accomplish this, but little is known about the gustatory transduction of calcium and still less about magnesium. Animal studies indicate that calcium and magnesium intakes are regulated by specific appetites (25, 35, 40) and that manipulations that perturb calcium metabolism also influence oral calcium sensitivity and palatability (14, 22–24, 33). The tastes of calcium and magnesium are not easily characterized. Humans can discriminate CaCl2 from MgCl2 but rate them as having similar tastes and cannot easily describe them in terms of the four (or five) basic taste qualities (19, 34, 41). Similarly, rats can discriminate calcium from magnesium (25), but there is strong cross-adaptation between the two divalent cations and little cross-adaptation or response generalization between calcium and sweet, salty, or sour tastes (27, 38). Rats fed low-calcium diet for several days have altered gustatory afferent nerve responses to, and increased intakes of, MgCl2 as well as CaCl2 (6, 14, 33). Mice have a group of gustatory afferent nerve fibers that respond more robustly to calcium and magnesium than to other taste stimuli (31). These findings are consistent with the existence of a common calcium-magnesium transduction mechanism that is distinct from those used for other tastes.
We have begun studies to identify the genes that underlie calcium and magnesium consumption and preference. In a survey of 40 inbred strains, the PWK/PhJ (PWK) strain had the highest preferences for several concentrations of calcium chloride (CaCl2) and lactate (CaLa), whereas the C57BL/6J (B6) strain was ranked in the lower half (43, 44). Based on this information, we conducted a genome screen of B6 × PWK F2 mice, and found seven sets of quantitative trait loci (QTLs) that were involved in calcium and magnesium consumption (46). The common peak of one set of QTLs (Drinkcacl21, Drinkcala1, and Drinkmgcl21) was coincident with the taste receptor gene, Tas1r3, on distal chromosome 4. We report here 1) the preferences of the B6 and PWK parental strains for several taste solutions, which characterize the specificity of the PWK strain's high preference for calcium, 2) details of the linkage of calcium and magnesium preference to distal chromosome 4 in B6 × PWK F2 mice, 3) studies with Tas1r3 congenic and knockout mice that implicate Tas1r3 as the gene responsible for this linkage, 4) data from the Mouse Phenome Database and other sources showing an inverse relationship among inbred mouse strains between preferences for calcium and sweeteners, and 5) an analysis of Tas1r3 haplotypes of inbred strains that suggests single nucleotide polymorphisms (SNPs) underlying conformational changes at I60T and V689A may account for strain differences in preferences for calcium and magnesium.
METHODS
General Procedures
Procedures involving animals were approved by the Animal Care and Use Committee of the Monell Chemical Senses Center. Mice from the C57BL/6J strain (B6; stock no. 000664) and PWK/PhJ strain (PWK; stock no. 003715) were purchased from The Jackson Laboratory (Bar Harbor, ME) or bred in-house.
Maintenance and diet.
All breeding and phenotyping was conducted in a vivarium maintained at 23°C with a 12:12 h light/dark cycle (lights off at 7 PM). The mice were housed in plastic “tub” cages (26.5 × 17 × 12 cm) with stainless steel wire lids and pine shavings scattered on the floor. They were housed in groups of three to six mice of the same strain and sex until at least a week before behavioral tests, when they were individually housed. The cage lids included space for pelleted food and a water bottle (see Ref. 42 for details). The food was AIN-76A, a semisynthetic diet containing by weight: 20% protein (casein), 65% carbohydrate (sucrose and cornstarch), 5% fat (corn oil), and 10% fiber (cellulose), minerals and vitamins (diet no. 100000; Dyets, Bethlehem, PA). Food was freely available at all times. When mice were not being tested, deionized water was available from an inverted 300-ml glass bottle with a neoprene stopper and a stainless steel drinking spout.
Two-Bottle choice tests.
Details of drinking tube construction, cage layout, and the position of drinking tubes during tests are described elsewhere (42). At the start of each test, two graduated tubes were placed on the lid of each mouse's cage so that the attached drinking spouts rested inside the cage at ∼3 cm above the floor and ∼2 cm apart. One drinking tube contained deionized water and the other contained a taste solution. The position of the tubes was switched every 24 h. In most experiments, the mice received a series of 48-h choices between water and ascending concentrations of three to nine taste compounds. These were purchased from Sigma Chemical (St. Louis, MO) except for CaLa, which was purchased from Fisher Scientific, and all were dissolved in deionized water. Concentrations of each compound were chosen based on previous work (e.g., Refs. 6, 43–45) with the aim of spanning the range from indifference to strong avoidance. The concentrations usually increased in 0.5-log steps (e.g., 1, 3.16, 10, 31.6 mM), but other concentrations were tested if prior studies suggested they might be useful. In the experiment involving B6 × PWK F2 mice, there were 96-h tests of single concentrations of several taste compounds. Fluid intakes were measured volumetrically to the nearest 0.1 ml.
Statistical analyses.
Taste solution preference scores were calculated as taste solution intake/(water intake + taste solution intake) × 100. Intakes and preference scores were analyzed by ANOVA with factors of strain (or genotype), sex, and concentration when appropriate. Almost identical results were produced whether daily intakes or preference scores were analyzed so only preference score data are reported here. None of the analyses revealed a sex difference so data for each sex are combined. If the interaction between strain and concentration was significant, least significant difference (LSD) post hoc tests were used to assess differences between the strains at specific concentrations. A criterion of P < 0.05 was used to determine statistical significance.
Preference scores can range from strong avoidance (0%) to strong liking (100%), with a score of 50% being indifference. We used one-sample t-tests to determine whether preference scores of a group differed from 50%. Because of the large number of comparisons involved, a criterion for statistical significance of P < 0.005 was used for these tests to provide protection from type II errors.
Two-Bottle Choice Tests of the B6 and PWK Parental Strains
Several experiments were conducted to assess the range of calcium concentrations over which differences between the strains existed and to examine the specificity of the response to calcium. In each experiment, groups of 9–13 male B6 and 10–12 male PWK mice aged 7–9 wk received a series of 48-h two-bottle choice tests. We selected two calcium salts (CaCl2 and CaLa) for study because these are soluble and both have been used to monitor calcium intake previously (e.g., Refs. 33, 44). To determine whether strain differences in calcium preference scores extended to other mineral salts, we tested MgCl2, NaCl, and sodium lactate (NaLa). Human psychophysical research indicates that the predominant taste qualities of CaCl2 and CaLa are bitter and sour (41) so three representative bitter compounds (denatonium benzoate, quinine hydrochloride, and caffeine) and two representative sour compounds (HCl and citric acid) were tested. We also tested a representative sweet compound (saccharin) and umami compound [inosine monophosphate (IMP)].
It was not possible to test each compound in naive mice because of mouse supply problems. In the order listed, one study involved B6 and PWK mice tested with the two calcium salts, NaCl and NaLa; one with IMP, denatonium, QHCl and caffeine; one with HCl, citric acid, and saccharin; and one with MgCl2. There were at least 2 days with a single drinking tube of water available between each test series.
The PWK mice were roughly two-thirds the body size of the B6 mice (body weights at start of tests: B6 = 23.2 ± 0.4 g, n = 40; PWK = 14.6 ± 0.2 g, n = 40), and consequently they consumed considerably less fluid. For example, total water intakes at the start of the first test series were B6 = 5.7 ± 0.4 ml/day, n = 40; PWK = 3.1 ± 0.1 ml/day, n = 40. We therefore present preference scores, which are independent of the volume of fluid consumed. Daily intakes of individual mice tested with CaCl2, CaLa, NaCl, and NaLa are available as part of the Mouse Phenome Database (43).
Two-Bottle Choice Tests and Linkage Analysis of B6 × PWK F2 Mice
Detailed procedures used for breeding, genotyping, and phenotyping B6 × PWK F2 mice are presented elsewhere (36, 46). In brief, B6 and PWK mice were intercrossed and their F1 offspring bred brother-to-sister to produce 244 male and 240 female B6 × PWK F2 mice. These were weaned at 21–23 days old and housed in same-sex groups of up to six mice until they were 6–8 wk old, when they were transferred to individual cages for taste phenotyping. After 7 days to adapt to individual housing, each mouse received a series of two-bottle choice tests with each test lasting 4 days. For the first test, two bottles of deionized water were provided. Subsequent tests involved one bottle of deionized water and one of the following taste solutions, in the order listed: 50 mM CaCl2, 50 mM CaLa, 50 mM MgCl2, and 2 mM saccharin. Each test was separated from the next by a day with just water to drink. Other taste compounds were tested in between the tests with MgCl2 and saccharin, but to maintain the focus of this report, these results are described elsewhere (see Ref. 46).
Genomic DNA was extracted from mouse tails and a genome scan was conducted. The initial genotyping of chromosome 4 was performed by the Center for Inherited Disease Research using seven microsatellite markers (see Ref. 46 for details), to which we added two SNPs. One was located in Tas1r3 (rs13478075 at 155,236,752 bp; all positions are according to MGI, Build 37); the other was located between Tas1r3 and the telomere (rs33244176 at 155,559,234 bp). Linkage between individual traits and genotypes was computed using algorithms implemented by the R/qtl 1.04-53 package of R (4). Means of groups assorted according to genotype at rs13478075 were compared by ANOVA and then by post hoc LSD tests. The percentage of phenotypic variation accounted for by genetic variation at the marker was calculated from the ratio of sums of squares (SS) of the ANOVA, SSbetween/SStotal.
Two-Bottle Choice Tests of 129.B6ByJ-Tas1r3 Congenic Mice and Tas1r3 Knockout Mice
129.B6ByJ-tas1r3 congenics.
We developed a strain of congenic mice as part of a project to clone Tas1r3 (2, 17). This involved using serial backcrossing to introgress a chromosomal fragment containing the Tas1r3 allele from the high sweetener-preferring C57BL/6ByJ (B6ByJ) strain onto the genetic background of the low sweetener-preferring 129P3/J strain. The B6 allele is dominant over the 129 allele and exerts a large effect on preferences for a variety of sweeteners (13, 17). The congenic interval is on chromosome 4 and is <194 kb. It begins proximally somewhere between markers 280G12-T7 and 4902-T7 (155,171,951-155,187,450 bp) and ends distally somewhere between markers 350D2-T7 and D4Mon1 (155,302,732-155,365,331 bp). Tas1r3 is at 155,233,379-155,237,462 bp.
The subjects were 11 heterozygous (B6ByJ/129) congenics and 19 homozygous (129/129) littermates from the N10F4, N11F3, and N11F4 segregating generations (see Ref. 15 for explanation of nomenclature). All were female, with similar ages and body weights (B6ByJ/129 = 18 ± 1 wk, 20.1 ± 0.5 g; 129/129 = 16 ± 1 wk, 20.0 ± 0.4 g), and had genotype confirmed at rs13478075. The mice received four series of 48-h two-bottle choice tests. Each series began with a choice between two bottles of water and then between water and an ascending series of 11 concentrations of saccharin or 3 concentrations each of CaCl2, CaLa, and MgCl2. There were 2–3 days with just water to drink between each test series.
Tas1r3 knockouts.
A line of Tas1r3 knockout mice was produced by homologous recombination in B6 embryonic stem cells (7) and was maintained on the B6 background. For the experiment described here, we first produced Tas1r3+/− hybrids by mating Tas1r3−/− mice with B6 mice, then mated brother-to-sister to yield a total of 19 +/+, 36 +/−, and 14 −/− offspring with approximately equal numbers of males and females in each group. This approach had the advantage of generating all three potential genotypes and providing littermate controls. Testing began when the mice were at ∼7 wk old, at which time the Tas1r3+/+, +/−, and −/− groups weighed 21.6 ± 0.8, 19.6 ± 0.6, and 20.9 ± 0.8 g, respectively. Each mouse received 48-h two-bottle tests with a choice between water and various concentrations of CaCl2, CaLa, MgCl2, NaCl, and saccharin (see below). The genotype of each mouse was determined by a commercial service (Transnetyx).
Gustatory Electrophysiology
The results of long-term two-bottle choice “taste” tests used in previous experiments can be influenced by nongustatory-chemosensory, postingestive, and experiential effects, as well as taste. To determine whether the effects observed involved changes in taste, we applied solutions of calcium and other compounds to the oral cavity while recording electrical activity of the chorda tympani nerve, which conveys gustatory information from the anterior two-thirds of the tongue to the brain. Eight male Tas1r3−/− mice and eight male wild-type littermates (Tas1r3+/+) were anesthetized with a mixture of 90 mg/kg ketamine, 20 mg/kg xylazine, and 3 mg/kg acepromazine (injected intraperitoneally, with further doses as necessary), tracheotomized, and placed in a supine position in a nontraumatic head holder. The right chorda tympani nerve was exposed between its exit from the lingual nerve and entry to the bulla. The nerve was cut near the bulla and placed on a platinum wire electrode, and a few drops of mineral oil were placed in the wound site to prevent desiccation. An indifferent electrode was positioned in nearby muscle tissue. The anterior tongue was placed in a flow chamber. Deionized water rinse and stimulus solutions (at room temperature) were applied by continuous flow with a rate of 0.5 ml/s. Each stimulus presentation lasted for 20 s and was followed by at least 60 s of water rinse. Concentration series of a given compound were applied in ascending order.
The whole nerve response was amplified and integrated with a time constant of 1.0 s. Net responses were quantified by calculating the integral of the voltage (area under the curve) for 10 s after stimulus onset and subtracting it from the area for the 10 s prior to onset (baseline). Because the response of individual animals depends on the preparation (e.g., the exact placing of the recording electrode), responses were expressed relative to the response produced by 100 mM NH4Cl, which was applied regularly throughout the recording session. NH4Cl is commonly used as a standard solution in studies of gustatory electrophysiology and seemed appropriate here. There was no difference between the two groups in raw mean responses of each nerve preparation to NH4Cl [means ± SE, Tas1r3+/+ = 8,222 ± 1,765 Vms, Tas1r3−/− = 5,570 ± 565 Vms, t(14) = 1.43, P = 0.17], and pilot work comparing 9 Tas1r3+/+ with 9 Tas1r3−/− mice found there was no difference in preferences for any concentration of NH4Cl tested (1, 3.16, 10, 31.6, 100, 178, and 316 mM).
Differences between the Tas1r3+/+ and −/− mice were assessed using t-tests for compounds tested at only one concentration and two-way, mixed-design ANOVAs for taste compounds tested at two or more concentrations. If the interaction term of the ANOVA was significant then LSD post hoc tests were used to assess differences between the strains at specific concentrations.
Tas1r3 Haplotype and Calcium Preference Scores
To identify sequence variants of Tas1r3 associated with calcium preference, we compared SNPs of 40 inbred mouse strains for which we had calcium preference scores (43, 44). Most of the SNP information was obtained from the Mouse Phenome Database (www.jax.org/phenome). Because SNP data from the PWK strain are scarce, we initially used data from the closely related PWD/PhJ strain. The PWK and PWD/PhJ strains diverged only recently (11), and the PWD/PhJ strain has the same high calcium preference scores as the PWK strain (data not shown). However, we subsequently sequenced several regions of the PWK strain to obtain all polymorphisms of Tas1r3 between B6 and PWK and found that the PWK and PWD/PhJ strains had an identical haplotype. Sequencing led us to discover a B6/PWK SNP at 155,234,806 bp, and so we also sequenced this in the other 38 strains. To eliminate sequencing errors, we confirmed every nucleotide variant by sequencing the forward and reverse strands and performed a second PCR reaction and resequenced the key variants.
To compare genotypes with phenotypes, for each SNP in Tas1r3, the 40 strains were assigned to one of two groups based on whether they had the B6 or alternate genotype, and their preference scores for 7.5, 25, and 75 mM CaCl2 and CaLa were compared with t-tests.
Relationship Between Preference Scores for Calcium and Sweetness
If Tas1r3 is involved in the preference for calcium then it seemed possible that there might be a relationship between the preference for calcium and sweeteners. To assess this we conducted analyses on five sets of data, each based on experiments involving two-bottle choice tests. We used Pearson correlation coefficients to compare preference scores of 1) the 484 F2 B6 × PWK mice described above. These mice were tested with 50 mM CaCl2, 50 mM CaLa, 50 mM MgCl2, and 2 mM saccharin. 2) Twenty-four inbred strain means from two studies conducted at our institution (32, 44). One study involved tests with six calcium solutions (7.5, 25, and 75 mM CaCl2 and 7.5, 25, and 75 mM CaLa) and the other with 1.6 mM saccharin solution. In this comparison, there were 24 strains and all mice were males. 3) Inbred strain means from mice tested with calcium (44) and mice tested with various concentrations of sucrose (16). In this comparison, there were 10 strains and all mice were males. 4) Data from 22 strains collected by Finn et al. and available as part of the Mouse Phenome Database (Ref. 39; Project 256; Finn1) involving 0.2% (∼10 mM) saccharin preference scores. This was compared with i) 22 inbred strains (both sexes) from Project 103 (Tordoff3) with preference scores for various concentrations of CaCl2 and CaLa, and ii) 13 inbred strains (males only) from Project 61 (Tordoff1) involving tests with four different concentrations of CaCl2.
RESULTS
Taste Preference Scores of B6 and PWK Inbred Mouse Strains
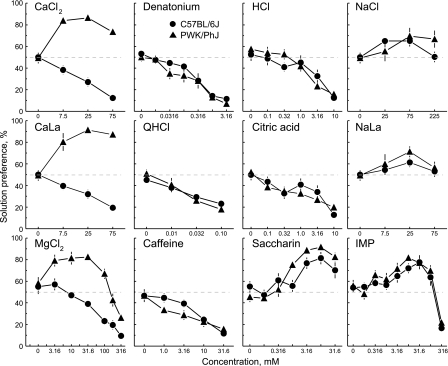
PWK mice had higher preference scores than did B6 mice for all concentrations tested of CaCl2, CaLa, and MgCl2 (Fig. 1, Table 1). Based on the results of one-sample t-tests comparing the strain means to indifference (i.e., 50% preference score), the PWK mice had preference scores significantly above indifference for all concentrations of CaCl2 and CaLa and most concentrations of MgCl2 (3.16, 10, 31.6, and 100 mM), whereas the B6 mice were either indifferent to or avoided all of these compounds.
Fig. 1.
Preferences of male C57BL/6J and PWK/PhJ mice for various taste solutions. CaCl2, calcium chloride; CaLa, calcium lactate; QHCl, quinine hydrochloride; NaLa, sodium lactate; IMP, inosine monophosphate (umami). Dotted horizontal lines show indifference (50% preference).
Table 1.
Preferences of B6 and PWK mice for 12 taste compounds: results of ANOVAs
| Compound |
Strain |
Concentration
|
Strain × Concentration
|
|||||
|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | F | P | |
| CaCl2 | 1,17 | 284.28 | 0.0000 | 3,51 | 11.69 | 0.0000 | 37.91 | 0.0000 |
| CaLa | 1,17 | 206.84 | 0.0000 | 3,51 | 3.39 | 0.0249 | 24.92 | 0.0000 |
| MgCl2 | 1,23 | 185.29 | 0.0000 | 6,132 | 21.40 | 0.0000 | 34.11 | 0.0000 |
| Denatonium | 1,23 | 1.79 | 0.1944 | 6,138 | 35.00 | 0.0000 | 0.76 | 0.6049 |
| QHCl | 1,23 | 0.00 | 0.9775 | 3,69 | 17.80 | 0.0000 | 0.94 | 0.4264 |
| Caffeine | 1,23 | 1.73 | 0.2011 | 4,92 | 25.98 | 0.0000 | 1.77 | 0.1419 |
| HCl | 1,22 | 0.51 | 0.4835 | 5,110 | 22.69 | 0.0000 | 1.28 | 0.2785 |
| Citric acid | 1,22 | 0.31 | 0.5813 | 5,110 | 17.70 | 0.0000 | 1.28 | 0.2764 |
| Saccharin | 1,23 | 1.44 | 0.2428 | 6,132 | 25.26 | 0.0000 | 3.28 | 0.0049 |
| NaCl | 1,17 | 0.22 | 0.6432 | 3,51 | 3.25 | 0.0292 | 1.82 | 0.1546 |
| NaLa | 1,17 | 1.87 | 0.1892 | 3,51 | 3.88 | 0.0142 | 2.00 | 0.1251 |
| IMP | 1,22 | 1.27 | 0.2728 | 8,176 | 29.25 | 0.0000 | 0.58 | 0.7912 |
Each row gives the output of a 2-way mixed-design ANOVA based on preferences of C57BL/6J (B6) and PWK/PhJ (PWK) mice (means and SE, and concentrations tested are given in Fig. 1). CaLa, calcium lactate; QHCl, quinine hydrochloride; NaLa, sodium lactate; IMP, inosine monophosphate. Degrees of freedom (df) for strain × concentration interaction are always the same as for the main effect of concentration.
There was an interaction between strain and concentration in preference scores for some concentrations of saccharin (Table 1), but post hoc tests could not determine a significant strain difference at any specific saccharin concentration. The two strains did not differ in response to any of the other compounds tested, indicating they had similar preference scores for bitter, sour, salty, and umami compounds.
Interval Mapping of Taste Preference Scores to Chromosome 4 in B6 × PWK F2 Mice
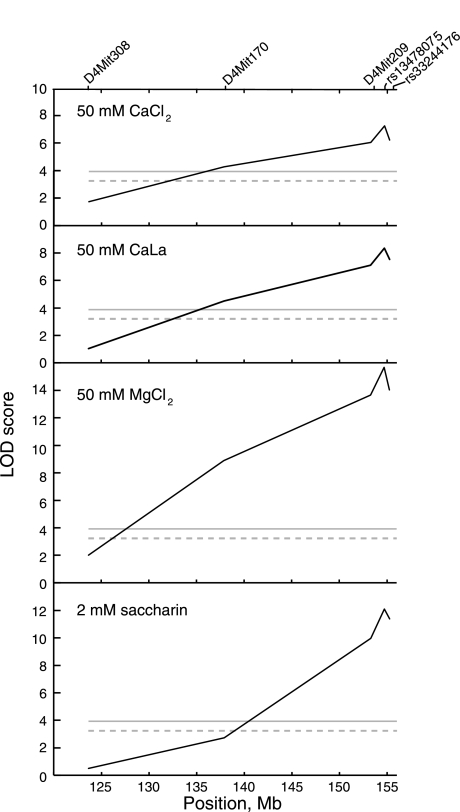
A whole genome screen revealed seven genetic loci involved in the preference for calcium or magnesium solutions, of which four were common to calcium and magnesium, two involved calcium only, and one involved magnesium only (46). Here, we focus on the linkages on distal chromosome 4 related to calcium, magnesium, and saccharin preference scores. The peaks of these QTLs were all at marker rs13478075, an SNP in Tas1r3 at 155,236,752 bp on chromosome 4 (Fig. 2). Genotyping an additional marker, rs33244176 at 155,559,234 bp, to our previously published description of these QTLs (46) raised logarithm of the odds ratio scores slightly (Table 2) and gave each a peak, although this was not very distinct because there was little opportunity for recombination between rs13478075 and the chromosome 4 telomere (at 155,630,120 bp). The region between the two markers flanking the peak (D4Mit209 and rs33244176) is <1.8-Mb and contains 72 known or predicted genes.
Fig. 2.
Interval maps of distal chromosome 4 based on genome screens of CaCl2, CaLa, MgCl2, and saccharin preference scores of 484 B6 × PWK F2 mice. Marker symbols are shown at top. Faint horizontal lines are genome-wide significance levels for P = 0.05 (lower) and P = 0.01 (upper). LOD, logarithm of the odds ratio.
Table 2.
Effects of Tas1r3 genotype on preference scores for calcium, magnesium, and saccharin in B6 × PWK F2 mice: QTL LOD scores, percentage of phenotypic variance accounted for, and mean preferences of genotype groups at SNP marker rs13478075 in Tas1r3
| Measure | LOD Score | % Variance | B6/B6 (n = 108) | B6/PWK (n = 256) | PWK/PWK (n = 107) | F(2,468), P |
|---|---|---|---|---|---|---|
| CaCl2 | 7.2* | 7.1 | 27±1 | 34±1a | 40±2ab | 17.9, P < 0.000001 |
| CaLa | 8.4* | 10.1 | 34±2 | 42±1a | 51±2ab | 19.3, P < 0.000001 |
| MgCl2 | 15.9* | 12.6 | 30±1 | 39±1a | 51±2ab | 39.9, P < 0.000001 |
| Saccharin | 12.1* | 10.3 | 82±1 | 83±1 | 74±1ab | 26.7, P < 0.000001 |
Preference values are means ± SE; n = group sizes. %variance = percentage of phenotypic variance accounted for, based on SSbetween/SStotal.
Significance of linkage, P < 0.01;
significant difference between groups of F2 mice with different Tas1r3 genotypes, P < 0.01 relative to B6/B6 group;
P < 0.01 relative to B6/PWK group. Group sizes and degrees of freedom for F-tests were slightly less in some cases because data were lost due to spillage or other technical errors. QTL, quantitative trait locus; LOD, logarithm of the odds ratio; SNP, single nucleotide polymorphism; SS, sum of squares.
Variation at the peak of the QTL (rs13478075) accounted for 7–13% of the total phenotypic variance, depending on the trait. The PWK allele exerted additive effects to increase preference scores for the divalent salts but was recessive and reduced preference scores for saccharin (Table 2).
Preference Scores of 129.B6ByJ-Tas1r3 Congenic Mice
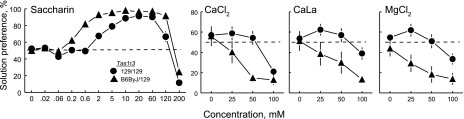
129.B6ByJ-Tas1r3 congenic mice carry the B6 allele of Tas1r3 on a 129P3/J (129) background; the B6 allele is dominant and confers high sweetener preference relative to littermates homozygous for 129 at Tas1r3 [i.e., (129/129); Refs. 13, 17]. Two-bottle choice tests of these mice with various concentrations of CaCl2, CaLa, and MgCl2 all showed the same pattern of results: Relative to mice with the 129/129 genotype, those with the 129/B6ByJ genotype at Tas1r3 had significantly lower preference scores for the divalent salts [strain × concentration interactions: CaCl2, F(3,84) = 4.06, P = 0.0095; CaLa, F(3,84) = 12.2, P < 0.0001; MgCl2, F(3,84) = 9.44, P < 0.0001], with the two genotype groups differing significantly at the following concentrations: 50 mM CaCl2, 25–100 mM CaLa, and 25–50 mM MgCl2 (Fig. 3). Mice with the 129/129 genotype avoided (i.e., had preference scores significantly <50%) 100 mM CaCl2 and 100 mM MgCl2. Those with the 129/B6ByJ genotype significantly avoided 50 and 100 mM CaCl2, 50 and 100 mM CaLa, and all three concentrations of MgCl2.
Fig. 3.
Preferences of 129.B6ByJ-Tas1r3 congenic mice possessing a B6ByJ allele of Tas1r3 (B6ByJ/129 heterozygotes; n = 11) and their littermate controls (129/129 homozygotes; n = 19) for various concentrations of saccharin, CaCl2, CaLa, and MgCl2. The 129.B6ByJ-Tas1r3 congenic mice carry a 194-kb chromosomal segment including Tas1r3 from the B6ByJ strain that is introgressed onto the 129 strain background. Dotted horizontal lines show indifference (50% preference).
Confirming earlier work (13), we found that mice with the 129/B6ByJ genotype at Tas1r3 had higher preference scores for several concentrations of saccharin than did mice with the 129/129 genotype [0.6, 2, 5, and 120 mM; strain × concentration interaction, F(11,297) = 5.29, P < 0.0001; Fig. 3]. According to one-sample t-tests, relative to indifference, mice with the 129/129 genotype significantly preferred 2–60 mM saccharin, whereas those with the 129/B6ByJ genotype significantly preferred 0.6–120 mM. Both genotype groups significantly avoided 200 mM saccharin.
Preference Scores of Tas1r3 Knockout Mice
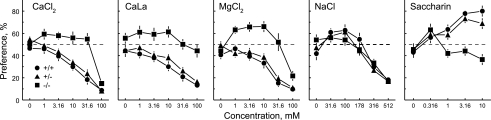
Relative to Tas1r3+/+ and +/− mice, Tas1r3−/− mice had significantly higher preference scores for all three divalent salts at all concentrations tested except for 100 mM MgCl2 [Fig. 4; group × concentration interactions: CaCl2, F(10,325) = 2.94, P = 0.0015; CaLa, F(10,325) = 2.05, P = 0.0278; MgCl2, F(10,325) = 3.55, P = 0.0002]. They had significantly lower preference scores for 1, 3.16, and 10 mM saccharin and did not differ from Tas1r3+/+ or +/− mice in preference scores for NaCl [group × concentration interactions; saccharin, F(8,260) = 6.13, P < 0.0001; NaCl, F(10,325) = 1.17, NS]. The preference scores of Tas1r3+/+ and +/− mice were always similar.
Fig. 4.
Preferences of Tas1r3 knockout (−/−; n = 14), heterozygous (+/−; n = 36), and wild type (+/+; n = 19) mice for various concentrations of 5 taste compounds. Dotted horizontal lines show indifference (50% preference).
The preference scores of Tas1r3−/− mice for some concentrations of calcium and magnesium were significantly above indifference (1 and 3.16 mM CaCl2, 1 and 10 mM CaLa, and 1, 3.16, and 10 mM MgCl2), but this was never the case for Tas1r3+/+ or +/− mice (Fig. 4).
Gustatory Electrophysiology of Tas1r3 Knockout Mice
There were significant differences between Tas1r3+/+ and −/− mice in the chorda tympani nerve response elicited by application of calcium to the oral cavity (Table 3). Responses to the CaCl2 and CaLa series were larger overall in the Tas1r3+/+ mice [F(1,14) = 4.70, P < 0.05 and F(1,14) = 28.2, P < 0.001, respectively]. There was a significant interaction between strain and concentration influencing the response to CaLa, F(1,14) = 12.0, P = 0.0038. Post hoc tests revealed differences between the strains at 100 mM CaLa but not at 10 mM CaLa.
Table 3.
Responses of the chorda tympani nerve evoked by oral application of various taste compounds in Tas1r3 +/+ and −/− mice
| Taste Solution | Tas1r3+/+ | Tas1r3−/− |
|---|---|---|
| 10 mM HCl | 0.93±0.13 | 0.66±0.04 |
| 20 mM QHCl | 0.74±0.07 | 0.64±0.05 |
| 500 mM Sucrose | 0.96±0.17 | 0.14±0.04* |
| 0.1 mM CaCl2 | 0.04±0.02 | 0.01±0.011 |
| 1 mM CaCl2 | 0.25±0.05 | 0.17±0.031 |
| 10 mM CaCl2 | 0.54±0.05 | 0.40±0.041 |
| 100 mM CaCl2 | 0.89±0.09 | 0.72±0.061 |
| 10 mM CaLa | 0.32±0.03 | 0.26±0.021 |
| 100 mM CaLa | 0.68±0.03 | 0.44±0.031* |
| 10 mM NaCl | 0.36±0.07 | 0.31±0.06 |
| 100 mM NaCl | 1.07±0.12 | 0.96±0.06 |
| 10 mM MgCl2 | 0.43±0.05 | 0.41±0.07 |
| 100 mM MgCl2 | 1.00±0.07 | 0.82±0.08 |
| 10 mM KCl | 0.30±0.03 | 0.24±0.05 |
| 100 mM KCl | 0.67±0.04 | 0.61±0.08 |
Values are means ± SE of 8 male mice/group. Each value represents the change in activity of the chorda tympani nerve during the 1st 10 s after the taste solution was applied to the tongue, relative to the previous 10 s, and is expressed relative to the response to 100 mM NH4Cl (i.e., NH4Cl response = 1.0).
Relative to the Tas1r3+/+ mice, the Tas1r3−/− mice had significantly lower responses overall to CaCl2, F(1,14) = 4.7, P < 0.05, and CaLa, F(1,14) = 28.2, P < 0.001.
P < 0.01 relative to response of wild-type mice according to least significant difference post hoc test.
As expected (7), the Tas1r3−/− mice also had strongly attenuated chorda tympani responses to sucrose relative to Tas1r3+/+ mice (Table 3). There were no differences between the two groups in the response of the chorda tympani nerve to HCl, QHCl, NaCl, KCl, or MgCl2. The similar responses of Tas1r3+/+ and −/− mice to HCl, quinine, and NaCl are consistent with the results of another study (7); KCl and MgCl2 have not been tested previously.
Tas1r3 Haplotype and Calcium Preference Scores
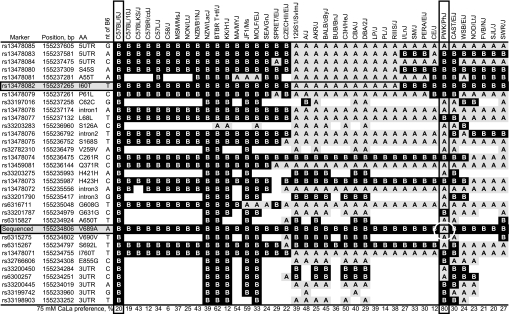
The linkage of calcium preference scores to Tas1r3 observed in the B6 × PWK F2 cross implies that the sequence of this gene should differ between the parental strains. Sequence analysis found 18 variant sites between B6 and PWK (Fig. 5). To distinguish which of these are likely functional polymorphisms, we extended the analysis to include the 40 strains that form the core of the Mouse Phenome Database. Nine of the 40 strains examined had Tas1r3 haplotypes identical to the C57BL/6J strain (C57BL/10J, C57BLKS/J, C57BR/cdJ, C57L/J, C58/J, MSM/MsJ, NON/LtJ, NZB/B1NJ, and NZW/LacJ), and two others (BTBR T+tf/J and KK/H1J) had the B6 haplotype at all except one SNP (rs33203283). A second haplotype group of 11 strains consisted of 129S1/SvImJ, A/J, AKR/J, BALB/cByJ, BUB/BnJ, C3H/HeJ, CBA/J, DBA/2J, LP/J, PL/J, and RIIIS/J (the A/J strain had one SNP that deviated from this haplotype). The 129P3/J strain, which was a parent for our Tas1r3 congenic mice but not a Mouse Phenome Database core strain, also had this haplotype. Two smaller haplotype groups were present (I/LnJ, SM/J, and PERA/EiJ; FVB/NJ, SJL/J, and SWR/J), and the remaining 11 strains had unique haplotypes.
Fig. 5.
Tas1r3 single nucleotide polymorphism (SNP) analysis of 40 inbred strains of mice. Left columns give each marker's name, position (MGI, Build 37), and effect on amino acid (AA) sequence, if any. Column labeled “nt of B6” shows nucleotide present in C57BL/6J strain. In body of table, B = same nucleotide as B6; A = alternative to B6 (i.e., other nucleotide); blank = no genotype available. Strains are arranged from left to right so as to display similarities. PWK/PhJ and PWD/Ph (not shown) have the same haplotype; 129S1/SvImJ and 129P3/J (not shown) have the same haplotype. The I60T substitution (at 155,237,265 bp; shaded) is believed to be primarily responsible for determining responsiveness of Tas1r3 to saccharin. The critical SNP at “Sequenced” (shaded) is unique to the PWK/PhJ and PWD/PhJ strains (highlighted with circle) and may thus be responsible for these strains high calcium preferences. Preference data for 75 mM CaLa in bottom row are extracted from Ref. 43.
The only SNP that was unique to the PWK and PWD/PhJ strains was in a region we sequenced at 155,234,806 bp (Fig. 5).
It has been suggested that an I60T amino acid substitution in Tas1r3 is most likely responsible for conferring high sweetener preference (i.e., B6) or low sweetener preference (i.e., 129 or DBA/2) to mice (30, 32). Based on genotype at the underlying SNP (rs13478082), there were no differences in calcium preferences of the 40 strains. For example, preferences for 25 mM CaCl2 of the 29 strains with B6 genotype at rs13478082 were 42 ± 2%, whereas those of the 11 strains with the alternate genotype were 37 ± 3%, t(38) = 0.90, P = 0.3727. However, we noticed that the B6 genotype group included seven “wild-derived” strains, whereas the other genotype group contained none. If only the “common” laboratory strains (which are primarily of Mus musculus domesticus origin) were included in the comparison, significant differences were present [e.g., 25 mM CaCl2 preference, B6 genotype = 31 ± 3% (n = 22), alternate genotype = 42 ± 2% (n = 11); t(31) = 2.25, P = 0.0319].
Relationship Between Preference Scores for Calcium and Sweetness
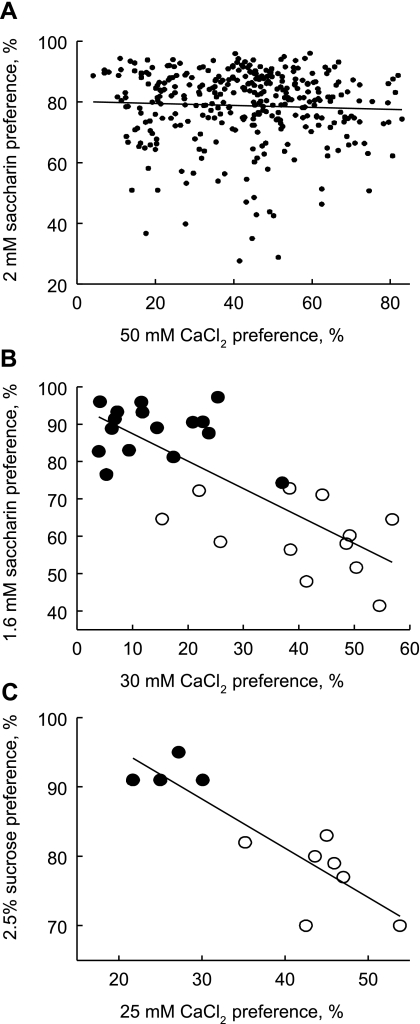
In B6 × PWK F2 mice, there were moderately strong and highly significant correlations between preference scores for CaCl2 and CaLa (r = 0.63), and between preference scores for each of these salts and MgCl2 (r = 0.68 and r = 0.67, respectively). However, there was no reliable association between preference scores for the divalent salts and for saccharin. The correlations of preference scores for 2 mM saccharin with preference scores for 50 mM CaCl2, CaLa, and MgCl2, were r = −0.12, r = −0.05, and r = 0.05, respectively (see Fig. 6A for an example).
Fig. 6.
Scatter plots showing relationship between preferences for calcium and sweeteners in: (A) 480 B6 × PWK F2 hybrid mice (r = −0.05, not significant); (B) males of 28 inbred strains with 30 mM CaCl2 preference from Ref. 1 and 1.6 mM saccharin preference from Ref. 32 (r = −0.77, P < 0.00001); (C) males of 11 strains with 25 mM CaCl2 preference from Ref. 44 and 2.5% sucrose preference from Fig. 2 of Ref. 16 (r = −0.87, P = 0.0005). •, B6 genotype at rs13478082, which is believed to confer high sweet preference; ○, alternate genotype at rs13478082.
Data from previously published experiments and the Mouse Phenome Database revealed several significant inverse correlations between preference scores for calcium and sweeteners among inbred mouse strains. Based on comparison of 28 strains tested by our group (1, 32), the correlation between preference scores for 30 mM CaCl2 and 1.6 mM saccharin was r = −0.77 (P < 0.00001; Fig. 6B). Based on a comparison of calcium preference scores from our group with sucrose preference scores published by Lewis et al. (16), the correlation between preference scores for 25 mM CaLa and 2.5% sucrose was r = −0.87 (P = 0.0005, n = 11 strains; Fig. 6C). Correlations derived from comparison of Mouse Phenome Database projects 103 and 256 (39) were available for each sex separately from 22 strains: For males, the associations between preference scores for 0.2% (∼10 mM) saccharin and 75 mM CaCl2 were r = −0.68 (P = 0.0005) and between 0.2% saccharin and 25 mM CaCl2 were r = −0.55 (P = 0.0080). For females, the corresponding correlations were r = −0.54 (P = 0.0095) and r = −0.52 (P = 0.0131). Finally, the correlations involving Mouse Phenome Database projects 61 and 256 involved male mice from 13 strains. The association between preference scores for 0.2% saccharin and 30 mM CaCl2 was r = −0.63 (P = 0.0210), and between 0.2% saccharin and 10 mM CaCl2 was r = −0.60 [P = 0.0302; scatter plots of these data can be generated on the Mouse Phenome Database website (39)].
In all eight of the comparisons between calcium and sweetener preference scores across strains, it was clear from the scatter plots (including Fig. 6, B and C) that different inbred strains segregated into two groups depending on genotype at rs13478082, the SNP responsible for I60T. One group of strains had high preference scores for sweeteners and low preference scores for calcium; the other group of strains had low preference scores for sweeteners and high preference scores for calcium. It is noteworthy that nearly all the mice in these strain comparisons were domesticus strains. None of the comparisons included the PWK strain, which has high preference scores for both sweeteners and calcium (Fig. 1).
DISCUSSION
The impetus for this work was the observation that the PWK strain of mice has higher preference scores for calcium solutions than do most other strains (43, 44). We found that this “calcium appetite” of the PWK strain encompasses preferences for CaCl2, CaLa, and MgCl2 but not representative solutions of bitter, sour, salty, or umami taste qualities. A genome screen of B6 × PWK F2 mice revealed QTLs responsible for the variation in calcium and magnesium preference scores on distal chromosome 4. There was also linkage at this locus to saccharin preference scores, which raised the possibility that Tas1r3 could be involved because this gene is located on distal chromosome 4, and its protein product, T1R3, is a component of a sweet taste receptor. Our finding that mice with a targeted disruption of Tas1r3 have higher preference scores for calcium and magnesium than do Tas1r3+/+ and +/− littermates argues that in the B6 wild-type mouse, T1R3 normally acts to reduce calcium and magnesium preference. Our finding that 129.B6ByJ-Tas1r3 congenic mice differed from their 129/129 littermates in calcium and magnesium preference scores argues that functional variants of Tas1r3 influence preference for the divalent ions.
The QTLs underlying calcium, magnesium, and saccharin preference scores had a common peak at rs13478075 (155.2 Mb), a SNP in Tas1r3. There are 72 known and predicted genes in the ∼1.8-Mb region between the two markers flanking this peak (D4Mit209 at 153.8 Mb and rs33244176 at 155.6 Mb), with Tas1r3 being the only gene that has a known function in taste transduction. The congenic segment of 129.B6ByJ-Tas1r3 mice is <194 kb and contains Tas1r3 and 11 other genes (Ccnl2, Aurkaip1, Mxra8, Dvl1, Gltpd1, Cpsf31, Pusl1, Centb5, Ube2j2, C1qdc2, and one predicted gene), most of which are involved in cell division and differentiation (see Ref. 2). The Tas1r3−/− mice were produced by homologous recombination of B6 stem cells, which confines the genetic lesion to Tas1r3. It is feasible but unlikely that different genes underlie the QTL, the congenic mouse phenotype, and the knockout mouse phenotype. Instead, the most parsimonious explanation is that Tas1r3 is responsible for each of them.
These are the first functional data to implicate T1R3 in the detection of calcium but they are in line with structural models. Calcium binds to related G proteins, including the γ-aminobutyric acid-B receptor, the metabotropic glutamate receptors, and the calcium-sensing receptor CaSR (review: Ref. 5), and a model predicts that a calcium-binding site is present in the Venus fly-trap domain of T1R3 (37). PWK, 129.B6ByJ-Tas1r3, and Tas1r3−/− mice had altered preferences for both calcium and magnesium relative to controls, which suggests that T1R3 is sensitive to both these cations. At least one other G protein-coupled receptor, CaSR, is sensitive to both divalent cations, although, in this case, the response to calcium is stronger than that to magnesium (review Ref. 5). A common transduction mechanism for calcium and magnesium is also supported by the significant correlations observed between preference scores for CaCl2, CaLa, and MgCl2 in B6 × PWK F2 mice, the strong cross-adaptation of calcium and magnesium salts in rats (38), and the existence of gustatory afferent nerve fibers in mice that respond more strongly to calcium and magnesium than to other taste stimuli (31).
Electrophysiological recordings from the chorda tympani, a peripheral gustatory nerve, showed that Tas1r3 knockout mice have attenuated responses to CaCl2 and 100 mM CaLa relative to wild-type littermate controls. Since calcium at the concentrations used is disliked by most rodents, this raises the hypothesis that Tas1r3 knockout mice have a reduced aversion for calcium because they cannot easily detect it. However, this cannot be the entire explanation because Tas1r3 knockout mice drank more of some concentrations of CaCl2, CaLa, and MgCl2 than water. This was similar to, although not as strong as, the preferences shown by the PWK strain, and was in marked contrast to Tas1r3+/+ and +/− mice, and the B6 strain, which did not prefer any concentration of calcium or magnesium salts to water. The observation of greater intake of CaCl2, CaLa, and MgCl2 than water by Tas1r3−/− mice is important because it demonstrates that they were competent to detect these salts. It implies that there must be at least one other transduction element that is responsible for giving calcium a positive hedonic valence, and that in the wild-type B6 mouse this is normally masked by the action of Tas1r3.
The SNP rs13478082 underlies an I60T amino acid substitution in the extracellular NH2 terminus of T1R3 and has been implicated as responsible for strain differences in sweetener preference (9, 32). Our observation that there is an inverse relationship between preference scores for calcium and sweeteners among inbred strains of primarily domesticus mice (Fig. 6, B and C, for examples) suggests that this SNP may also influence calcium preference. However, there are several reasons to believe that rs13478082 is not the only SNP influencing the function of Tas1r3 (see Ref. 32). Most pertinently, saccharin preference differed between the B6 and PWK strains and was linked to Tas1r3 in B6 × PWK F2 mice, yet the two strains have identical genotypes at rs13478082. Moreover, there was no association between saccharin and calcium preference scores in B6 × PWK F2 mice (Fig. 6A), and the PWK parental strain had higher preferences for both calcium and saccharin than did the B6 strain. There were 18 SNPs in Tas1r3 between the B6 and PWK strains, of which five were nonsynonymous (underlying S126A, Q371R, A650T, V689A, and S962L). Although any of these could potentially influence saccharin and calcium preferences, the SNP that underlies V689A was particularly noteworthy because it was unique to the PWK strain. This SNP is located in the 4th transmembrane domain of the protein, which in other G protein-coupled receptors has been implicated in ligand binding [e.g., Refs. 3, 10) and can also be a dimer interface (e.g., Ref. 12)]. Thus, it is a good candidate to have functional significance and consequently may account for the PWK strain's strong preferences for calcium and magnesium. Whether it also accounts for the PWK strain's strong saccharin preference is unknown.
rs13478082 was initially implicated as a source of strain differences in sweetener preferences based on genotype-phenotype associations in a panel of 30 strains of which 28 were domesticus. The inverse association between preference scores for saccharin and calcium is also based primarily on domesticus strains, and rs13478082 genotype influenced calcium preference scores of inbred strains only if wild-derived strains were excluded from the comparison. Based on these considerations, we speculate that the I60T substitution in Tas1r3 confers linked “sweet-liker” and “calcium-avoider” status to domesticus mice, but this is negated by one or more mutations in other regions of Tas1r3 that occurred in a common ancestor to the castaneus, molossinus, and musculus subspecies (which includes the PWK strain) after these diverged from the domesticus subspecies. Genotype-phenotype association analyses based on 30 or 40 strains have insufficient statistical power to distinguish multiple functional polymorphisms so to confirm this and to identify additional loci influencing saccharin and calcium preference will require other approaches, such as heterologous expression assays coupled with directed mutagenesis.
Irrespective of the specific polymorphisms involved, the present findings raise intriguing questions about the connection between the perception of sweetness and calcium. There is little cross-adaptation or generalization between calcium and sweeteners in discrimination studies (19, 27, 34, 38, 41), and calcium deprivation increases preferences for calcium but decreases preferences for sweet compounds (6, 45). Moreover, in unpublished work, we have been unable to demonstrate generalization of a conditioned taste aversion between calcium and sweetness in either B6 or PWK mice. Thus, it seems highly unlikely that mammals in general, or PWK mice in particular, confuse the taste of calcium with sweetness.
How can one receptor, T1R3, contribute to the perception of compounds with different taste qualities? In vitro studies demonstrate that T1R3 dimerizes with T1R2 to produce a receptor, which recognizes sugars and other sweeteners and dimerizes with T1R1 to produce a receptor that responds to L-amino acids and thus functions as a detector of umami taste (8, 18, 21, 26, 28, 29, 47). T1R1 and T1R2 show little coexpression in taste receptor cells, which allows them to influence different sets of neurons in the periphery and brain and thus provide differential signals for sweet and umami compounds. We propose that T1R3 dimerizes with another G protein-coupled receptor to produce a functional receptor for calcium and magnesium, presumably in different receptor cells than those expressing T1R1 or T1R2 and thus activating a gustatory pathway distinct from those for sweet and umami taste. One attractive possibility is that the PWK form of V689A allows T1R3 to dimerize with the calcium-sensing receptor, CaSR, but there is no experimental support for this.
Our behavioral studies used long-term tests to assess taste preferences, and it is therefore possible that postingestive effects contributed to these results. Our gustatory electrophysiological study demonstrates that T1R3-mediated calcium transduction can occur in the mouth, but this does not rule out actions elsewhere. T1R3 is found in several sites besides the tongue, most notably in the gastrointestinal tract (e.g., Ref. 20). It remains to be seen whether alleles of Tas1r3 influence the absorption or metabolism of calcium or magnesium.
Although behavioral and electrophysiological results were largely congruent, there was one discrepancy: Relative to wild-type controls, Tas1r3 knockout mice had elevated MgCl2 preference scores but similar chorda tympani responses to MgCl2. The reason for this is unknown. Perhaps the electrophysiological response elicited by T1R3 receptors was masked by neural activity produced by other taste components, such as strong bitterness, that are elicited by MgCl2 but not by CaCl2 or CaLa. This is consistent with several findings that support the existence of calcium- and magnesium-specific transduction elements in addition to T1R3. For example, several mouse strains differ in calcium preference despite identical Tas1r3 sequences (Fig. 5). Moreover, some QTLs found in the B6 × PWK F2 genome screen were unique to CaCl2 preference scores (Drinkcacl23, Drinkcacl25) or MgCl2 preference scores (Drinkmgcl25) (46). It is very likely that multiple receptor mechanisms are involved in the detection of calcium and magnesium, as is the case for most other, perhaps all, taste stimuli. T1R3 is the first receptor to be implicated in mammalian calcium and magnesium gustation but it is unlikely to be the only one.
GRANTS
Supported by NIH Grants DK-46791 (M. G. Tordoff) and DC-00882 (A. A. Bachmanov).
DISCLOSURES
Dr. Margolskee has a personal financial interest in the form of stock ownership in the Redpoint Bio company, receives consulting fees from Redpoint Bio, and is an inventor on patents and patent applications that have been licensed to Redpoint Bio.
Acknowledgments
Genotyping services were provided by the Center for Inherited Disease Research, which is funded through National Institutes of Health (NIH) contract NO1-HG-65403. We thank Dr. Natalia Bosak for genotyping the 129.B6ByJ-Tas1r3 congenic mice. Excellent technical assistance was provided by Diane Pilchak, Fred Ollinger, Maureen Lawler, Stephanie Craw, Sara Lehmann, and Maria Theodorides.
Address for reprint requests and other correspondence: M. G. Tordoff, Monell Chemical Senses Center, 3500 Market St., Philadelphia, PA 19104-3308 (e-mail: tordoff@monell.org).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2 and NH4Cl solutions by 28 mouse strains. Behav Genet 32: 445–457, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucard AA, Sauve SS, Guillemette G, Escher E, Leduc R. Photolabelling the rat urotensin II/GPR14 receptor identifies a ligand-binding site in the fourth transmembrane domain. Biochem J 370: 829–838, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broman KW, Wu H, Sen S, Churchill G. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. Am J Physiol Regul Integr Comp Physiol 271: R1–R10, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. [DOI] [PubMed] [Google Scholar]
- 8.DuBois GE Unraveling the biochemistry of sweet and umami tastes. Proc Natl Acad Sci USA 101: 13972–13973, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller JL Single-locus control of saccharin preference in mice. J Hered 65: 33–36, 1974. [DOI] [PubMed] [Google Scholar]
- 10.Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem 268: 23116–23121, 1993. [PubMed] [Google Scholar]
- 11.Gregorova S, Forejt J. PWD/Ph and PWK/Ph inbred mouse strains of Mus M. musculus subspecies–a valuable resource of phenotypic variations and genomic polymorphisms. Folia Biol (Praha) 46: 31–41, 2000. [PubMed] [Google Scholar]
- 12.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem 278: 4385–4388, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics 32: 82–94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue M, Tordoff MG. Calcium deficiency alters chorda tympani nerve responses to oral calcium chloride. Physiol Behav 63: 297–303, 1998. [DOI] [PubMed] [Google Scholar]
- 15.International Committee on Standardized Genetic Nomenclature for Mice. Rules and Guidelines for Nomenclature of Mouse and Rat Strains: MGI, 2007. (http://www.informatics.jax.org/mgihome/nomen/strains.shtml#ioi).
- 16.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav 85: 546–556, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mamm Genome 12: 13–16, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim J, Lawless HT. Qualitative differences of divalent salts: multidimensional scaling and cluster analysis. Chem Senses 30: 719–726, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, and Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001. [DOI] [PubMed] [Google Scholar]
- 22.McCaughey SA, Forestell CA, Tordoff MG. Calcium deprivation increases the palatability of calcium solution in rats. Physiol Behav 84: 335–342, 2005. [DOI] [PubMed] [Google Scholar]
- 23.McCaughey SA, Tordoff MG. Calcium-deprived rats sham-drink CaCl2 and NaCl. Appetite 34: 305–311, 2000. [DOI] [PubMed] [Google Scholar]
- 24.McCaughey SA, Tordoff MG. Calcium deprivation alters gustatory-evoked activity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 281: R971–R978, 2001. [DOI] [PubMed] [Google Scholar]
- 25.McCaughey SA, Tordoff MG. Magnesium appetite in the rat. Appetite 38: 29–38, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–498, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Morrison GR Behavioral response patterns to salt stimuli in the rat. Can J Psychol 21: 141–152, 1967. [Google Scholar]
- 28.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol 15: 1948–1952, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ninomiya Y, Tonosaki K, Funakoshi M. Gustatory neural response in the mouse. Brain Res 244: 370–373, 1982. [DOI] [PubMed] [Google Scholar]
- 32.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci 24: 938–946, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter CP, Eckert JF. Mineral appetite of parathyroidectomized rats. Am J Med Sci 9: 9–16, 1932. [Google Scholar]
- 34.Schiffman SS, Erickson RP. A psychophysical model for gustatory quality. Physiol Behav 7: 617–633, 1971. [DOI] [PubMed] [Google Scholar]
- 35.Schulkin J Calcium Hunger. Cambridge: Cambridge University Press, 2001.
- 36.Shao H, Reed DR, Tordoff MG. Genetic loci affecting body weight and fatness in a C57BL/6J × PWK/PhJ mouse intercross. Mamm Genome 18: 839–851, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silve C, Petrel C, Leroy C, Bruel H, Mallet E, Rognan D, Ruat M. Delineating a Ca2+ binding pocket within the Venus flytrap module of the human calcium-sensing receptor. J Biol Chem 280: 37917–37923, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Smith DV, Frank M. Cross adaptation between salts in the chorda tympani nerve of the rat. Physiol Behav 8: 213–220, 1972. [DOI] [PubMed] [Google Scholar]
- 39.The Jackson Laboratory. Mouse Phenome Database web site, 2001. http://www.jax.org/phenome.
- 40.Tordoff MG Calcium: taste, intake and appetite. Physiol Rev 81: 1567–1597, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Tordoff MG Some basic psychophysics of calcium salt solutions. Chem Senses 21: 417–424, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Tordoff MG, Bachmanov AA. Monell mouse taste phenotyping project: Monell Chemical Senses Center, 2001. (www.monell.org/MMTPP).
- 43.Tordoff MG, Bachmanov AA. Survey of calcium and sodium intake and metabolism with bone and body composition data (MPD:103): Mouse Phenome Project, 2002. (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&id=103).
- 44.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol Behav 91: 632–643, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tordoff MG, Rabusa SH. Calcium-deprived rats avoid sweet compounds. J Nutr 128: 1232–1238, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Tordoff MG, Reed DR, Shao H. Calcium taste preferences: Genetic analysis and genome screen of C57BL/6J × PWK/PhJ hybrid mice. Genes Brain Behav 18: 839–851, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]