Abstract
Integrin-mediated cell adhesion to extracellular matrices provides signals essential for cell cycle progression and differentiation. We demonstrate that substrate-dependent changes in the conformation of adsorbed fibronectin (Fn) modulated integrin binding and controlled switching between proliferation and differentiation. Adsorption of Fn onto bacterial polystyrene (B), tissue culture polystyrene (T), and collagen (C) resulted in differences in Fn conformation as indicated by antibody binding. Using a biochemical method to quantify bound integrins in cultured cells, we found that differences in Fn conformation altered the quantity of bound α5 and β1 integrin subunits but not αv or β3. C2C12 myoblasts grown on these Fn-coated substrates proliferated to different levels (B > T > C). Immunostaining for muscle-specific myosin revealed minimal differentiation on B, significant levels on T, and extensive differentiation on C. Differentiation required binding to the RGD cell binding site in Fn and was blocked by antibodies specific for this site. Switching between proliferation and differentiation was controlled by the levels of α5β1 integrin bound to Fn, and differentiation was inhibited by anti-α5, but not anti-αv, antibodies, suggesting distinct integrin-mediated signaling pathways. Control of cell proliferation and differentiation through conformational changes in extracellular matrix proteins represents a versatile mechanism to elicit specific cellular responses for biological and biotechnological applications.
INTRODUCTION
The adhesion of cells to their substrate through an extracellular matrix provides signals that influence their ability to survive, proliferate, and express specific developmental phenotypes (Menko and Boettiger, 1987; Werb et al., 1989; Adams and Watt, 1990; Streuli et al., 1991; Zhu et al., 1996; Chen et al., 1997). One of the early examples was the development of in vitro culture conditions that permitted the differentiation of avian myogenic cells into contracting myotubes (Hauschka and Konigsberg, 1966; Bischoff and Holtzer, 1968). The critical element for this system was the precoating of tissue culture surfaces with rat tail collagen. This general principle of providing an appropriate substrate to permit the expression of developmental phenotypes has been applied to a wide variety of cells. These include systems that allow the maintenance of neurons and outgrowth of growth cones (Westerfield, 1987) and the recapitulation of the stages of mammary gland development and involution (Li et al., 1987; Barcellos-Hoff et al., 1989). These findings indicate that critical elements of the message directing the expression of a differentiated phenotype are encoded in the extracellular matrix.
Cells interact with extracellular matrices primarily through integrins, a widely expressed family of cell surface receptors (Hynes, 1987), and integrin binding to its extracellular ligand is responsible for the downstream effects of the matrix on cell function. For example, in the muscle differentiation system, antibodies to β1 integrin reversibly block differentiation and retain cells in a proliferating state (Menko and Boettiger, 1987). This fundamental principle of regulation of developmental phenotype through binding of integrin receptors has been demonstrated for a variety of other systems, including mammary (Streuli et al., 1991) and kidney (Sorokin et al., 1990) epithelial cells and keratinocytes (Adams and Watt, 1990). This interaction is governed by the surface densities of integrin receptors and their ligands and the receptor–ligand binding affinities. Integrin receptors undergo changes in conformation in response to intracellular signals that are capable of modulating their ligand binding affinity (Shattil et al., 1985). This modulation of integrin binding has been shown to play roles in epithelial and muscle differentiation (Adams and Watt, 1990; Boettiger et al., 1995).
Fibronectin (Fn)1 is one of the most intensively studied components of the extracellular matrix, particularly in terms of its effects on cells. Fn plays a central role in the adhesion of many cell types to extracellular matrices and artificial substrata, including tissue culture plastic dishes. Fn is an essential component for normal development, and Fn knockout mice fail to develop beyond embryonic day 10 or 11 (George et al., 1993). The Fn molecule is folded into globular domains specialized for particular functions, such as binding to integrins, collagen, heparan sulfate, hyaluronic acid, and itself to form self-assembled fibrils (Engvall and Ruoslahti, 1977; Hayman et al., 1982; Laterra et al., 1983; Morla and Ruoslahti, 1992). Fn exhibits multiple, complex interactions both in vitro and in vivo. Upon adsorption to surfaces, Fn undergoes conformational changes that affect its biological activity (Grinnell and Feld, 1981; Iuliano et al., 1993; Underwood et al., 1993; Pettit et al., 1994; García et al., 1998a). For example, Grinnell and Feld (1981, 1982) demonstrated that Fn adsorbed onto tissue culture polystyrene supports higher cell-spreading rates and Fn antibody binding compared with bacterial polystyrene. In vivo, Fn is found in many sites of extracellular matrix deposition and in association with different matrix components (Hynes, 1990). In addition, it is expressed in different splice variants (Norton and Hynes, 1987), and recent evidence suggests that these variants affect the conformation of the molecule and modulate its interaction with other proteins (Manabe et al., 1997). Thus, its role as an adapter molecule for binding different elements in the extracellular space may be analogous to the growing collection of adapter molecules, such as Grb2 and cas, which are thought to participate in intracellular signaling pathways (Schlaepfer et al., 1997).
In this study, we demonstrate that Fn adsorption onto different surfaces results in conformational changes that lead to differences in integrin receptor binding and modulate the switch between cell proliferation and myogenic differentiation. This demonstrates that the conformation of the extracellular matrix ligand, like the conformation of the integrin receptor, can be modified to regulate the integrin–ligand interaction and integrin-mediated signaling. This may be particularly important in the case of Fn because of the large variety of processes that it controls, its widespread expression in different tissues, and its ability to associate with a variety of other extracellular molecules. In addition, control of integrin-ligand interactions and signaling through substrate-dependent conformational changes in the extracellular matrix represents a versatile approach to manipulate cellular responses in biomaterial and tissue engineering applications.
MATERIALS AND METHODS
Cells and Reagents
Mouse C2C12 myoblasts (ATCC CRL-1772) were kindly provided by C. Emerson (University of Pennsylvania) and grown in Dulbecco’s modified Eagle’s medium (DMEM), 15% FBS, and 1% penicillin-streptomycin. Human IMR-90 fibroblasts (ATCC CCL-186) were grown in DMEM, 10% FBS, and antibiotics. Fn- and vitronectin-depleted serum was prepared by sequential affinity chromatography through gelatin, Fn antibody, and glass columns. Human plasma Fn and tissue culture reagents were obtained from Life Technologies (Grand Island, NY). Bacterial (B, number 1007; Falcon, Lincoln Park, NJ) and tissue culture grade (T, number 25000; Corning, Corning, NY) polystyrene plates were used. Collagen (C) plates were prepared by drying 0.1% collagen type I (Vitrogen-100; Celtrix Laboratories, Palo Alto, CA) from a dilute acetic acid solution onto T plates. Ethidium homodimer was obtained from Molecular Probes (Eugene, OR). All other reagents were obtained from Sigma (St. Louis, MO).
Antibodies
HFN7.1 and MF20 hybridomas were obtained from American Type Culture Collection (Manassas, VA). HFN7.1 antibody was affinity purified on a protein G-Sepharose column. Adhesion-blocking polyclonal antibody against Fn was obtained from Cappel (Durham, NC). mAbs 3E1 and 4B2 were purchased from Life Technologies. Adhesion-blocking hamster anti-mouse integrin α5 and αv mAbs were obtained from Pharmigen (San Diego, CA). For Western blotting, polyclonal antibodies against α5, αv, and β3 integrin subunits were purchased from Chemicon (Temecula, CA), whereas antibodies against α3 and β1 were raised in this laboratory by standard procedures (Enomoto-Iwamoto et al., 1993). Alkaline phosphatase–conjugated antibodies were obtained from Jackson ImmunoResearch (West Grove, PA).
Characterization of Fn Adsorption and Conformation
Lyophilized Fn was reconstituted with sterile distilled H2O to 1 mg/ml. Substrates (B, T, and C) were coated with Fn diluted in Dulbecco’s PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, 1.5 mM KH2PO4, 0.9 mM CaCl2·2H2O, 1 mM MgCl2·6H2O, pH 7.4) for 30 min at 22°C and blocked in 1% BSA for 30 min. Adsorbed Fn for different coating concentrations was measured using Fn iodinated with the Bolton–Hunter reagent (DuPont NEN, Boston, MA).
The conformation of Fn adsorbed onto the different substrates was examined by a modified ELISA. Ninety-six–well plates were coated with Fn, blocked in blocking buffer (Dulbecco’s PBS, 0.25% BSA, and 0.05% Tween 20) for 1 h, and incubated in anti-Fn antibodies (1:1000 dilution) for 1 h at 37°C. After washing, wells were incubated in alkaline phosphatase–conjugated anti-mouse immunoglobulin G (1:4000) for 1 h at 37°C. Substrate (4-methyl-umbelliferyl-phosphate, 60 μg/ml) was then incubated for 15 min. Reaction products from the different substrates were transferred to a clean plate, and fluorescence was read in a microwell plate reader (365-nm excitation, 450-nm emission; Dynatech, Alexandria, VA).
Integrin Binding Analysis
Bound integrins were analyzed using a modification of the biochemical method of Enomoto-Iwamoto et al. (1993). Briefly, IMR-90 cells were plated (6400 cells/cm2) overnight in DMEM and antibiotics on dishes coated with 10 μg/ml Fn and blocked in 1% BSA. Cells were washed three times in Dulbecco’s PBS and incubated in 1 mM cell-impermeable sulfo-BSOCOES cross-linker (Pierce, Rockford, IL) for 15 min at 4°C. After quenching unreacted cross-linker with 50 mM Tris, cells were extracted in 0.1% SDS, 350 μg/ml PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Proteins cross-linked to the dish were recovered by reversing the cross-linking in 50 mM NaHCO3 (pH 11.6) and 0.1% SDS at 37°C for 2 h and concentrated by size exclusion filtration (Microcon 30; Amicon, Bervely, MA). Recovered integrins were separated by SDS-PAGE (7% acrylamide gels) and transferred to polyvinylidene difluoride membranes using a Xcell Mini-Cell (Novex, San Diego, CA). Integrins were quantified by Western blotting with alkaline phosphatase–conjugated secondary antibodies and ECF substrate (Amersham, Arlington Heights, IL) using the Storm fluorescence imaging system (Molecular Dynamics, Sunnyvale, CA). Soluble fractions were used as positive controls, and soluble fractions for β1 were used to normalize for differences in cell number among substrates.
Normalized intensities for bound integrins were computed using the formula: intensity = (signal − background)/background. For each integrin subunit, differences in integrin binding among substrates were analyzed using ANOVA and Scheffé’s test for pairwise comparisons.
For immunofluorescent staining, IMR-90 cells were plated on Fn-coated substrates as described above. Parallel plates were washed in Dulbecco’s PBS, cross-linked using sulfo-BSOCOES, and either extracted with 0.1% SDS or permeabilized with 1% Triton X-100. Integrins were then stained using polyclonal antibodies (1:50) directed against integrin subunits followed by fluorescein-conjugated secondary antibody (1:50).
Muscle Cell Differentiation Assay
Substrates (35-mm2 dishes) were coated with 10 μg/ml Fn and blocked in BSA. C2C12 cells (1500 cells/cm2) were grown in DMEM, 0.1% FBS, 1% penicillin-streptomycin and 6 μg/ml insulin. After 3 d, cells were fixed in 70% ethanol:37% formaldehyde:glacial acetic acid (20:2:1) for 10 min and blocked in 5% horse serum for 1 h. Cells were stained for myosin and DNA with MF20 hybridoma supernatant (1:5 dilution) and ethidium homodimer (1 μg/ml) for 1 h. Plates were then incubated in fluorescein-conjugated anti-mouse immunoglobulin G (1:50) for 1 h. Substrates were scored for percent nuclei positive for myosin (200–300 cells were counted for each plate) and analyzed using ANOVA and Scheffé’s test for pairwise comparisons. Measurements represent percentage of attached and spread cells expressing sarcomeric myosin.
For blocking experiments, cells were seeded onto Fn-coated substrates in the presence or absence of different concentrations of antibodies specific for either Fn or integrin subunits. Experiments with Fn antibodies were performed in Fn-depleted serum. After 3 d in culture, cells were stained and scored as described above. For experiments designed to test the reversibility of the anti-α5 block on differentiation, cells were cultured on Fn-coated C in the presence or absence of α5-specific mAbs. After 3 d, cells were washed and cultured in fresh media with and without anti-α5 for an additional 3 d. Cells were stained and scored as before.
RESULTS
Substrate-dependent Changes in the Conformation of Adsorbed Fn
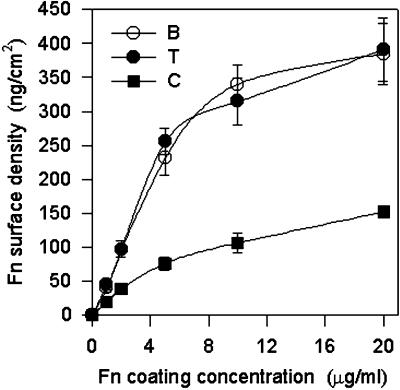
Fn adsorbs and mediates cell adhesion to a variety of natural and synthetic substrates (Klebe et al., 1981). Surfaces routinely used for Fn adsorption and cell adhesion studies include bacterial and tissue culture grade polystyrenes and type I collagen-coated plates. Bacterial or untreated polystyrene (referred to below as B) is highly hydrophobic, whereas tissue culture grade polystyrene (T) has been surface treated to present a negative charge and reduce hydrophobicity. Adsorption of purified Fn to these synthetic surfaces was measured using 125I-Fn. Adsorption increased linearly up to a coating concentration of 10 μg/ml, at which it reached saturation values (Figure 1). There were no significant differences in adsorbed Fn density between B and T, and these values are in agreement with previous measurements (Grinnell and Feld, 1981). Saturation levels of 350–400 ng/cm2 represent approximately the amount of Fn necessary to produce a monolayer coating based on the dimensions of the molecule (Williams et al., 1982). The binding of Fn to type I collagen (C) saturates at about one-third of the levels for the polystyrenes (Figure 1). This lower saturation limit reflects the binding of Fn to specific domains on collagen, whereas other areas of the substrate are blocked by regions on collagen that do not bind Fn.
Figure 1.
Fn adsorption (mean ± SD; three separate experiments in duplicate) as a function of coating concentration for different substrates. Surfaces were coated with different concentrations of Fn for 30 min and blocked in 1% BSA for 30 min. Adsorption of 125I-Fn increased linearly with coating concentration until saturation levels reached ∼10 μg/ml.
Protein adsorption to surfaces involves multiple electrostatic, hydrophobic, hydrogen bonding, and van der Waals interactions. The adsorption of Fn onto the synthetic polystyrenes is relatively nonspecific, and it is expected to occur with Fn molecules in different orientations relative to the surface. Because Fn adsorption onto either B or T is essentially irreversible, this process presumably involves changes in the conformation, or partial denaturation, of Fn. It is likely that only a portion of the adsorbed molecules will display any particular epitope in a position that is accessible to antibody binding. For Fn molecules in which the cell binding domain is exposed, the average conformation of this domain could be influenced by the surface properties (charge and hydrophobicity) of the underlying substrate. For instance, because the binding domain for α5β1 integrin in Fn involves recognition sites on both the 9th and 10th type III repeats (Pierschbacher et al., 1981; Aota et al., 1994), which are connected by a flexible linkage (Leahy et al., 1996), the relative orientation of these domains could be altered by the physicochemical properties of the surface.
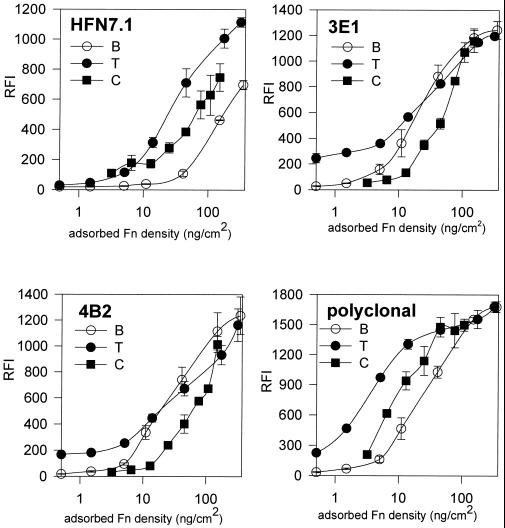
We used a modified ELISA to compare the adsorption of Fn to uncharged, bacterial (B; Figure 2, open circles) and charged, tissue culture (T; Figure 2, closed circles) polystyrenes using a polyclonal and three mAbs specific for distinct epitopes in Fn. HFN7.1 is directed against an epitope that lies between the PHSRN synergy and RGD sites (Bowditch et al., 1991) and blocks cell adhesion to Fn (Schoen et al., 1982). 3E1 reacts with the C-terminal heparin binding domain, and 4B2 binds near the gelatin binding site (Pierschbacher et al., 1981). In Figure 2, the x-axis has been normalized to the amount of adsorbed Fn based on the adsorption profiles from Figure 1. The y-axis is proportional to the amount of antibody bound. The mAbs 3E1 and 4B2 showed no significant differences between B and T in the amount of adsorbed Fn required for 50% saturation binding for each antibody. These data suggest that these epitopes are not affected differentially in the adsorption to these surfaces. In contrast, for HFN7.1, ∼10 times more Fn is required to bind the same amount of antibody for Fn adsorbed to B compared with Fn on T. This difference implies that the average binding affinity of HFN7.1 for its epitope in Fn adsorbed to B is significantly less than that for Fn on T. We interpret this difference in binding affinity to reflect a difference in the average conformation of Fn adsorbed to B compared with Fn adsorbed to T. The polyclonal antibody also exhibited significant differences (10-fold) in binding between Fn adsorbed to B and T, suggesting that changes in the conformation of adsorbed Fn extend outside the epitope for HFN7.1.
Figure 2.
Antibody binding assay for Fn conformation. Relative fluorescent intensity (RFI, mean ± SD; n = 3) for antibody binding as a function of adsorbed Fn surface density is shown. Different anti-Fn antibodies were examined: HFN7.1, 3E1, 4B2, and Cappel polyclonal antibody. Antibody binding increased sigmoidally with the log of Fn surface density. Shifts in antibody binding profiles represent variations in binding efficiency and reflect differences in the conformation of Fn adsorbed to the different substrates.
To provide one step toward a more physiological context for Fn, we analyzed the binding of these antibodies to collagen-bound Fn (Figure 2). The binding kinetics for HFN7.1 and the polyclonal antibody to Fn adsorbed to collagen (C; Figure 2, closed squares) were distinct from the binding to Fn adsorbed to either polystyrene surface, and the binding was consistently lower than the binding to Fn on T. Based on the rationale developed above, we conclude that the average conformation of Fn adsorbed to collagen is distinct from that on B or T. Because the antisera were made to soluble Fn (Pierschbacher et al., 1981; Schoen et al., 1982), their lower affinities for Fn adsorbed to C suggest that the conformation of soluble Fn is also altered in the process of binding to collagen. In this assay, it is possible that some epitopes are inaccessible because of the orientation of the bound Fn. However, masking of epitopes by the substrate cannot explain both the large differences in binding affinity observed for two antibodies (HFN7.1 and polyclonal) and the small differences in affinity for two other antibodies (3E1 and 4B2). We thus conclude that adsorption of Fn to different surfaces, uncharged polystyrene, negatively charged polystyrene, or collagen, produces different effects in the conformation of the adsorbed Fn. Furthermore, these results also suggest that not all domains of Fn are equally influenced by the interaction of the molecule with the substrate. Finally, differences in the conformation of Fn adsorbed onto different surfaces have been independently demonstrated using biophysical techniques, including electron spin resonance (Narasimhan and Lai, 1989), infrared spectroscopy (Pitt et al., 1987), total internal reflection fluorescence (Iwamoto et al., 1985), fluorescence polarization (Williams et al., 1982), and rotary shadowing (Price et al., 1982; Erickson and Carrell, 1983), as well as biological assays, such as antibody binding (Grinnell and Feld, 1982; Underwood et al., 1993; Pettit et al., 1994) and cell adhesion strength (Iuliano et al., 1993; García et al., 1998a).
Variations in Fn Conformation Lead to Differences in Integrin Binding
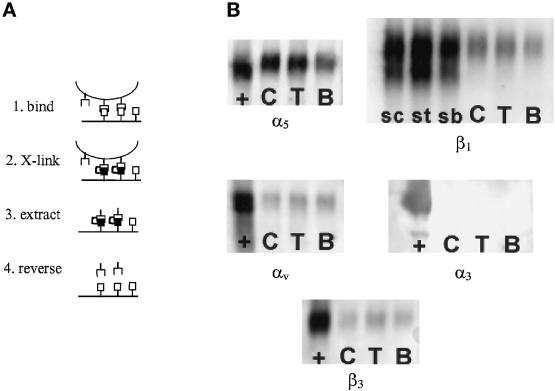
Because there were differences in the conformation of Fn adsorbed onto B, T, and C, and these differences influenced the binding of a mAb that recognizes an epitope within the cell binding domain, it is possible that these substrate-dependent conformational changes would modify integrin binding to the adsorbed Fn. Because it is extremely difficult to measure the binding constants of integrins on cell surfaces to adsorbed Fn, we chose to examine the quantity of bound α5β1, α3β1, and αvβ3 integrins, which interact with the cell binding domain in Fn (Sonnenberg, 1993), for cells plated on the Fn-coated surfaces. This approach uses a cross-linking and extraction procedure in which bound integrins are cross-linked to substrate-bound Fn using a reversible, cell-impermeable reagent (Figure 3A). Taking advantage of the fact that adsorbed Fn is resistant to extraction by detergents (Grinnell and Feld, 1981; Haas and Culp, 1982), the bulk of cell components were then extracted using 0.1% SDS, leaving behind extracellular matrix bound to the dish and its associated integrins. Bound integrins were recovered by reversing the cross-linking and quantified by Western blotting. Previous experiments have demonstrated that surface-expressed integrins that are not activated either because of the absence of an activation signal or an appropriate substrate cannot be cross-linked to the extracellular matrix (Enomoto-Iwamoto et al., 1993; García et al., 1998b). Immunofluorescent staining experiments conducted before and after alkaline cleavage demonstrated that all detectable integrins were removed for all substrates. Based on our measurements, we expect >90% of the cross-linked receptors to be recovered.
Figure 3.
Integrin binding analysis for IMR-90 fibroblasts plated on different Fn-coated substrates for 16 h under serum-free conditions. (A) Schematic diagram of cross-linking and extraction procedure. (1) Cells are plated on Fn-coated substrates. (2) Bound integrins are cross-linked to extracellular matrix using sulfo-BSOCOES. (3) Cellular components, including unbound integrins, are extracted using 0.1% SDS, leaving behind Fn and its cross-linked integrins. (4) Cross-linking is reversed, and integrins are recovered and quantified by Western blotting. (B) Representative Western blots for integrin subunits (α3, α5, αv, β1, and β3). Blots show soluble fractions (+) and cross-linked fractions for C, T, and B. For β1, soluble fractions for each substrate (sc, st, and sb) were used to normalize for cell numbers and show two bands: surface-expressed integrin (slow) and intracellular integrin precursor (fast).
IMR-90 human fibroblasts were plated for 16 h on the different Fn-coated substrates under serum-free conditions. These cells were chosen because they express constant levels of integrins under these experimental conditions. Soluble and cross-linked integrin fractions were extracted, analyzed by Western blotting, and quantified by fluorescent imaging. Soluble fractions (∼80–90% of the total cellular pool of integrins) were used to normalize for differences in the number of extracted cells among the substrates. This biochemical method showed differences in integrin binding to Fn adsorbed onto the different substrates (Figure 3B). Quantification of bound integrins (three independent experiments; Table 1) revealed significant differences among the substrates for α5 (p < 0.006) and β1 (p < 0.05), whereas no differences were detected for αv (p < 0.24) or β3 (p < 0.83). α3 was only detected in the soluble fractions. Pairwise comparisons showed significant differences in α5 binding between B and C (p < 0.007) and B and T (p < 0.04) and in β1 binding between B and C (p < 0.05).
Table 1.
Relative levels of cross-linked integrin subunits for IMR-90 fibroblasts plated on Fn-coated B, T, and C for 16 h as shown in Figure 3
| α5a | αv | α3 | β1b | β3 | |
|---|---|---|---|---|---|
| B | 1.53 ± 0.27 | 0.34 ± 0.09 | 0 | 1.59 ± 0.42 | 0.43 ± 0.32 |
| T | 2.15 ± 0.04 | 0.37 ± 0.05 | 0 | 2.06 ± 0.25 | 0.38 ± 0.22 |
| C | 2.43 ± 0.25 | 0.27 ± 0.06 | 0 | 2.34 ± 0.09 | 0.32 ± 0.21 |
Relative levels were quantified using fluorescent substrates and a Storm imager. Data from three separate experiments are shown (mean ± SD). Statistical significance among substrates was evaluated using ANOVA and pairwise comparisons.
p < 0.006.
p < 0.05.
These experiments were carried out with substrates coated with saturating amounts of Fn to maximize the signal. Because the Fn saturation density on C is approximately one-third of the saturation density on B or T, experiments were also performed on substrates coated with the same amount of Fn (100 ng/cm2). As before, there were significant differences in bound α5 and β1 among the substrates, and the differences between C and the synthetic substrates were even greater; the ratio B:T:C for α5 was 1.0:1.4:3.0.
Because α5β1 and αvβ3 integrins both bind to the RGD site in Fn, the ratios α5:αv and β1:β3 were calculated for each substrate and normalized to B. These ratios provide a relative measure of the competition of these integrins for Fn adsorbed onto the different substrates. Table 2 shows that, compared with their binding to Fn on B, α5β1 shows an increase in binding relative to αvβ3 for Fn on T and C. These differences in the levels of bound integrins support the hypothesis that integrin binding affinity can be modulated not only by varying the specific ligand but also by varying the conformation of the ligand, which is dependent on its interactions with the underlying substrate.
Table 2.
Integrin subunit binding ratios for Fn adsorbed onto different substrates reflecting differences in the relative affinity of α5β1 and αvβ3 for adsorbed Fn.
| α5:αv | β1:β3 | |
|---|---|---|
| B | 1.0 | 1.0 |
| T | 1.4 | 1.6 |
| C | 2.3 | 2.1 |
Ratios were calculated using normalized values for integrin binding (Table 1) and normalized to ratios for B.
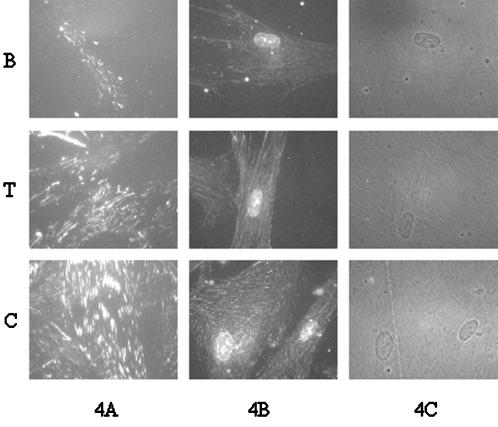
The cross-linking and extraction procedure was also combined with immunofluorescent staining to visualize substrate-bound α5β1. This extraction procedure has the advantage that it removes cytoplasmic proteins that can block antibody access to the integrin cytoplasmic domains (Enomoto-Iwamoto et al., 1993; DiPersio et al., 1995). Figure 4A shows immunofluorescent staining for cells plated on Fn-coated surfaces, cross-linked, extracted with 0.1% SDS, and stained using a polyclonal antibody specific for the cytoplasmic domain of α5. All photographs are at the same magnification and exposure. There is an obvious increase in both staining intensity and size of the focal adhesions in going from B to T to C. This is in agreement with the biochemical data showing differences in integrin binding for Fn adsorbed onto the different substrates. Experiments in which the cells were permeabilized with Triton X-100 instead of 0.1% SDS also showed similar differences in integrin binding to adsorbed Fn (Figure 4B). There were no apparent differences in cell spreading among the substrates (Figure 4C). Because a 16-h incubation time was used for these experiments, it is expected that there would be both synthesis and accumulation of extracellular components produced by the cells. Deposition of synthesized Fn would be expected to reduce differences in integrin binding among substrates. However, significant differences in the recruitment of α5 to focal adhesions are evident among the substrates, probably because any deposited Fn still interacts with the underlying substrate, which influences its conformation.
Figure 4.
Immunofluorescent staining for α5 integrin subunit on IMR-90 cells cultured on different Fn-coated substrates for 16 h and cross-linked as in Figure 3. Cells were extracted with either 0.1% SDS (A) or 1% Triton X-100 (B) before staining using an antibody against the α5 cytoplasmic domain. (C) Phase contrast micrographs of Triton X-100–extracted cells. All photographs are at the same magnification (600×) and exposure. More intense staining of the SDS-extracted cells reflects more complete removal of cytoplasmic proteins that can block antibody access to epitopes in the cytoplasmic domain of α5.
Fn Conformation Modulates Switching between Cell Proliferation and Differentiation
C2C12 mouse myoblasts were used to examine whether Fn conformation-sensitive changes in integrin binding influence cell proliferation and differentiation. Cells were propagated at high serum and then plated and grown on the Fn-coated substrates at low serum concentrations to induce differentiation (Silberstein et al., 1986). Previous studies have shown that β1 integrin-mediated signaling is important for this switch to differentiation, and additional data support a role for Fn in this process (Menko and Boettiger, 1987; Boettiger et al., 1995). C2C12 cells were plated at low density on the Fn-coated substrates, and examination of the plates 16 h after plating showed no evident differences in plating efficiency among the substrates (Figure 5A). The initial cell density was kept low to allow for cell proliferation and because plating at high density has been reported to promote differentiation (Yaffe and Saxel, 1977; Blau et al., 1983; Silberstein et al., 1986).
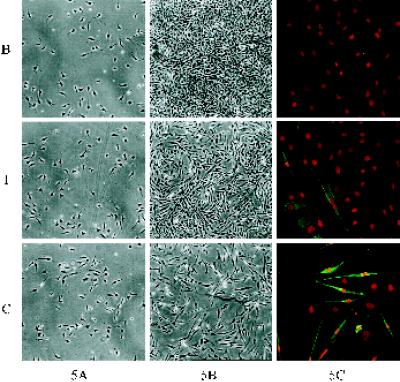
Figure 5.
C2C12 myoblast proliferation and differentiation on Fn-coated substrates. Phase contrast micrographs showing cell density and morphology at 16 h (A) and after 3 d (B) in culture (both at 40× magnification). (C) At 3 d, cultures were stained with MF20 for sarcomeric myosin (green) and ethidium homodimer for nuclei (red) (200× magnification). Levels of myogenic differentiation are quantified in Table 3.
Examination of the cultures after 16 h revealed no detectable differences in initial cell density or morphology among the substrates at the light microscope level (Figure 5A). After 3 d in culture, cells exhibited significant differences in the levels of proliferation for the different substrates (Figure 5B). Cells grown on Fn-coated C maintained their low density, and many cells showed a differentiated bipolar morphology, whereas cells grown on Fn-coated B had grown to confluence, and very few cells exhibited a bipolar morphology. Cells on Fn-coated T proliferated to reach subconfluent levels, and some bipolar cells were present in the culture.
Cell differentiation at 3 d was examined by immunofluorescent staining for sarcomeric myosin, a muscle-specific marker. Figure 5C shows double fluorescent staining with ethidium homodimer to label nuclei (red) and MF20 mAb specific for myosin (green). Switching between proliferation and differentiation was substrate dependent. The levels of differentiation varied for the Fn-coated substrates in an inverse pattern compared with proliferation. Although few cells expressed myosin on Fn-coated B, significant numbers of cells differentiated on Fn-coated T, and extensive differentiation was observed on Fn-coated C. Plates were scored to calculate the percent of nuclei positive for myosin (percent differentiation; Table 3). ANOVA statistical tests revealed significant differences in differentiation among the substrates (p < 0.001). Pairwise comparisons showed significant differences in differentiation between B and C (p < 0.001), B and T (p < 0.007), and T and C (p < 0.05). Comparable results were obtained with cells grown in medium containing Fn- and vitronectin-depleted serum. Similar trends in differentiation (C > T > B) were observed on substrates coated with approximately the same Fn surface density (100 ng/cm2). These results indicate that the major differences in the levels of differentiation among the substrates arise from variations in the conformation of Fn rather than differences in Fn surface density. These differences in differentiation cannot be explained by the previously observed cell density dependence of differentiation, because that would predict the highest differentiation on the substrate with the highest cell density, i.e., B.
Table 3.
Percent of nuclei in myosin-positive cells
| % differentiation | |
|---|---|
| B | 6.0 ± 2.0 |
| T | 21 ± 5.1 |
| C | 53 ± 13 |
Values are percent differentiation (mean ± SD; three separate experiments in duplicate) for C2C12 myoblasts grown on different Fn-coated substrates as shown in Figure 5 (ANOVA, p < 0.001).
Binding of α5β1 Integrin to Adsorbed Fn Controls Differentiation
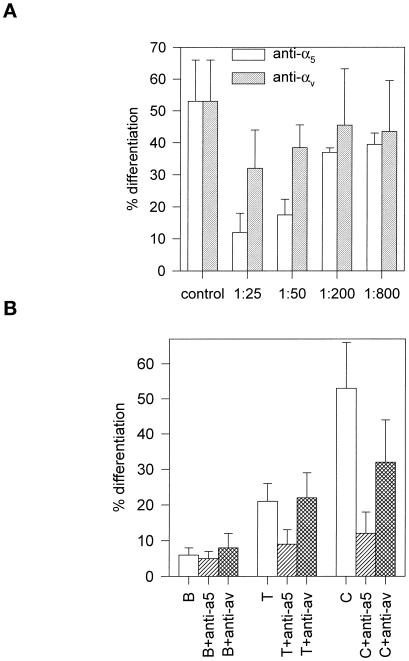
The differences in differentiation among the substrates correlated with the levels of α5 and β1 integrin subunits bound to the adsorbed Fn. To examine whether α5β1 binding to adsorbed Fn controls differentiation, experiments were conducted with function blocking mAbs against specific murine integrin subunits. Because Menko and Boettiger (1987) previously demonstrated that blocking antibodies against β1 inhibit myogenic differentiation, we focused on the role of α5. Cells were plated on Fn-coated substrates in the presence or absence of blocking mAbs specific for α5 or αv. Antibodies against α5 integrin inhibited muscle cell differentiation in a dose-dependent manner, whereas anti-αv antibodies had no effect (Figure 6A). At saturating antibody concentrations, α5-specific, but not αv-specific, antibodies reduced differentiation on T and C to the levels observed on B (Figure 6B) without influencing overall cell adhesion. Furthermore, the inhibition of differentiation by α5-specific antibodies was reversible (Table 4), demonstrating that α5 integrin binding to Fn is a control point in the transition between muscle cell proliferation and differentiation.
Figure 6.
Effect of α5 and αv integrin-specific blocking antibodies on C2C12 differentiation for Fn-coated substrates. Cells were plated as for Figure 5 and incubated for 3 d in the presence or absence of the antibodies. (A) Dose-dependent inhibition of myogenic differentiation on Fn-coated C by anti-α5, but not anti-αv, antibodies (mean ± SD; two separate experiments in duplicate). (B) Inhibition of myogenic differentiation for Fn-coated B, T, and C with saturating levels of anti-α5, but not anti-αv, antibodies (mean ± SD; two separate experiments in duplicate).
Table 4.
The block of differentiation by α5-specific antibodies is reversible
| Group | Treatment
|
% differentiation | |
|---|---|---|---|
| Initial 3 d | Subsequent 3 d | ||
| Control, 3 d | No Ab | 53 ± 13 | |
| Anti-α5, 3 d | Anti-α5 | 5 ± 2 | |
| Anti-α5, 6 d | Anti-α5 | Anti-α5 | 6 ± 4 |
| Reversed | Anti-α5 | No Ab | 45 ± 12 |
Values are mean ± SD (two separate experiments in duplicate). Cells were cultured on Fn-coated C in the presence or absence of α5-blocking mAbs for 3 d. Cells were then washed and cultured in fresh media with and without anti-α5 antibody for an additional 3 d.
Analysis of the Role of Adsorbed Fn in Myogenic Differentiation
Although the interaction of Fn with synthetic surfaces, such as bacterial and tissue culture polystyrenes, is common in experimental procedures, the interaction of Fn with other elements of the extracellular matrix is more important in vivo. The use of the collagen substrate in these experiments provides a step toward the separation of the individual roles of Fn and collagen. Whereas Fn adsorption to the plastics is nonspecific, the interaction of Fn with collagen involves specific binding sites and is expected to produce a conformation closer to that in developing muscle. The analysis is complicated by the ability of cells to interact directly with collagen as well as indirectly through the collagen-bound Fn. We used function-blocking antibodies to isolate the contribution of Fn to the differentiation stimulus.
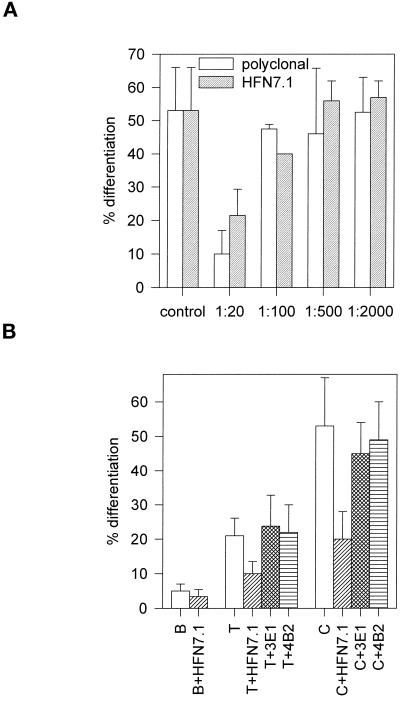
Adhesion-blocking HFN7.1 monoclonal and polyclonal antibodies inhibited differentiation in a dose-dependent manner (Figure 7A). At the highest concentrations used, these antibodies reduced differentiation on C and T to the levels observed on B (Figure 7B), indicating that differences in differentiation are controlled by binding to Fn. For these experiments, antibody concentrations were titrated to levels that did not perturb overall cell adhesion. On the other hand, 3E1 and 4B2 mAbs, which bind to epitopes outside the cell binding domain in Fn and do not inhibit cell adhesion, had no effect in C2C12 differentiation (Figure 7B). Blocking of differentiation by HFN7.1 antibody implicates binding to the central cell binding domain in Fn as a critical step in myoblast differentiation. Furthermore, HFN7.1 is specific for human Fn and does not cross-react with mouse or bovine Fn. This antibody reduced differentiation by 80%, indicating that the human Fn bound to the substrates before the addition of C2C12 cells and serum-containing media provided the dominant signal for differentiation with lesser contributions from either cell or serum Fn.
Figure 7.
Effect of Fn-specific antibodies on C2C12 myogenic differentiation for Fn-coated substrates. Cells were plated as for Figure 5 and 6 and incubated for 3 d in the presence or absence of antibodies against Fn. The cells remained normally spread for all antibody concentrations reported. (A) Dose-dependent inhibition of C2C12 differentiation on Fn-coated C with adhesion-blocking polyclonal and HFN7.1 monoclonal antibodies (mean ± SD; two separate experiments in duplicate). (B) Comparison of inhibition by mAbs to different domains in Fn. Adhesion-blocking HFN7.1 inhibited differentiation (mean ± SD; two separate experiments in duplicate), whereas 3E1 and 4B2 (mean ± SD; n = 2), which bind to epitopes outside the cell binding domain, did not affect differentiation.
The experiments with function-blocking antibodies directed against the receptor or the ligand demonstrated that binding of α5β1 integrin to adsorbed Fn is essential for myogenic differentiation. The contribution of collagen to myoblast differentiation, however, was not addressed in these experiments. It is possible that direct interactions between collagen and the cells modulate integrin binding and/or act synergistically to influence differentiation. To address this possibility, we conducted adhesion experiments to examine C2C12 myoblast adhesion to collagen. In the absence of Fn, these cells did not adhere to collagen in a 4-h adhesion assay. This lack of direct interaction with collagen is consistent with a previous study that reported no detectable levels of either α1 or α2 integrins, components of receptors that bind to collagen, on C2C12 cells (Yao et al., 1996). Based on these results, we do not expect any Fn-independent contributions from collagen in the differentiation of these cells.
DISCUSSION
Two important issues are raised by these results: 1) Fn adsorption to different surfaces can influence its conformation and alter its binding to specific integrins; and 2) the altered Fn-integrin binding has a profound effect on the cellular response to adhesion-mediated signals and can control proliferation and differentiation pathways.
Substrate-dependent Changes in Fn Conformation
The binding of integrins to Fn is a highly regulated process. It can be controlled by the expression levels of specific integrins, activation state of the integrins, and specific integrin heterodimers used in the binding (Hynes, 1992). This diversity of regulatory mechanisms suggests that the specific manner of interaction between the cell and Fn is critical to the control of cellular behavior. Here we describe a new level of potential regulation of integrin binding to Fn at the level of Fn conformation. Although the model system used in this study uses artificial substrates to provide a controlled environment, Fn interacts with many other elements of the extracellular matrix, including collagens, proteoglycans, and hyaluronic acid (Engvall and Ruoslahti, 1977; Hayman et al., 1982; Laterra et al., 1983), which could also influence the conformation of Fn. The primary cell binding domain of Fn is found in the 9th and 10th type III repeats where the PHSRN synergy and the RGD domains are located (Pierschbacher et al., 1981; Aota et al., 1994). X-ray crystallographic data on this region suggests that the 9th and 10th type III repeats are oriented so that the synergy and RGD sites are exposed on the same face of the molecule (Leahy et al., 1996). However, there is potential for rotation or extension of the bonds joining these repeats, which could be exerted by the energetics of the adsorption process. Such changes would be likely to influence the binding of HFN7.1 mAb, whose epitope maps to a segment spanning the connection between the 9th and 10th type III repeats and lies outside the RGD binding site (Bowditch et al., 1991). This could explain the relative changes in binding affinity of HFN7.1 to Fn molecules adsorbed onto the three different substrates. α5β1 integrin binds to both the RGD and the synergy sites of Fn, whereas αvβ3 binds to the RGD but not to the synergy site (Danen et al., 1995). This is consistent with our experiments, which show that the binding of α5β1 to Fn was sensitive to differences in Fn conformation, whereas the binding of αvβ3 was not.
Changes in the conformation of Fn resulting from adsorption to different surfaces have been previously investigated, and biophysical analyses of adsorbed Fn suggest that the adsorption process results in unfolding of the protein (Erickson and Carrell, 1983; Pitt et al., 1987; Narasimhan and Lai, 1989). These changes in conformation are more drastic on hydrophobic surfaces than on hydrophilic substrates (Iwamoto et al., 1985; Jonsson et al., 1987; Pitt et al., 1987; Narasimhan and Lai, 1989) and reflect differences in surface properties, such as surface energy and charge, which influence the Fn–substrate interaction. These changes in Fn conformation have been correlated with differences in antibody binding and cell adhesion and spreading rates (Grinnell and Feld, 1981; Iuliano et al., 1993; Underwood et al., 1993; Pettit et al., 1994; García et al., 1998a).
Fn Conformation Modulates Integrin Binding
Numerous studies have analyzed the binding of purified integrins to specific substrates using column chromatography (Buck et al., 1986; Gailit and Ruoslahti, 1988; Elices et al., 1991). This approach has certain disadvantages for the current analysis. First, integrin binding to extracellular matrix ligands is governed by cellular signals that can activate and inactivate the binding function of the expressed integrins (Adams and Watt, 1990; Faull et al., 1993; Boettiger et al., 1995). Second, only a fraction of the total pool of surface-expressed integrin is usually involved in substrate binding. Therefore, we have developed an alternative approach by using cell-impermeable, bifunctional, reversible chemical cross-linkers. We have demonstrated that these reagents can cross-link the Fn receptors α5β1 and αvβ3 to adsorbed Fn but not α6β1, which does not bind to Fn (Enomoto-Iwamoto et al., 1993). Application of this method to the analysis of the levels of α5β1 bound to Fn has also shown increases in bound α5β1 as a function of adsorbed Fn. Here we show that there is an increase in the levels of α5β1 bound, but not αvβ3, as the substrate is modified from Fn adsorbed to B, to Fn-coated T, and to Fn-coated C. From chemical equilibrium principles, the concentration of integrin–Fn bonds is equal to a constant (binding affinity) times the product of ligand and receptor concentrations (densities). Because the same cell suspension was plated on the different surfaces, the number of cells and, consequently, the total number of integrin receptors were constant. Thus, the differences in bound integrins can only be explained by differences in binding affinity constant and/or ligand density. The fact that differences in bound integrins among the substrates were specific for α5β1 but not αvβ3 argue that differences in binding affinity contribute to the differences in the levels of bound integrins. These values, however, do not measure actual binding constants and demonstrate only a relative order of binding affinities. This analysis is consistent with the interpretation that the binding site for α5β1 in Fn is modulated by the different substrates.
Quantification of integrin binding to the different Fn-coated substrates was done on IMR-90 fibroblasts, because these cells maintain constant levels of integrin expression. On the other hand, C2C12 cells, like many other myogenic cells, modulate their integrin expression levels in response to differentiation stimuli (Enomoto et al., 1993; Blaschuk and Holland, 1994). These alterations in integrin expression would have complicated the analysis considerably. However, for the IMR-90 cells, the pattern of immunofluorescent staining for bound integrins on the different substrates correlated well with the biochemical analysis. Compared with the IMR-90 cells, we observed similar patterns of immunofluorescent staining of integrins on the different surfaces for C2C12 myoblasts, suggesting similar integrin binding to Fn adsorbed onto the different substrates.
Control of Cell Proliferation and Differentiation Signals
In the original development of culture conditions for the differentiation of myoblasts to myotubes, a critical element was the use of collagen as a substrate for the cells (Hauschka and Konigsberg, 1966; Bischoff and Holtzer, 1968). More recent data have provided evidence for a role for β1 integrin and the extracellular matrix in this process (Chiquet et al., 1981; Kuhl et al., 1982; Sanes and Cheyney, 1982; Olwin and Hall, 1985; Foster et al., 1987; Menko and Boettiger, 1987; von der Mark and Ocalan, 1989; Boettiger et al., 1995). However, the specific receptors, matrix elements, and signaling mechanism have remained controversial. In this study, we demonstrate that, for C2C12 myogenic cells, differentiation requires the binding of α5β1 to the RGD site in Fn.
What is the role for collagen in myogenic differentiation? Most systems for the in vitro differentiation of myogenic cells use collagen as the substrate for cell plating, although gelatin appears to be equally effective for some cell systems. In the absence of a collagen substrate, other serum proteins compete with Fn present in serum-containing medium for adsorption onto the synthetic substrates, resulting in very little adsorption of Fn (Grinnell and Feld, 1982). On a collagen (or gelatin) substrate, nonspecific protein binding sites on the polystyrene are blocked by collagen (or gelatin), and specific Fn binding sites are available, which saturate with serum or cell-synthesized Fn. Thus, collagen is an efficient presenter of Fn to the cell. In the present study, we have shown that Fn bound to collagen is also in a conformation that is particularly favorable to the binding of α5β1 integrin. Our experiments with function-blocking antibodies demonstrate that binding of α5β1 integrin to Fn is essential to myogenic differentiation. Furthermore, for the particular cell system used in this study, collagen does not appear to have a direct, Fn-independent role in myogenic differentiation.
It has been proposed that laminin plays an essential role in the differentiation of myoblasts to myotubes (Kuhl et al., 1982; Sanes and Cheyney, 1982; Olwin and Hall, 1985; Foster et al., 1987; von der Mark and Ocalan, 1989). In general, these studies consider experimental conditions in which mixed substrates of laminin, Fn, and collagen were present. The data presented here show that the presence of other extracellular matrix components can influence the ability of Fn to promote differentiation. This may also apply to laminin- or Matrigel-coated surfaces and could explain the different results. In contrast to the ability of antibodies specific for α5 integrin to reversibly inhibit myoblast differentiation, as shown in the present study, no receptor-specific inhibition data have been presented for laminin. There is increasing evidence for an important role for merosin in the interaction with α7β1D in the survival of myotubes (Vachon et al., 1997). Sastry et al. (1996) used overexpression of α5 and α6 into quail myoblasts to examine their roles in the control of myoblast proliferation and differentiation. Their results show that transfection of α6 promotes differentiation, whereas α5 promotes cell proliferation. One interpretation of these results is that α6β1 and laminin promote differentiation. However, the effects of the transfections on cell adhesion were not examined, and other reports have shown that overexpression of cytoplasmic domains can inhibit integrin function (LaFlamme et al., 1994).
Integrin binding to the extracellular matrix triggers signals that involve both physical and biochemical components, including cell morphology, tyrosine phosphorylation, and second messengers (Roskelley et al., 1994; Clark and Brugge, 1995). For example, integrin-mediated adhesion of mammary epithelial cells to laminin, but not Fn or type I collagen, triggers tissue-specific expression of β-casein, demonstrating the specificity of the matrix components (Streuli et al., 1995). By varying cell spreading while maintaining a constant cell–matrix contact area, Chen et al. (1997) elegantly demonstrated that cell shape can control decisions between cell proliferation and apoptosis. Here, we demonstrate a novel mechanism of extracellular matrix control of cell proliferation and differentiation. Control of cell fate through conformational changes in the extracellular matrix represents a versatile mechanism to elicit specific cellular responses. For instance, the interaction and association of Fn with different matrix proteins in vivo may result in multiple Fn conformations that have distinct biological functions. Furthermore, this principle can be applied to engineer and tailor substrates for biotechnological applications, including biomaterials and tissue engineering. For example, we have shown that surface modification of bioactive glass substrates results in different conformations of adsorbed Fn that produce different levels of adhesion and modulate the expression of the osteoblastic phenotype (El-Ghannam et al., 1995; García et al., 1998a).
ACKNOWLEDGMENTS
We thank C. Emerson for kindly providing C2C12 cells. This work was funded by National Cancer Institute grants CA-16502 and CA-49866. A.J.G. acknowledges support under a Ford Foundation postdoctoral fellowship.
Abbreviations used:
- B
bacterial grade polystyrene
- C
collagen type I
- DMEM
Dulbecco’s modified Eagle’s medium
- Fn
fibronectin
- T
tissue culture polystyrene
REFERENCES
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes α5β1 integrin loss from the cell surface. Cell. 1990;63:425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Aota S, Nomizu M, Yamada KM. The short amino acid sequence ProHis-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R, Holtzer H. The effect of mitotic inhibitors on myogenesis in vitro. J Cell Biol. 1968;36:111–127. [PubMed] [Google Scholar]
- Blaschuk KL, Holland PC. The regulation of α5β1 integrin expression in human muscle cells. Dev Biol. 1994;164:475–483. doi: 10.1006/dbio.1994.1217. [DOI] [PubMed] [Google Scholar]
- Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Boettiger D, Enomoto-Iwamoto M, Yoon HY, Hofer U, Chiquet-Ehrismann R, Menko AS. Regulation of integrin α5β1 affinity during myogenesis. Dev Biol. 1995;169:261–272. doi: 10.1006/dbio.1995.1142. [DOI] [PubMed] [Google Scholar]
- Bowditch RD, Halloran CE, Aota S, Obara M, Plow EF, Yamada KM, Ginsberg MH. Integrin alpha IIb beta 3 (platelet GPIIb-IIIa) recognizes multiple sites in fibronectin. J Biol Chem. 1991;266:23323–23328. [PubMed] [Google Scholar]
- Buck CA, Shea E, Duggan K, Horwitz AF. Integrin (the CSAT antigen): functionality requires oligomeric integrity. J Cell Biol. 1986;103:2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides G, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Eppenberger HM, Turner DC. Muscle morphogenesis: evidence for an organizing function of exogenous fibronectin. Dev Biol. 1981;88:220–235. doi: 10.1016/0012-1606(81)90166-4. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Danen EH, Aota S, van Kraats AA, Yamada KM, Ruiter DJ, van Muijen GN. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin alpha 5 beta 1. J Biol Chem. 1995;270:21612–21618. doi: 10.1074/jbc.270.37.21612. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Shah S, Hynes RO. Alpha 3A beta 1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J Cell Sci. 1995;108:2321–2336. doi: 10.1242/jcs.108.6.2321. [DOI] [PubMed] [Google Scholar]
- El-Ghannam A, Ducheyne P, Shapiro IM. A bioactive glass template for the in vitro synthesis of bone. J Biomed Mater Res. 1995;29:359–370. doi: 10.1002/jbm.820290311. [DOI] [PubMed] [Google Scholar]
- Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by ARG-GLY-ASP peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Boettiger D, Menko AS. α5 integrin is a critical component of adhesion plaques in myogenesis. Dev Biol. 1993;155:180–197. doi: 10.1006/dbio.1993.1017. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Menko AS, Philp NJ, Boettiger D. Evaluation of integrin molecules involved in substrate adhesion. Cell Adhes Commun. 1993;1:191–202. doi: 10.3109/15419069309097253. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J Biol Chem. 1983;258:14539–14544. [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan J, Ginsberg MH. Affinity modulation of integrin α5β1: regulation of the functional response to fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122:11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- García AJ, Ducheyne P, Boettiger D. The effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J Biomed Mater Res. 1998a;40:48–56. doi: 10.1002/(sici)1097-4636(199804)40:1<48::aid-jbm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- García AJ, Huber F, Boettiger D. Force required to break α5β1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J Biol Chem. 1998b;273:10988–10993. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Feld MK. Adsorption characteristics of plasma fibronectin in relationship to biological activity. J Biomed Mater Res. 1981;15:363–381. doi: 10.1002/jbm.820150308. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Feld MK. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982;257:4888–4893. [PubMed] [Google Scholar]
- Haas R, Culp LA. Properties and fate of plasma fibronectin bound to the tissue culture substratum. J Cell Physiol. 1982;113:289–297. doi: 10.1002/jcp.1041130217. [DOI] [PubMed] [Google Scholar]
- Hauschka SD, Konigsberg IR. Influence of collagen on the development of muscle colonies. Proc Natl Acad Sci USA. 1966;55:119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman EG, Oldberg A, Martin GR, Ruoslahti E. Codistribution of heparin sulfate proteoglycan, laminin and fibronectin in the extracellular matrix of normal rat kidney cells and their coordinate absence in transformed cells. J Cell Biol. 1982;94:28–35. doi: 10.1083/jcb.94.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iuliano DJ, Saavedra SS, Truskey GA. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J Biomed Mater Res. 1993;27:1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- Iwamoto GK, Winterton LC, Stoker RS, van Wagenen RA, Andrade JD, Mosher DF. Fibronectin adsorption detected by interfacial fluorescence. J Colloid Interface Sci. 1985;106:459–464. [Google Scholar]
- Jonsson U, Lundstrom I, Ronnberg I. IgG and secretory fibronectin adsorption to silica. J Colloid Interface Sci. 1987;117:127–138. [Google Scholar]
- Klebe RJ, Bentley KL, Schoen RC. Adhesive substrates for fibronectin. J Cell Physiol. 1981;109:481–488. doi: 10.1002/jcp.1041090314. [DOI] [PubMed] [Google Scholar]
- Kuhl U, Timpl R, von der Mark K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev Biol. 1982;93:344–354. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas L, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterra J, Silbert JE, Culp LA. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J Cell Biol. 1983;96:113–123. doi: 10.1083/jcb.96.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe R, Ohe N, Maeda T, Fukuda T, Sekiguchi K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J Cell Biol. 1997;139:295–307. doi: 10.1083/jcb.139.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Occupation of the extracellular matrix receptor integrin is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Morla A, Ruoslahti E. A fibronectin self-assembly site involved in matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992;118:421–429. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan C, Lai CS. Conformational changes of plasma fibronectin detected upon adsorption to solid substrates: a spin-label study. Biochemistry. 1989;28:5041–5046. doi: 10.1021/bi00438a021. [DOI] [PubMed] [Google Scholar]
- Norton PA, Hynes RO. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987;7:4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin BB, Hall ZW. Developmental regulation of laminin accumulation in the extracellular matrix of a mouse muscle cell line. Dev Biol. 1985;112:359–367. doi: 10.1016/0012-1606(85)90407-5. [DOI] [PubMed] [Google Scholar]
- Pettit DK, Hoffman AS, Horbett TA. Correlation between corneal epithelial cell outgrowth and monoclonal antibody binding to the cell binding domain of adsorbed fibronectin. J Biomed Mater Res. 1994;28:685–691. doi: 10.1002/jbm.820280605. [DOI] [PubMed] [Google Scholar]
- Pierschbacher MD, Hayman EG, Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981;26:259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pitt WG, Spiegelberg SH, Cooper SL. Adsorption of fibronectin to polyurethane surfaces: Fourier transform infrared spectroscopy studies. In: Horbett TA, Brash JL, editors. Proteins at Interfaces. Washington, DC: American Chemical Society; 1987. pp. 324–338. [Google Scholar]
- Price TM, Rudee ML, Pierschbacher M, Ruoslahti E. Structure of fibronectin and its fragments in electron microscopy. Eur J Biochem. 1982;129:359–363. doi: 10.1111/j.1432-1033.1982.tb07058.x. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Cheyney JM. Laminin, fibronectin and collagen in synaptic and extrasynaptic portions of muscle fiber basement membrane. J Cell Biol. 1982;93:442–451. doi: 10.1083/jcb.93.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen RC, Bentley KL, Klebe RJ. Monoclonal antibody against human fibronectin inhibits cell attachment. Hybridoma. 1982;1:99–108. doi: 10.1089/hyb.1.1982.1.99. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb-IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- Silberstein L, Webster SG, Travis M, Blau HM. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986;46:1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A. Integrins and their ligands. Curr Top Microbiol Immunol. 1993;184:7–35. doi: 10.1007/978-3-642-78253-4_2. [DOI] [PubMed] [Google Scholar]
- Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the α6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: Basement membrane induced tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood PA, Steele JG, Dalton BA. Effects of polystyrene surface chemistry on the biological activity of solid phase fibronectin and vitronectin, analyzed with monoclonal antibodies. J Cell Sci. 1993;104:793–803. doi: 10.1242/jcs.104.3.793. [DOI] [PubMed] [Google Scholar]
- Vachon PH, et al. Integrins (alpha7beta1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870–1881. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K, Ocalan M. Antagonistic effects of laminin and fibronectin on the expression of the myogenic phenotype. Differentiation. 1989;40:150–157. doi: 10.1111/j.1432-0436.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. Substrate interactions affecting motor growth cone guidance during development and regeneration. J Exp Biol. 1987;132:161–175. doi: 10.1242/jeb.132.1.161. [DOI] [PubMed] [Google Scholar]
- Williams EC, Janmey PA, Ferry JD, Mosher DF. Conformational states of fibronectin. Effects of pH, ionic strength and collagen binding. J Biol Chem. 1982;257:14973–14978. [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cell lines isolated from dystrophic mouse muscle. Nature. 1977;270:725–726. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci. 1996;109:3139–3150. doi: 10.1242/jcs.109.13.3139. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]