Abstract
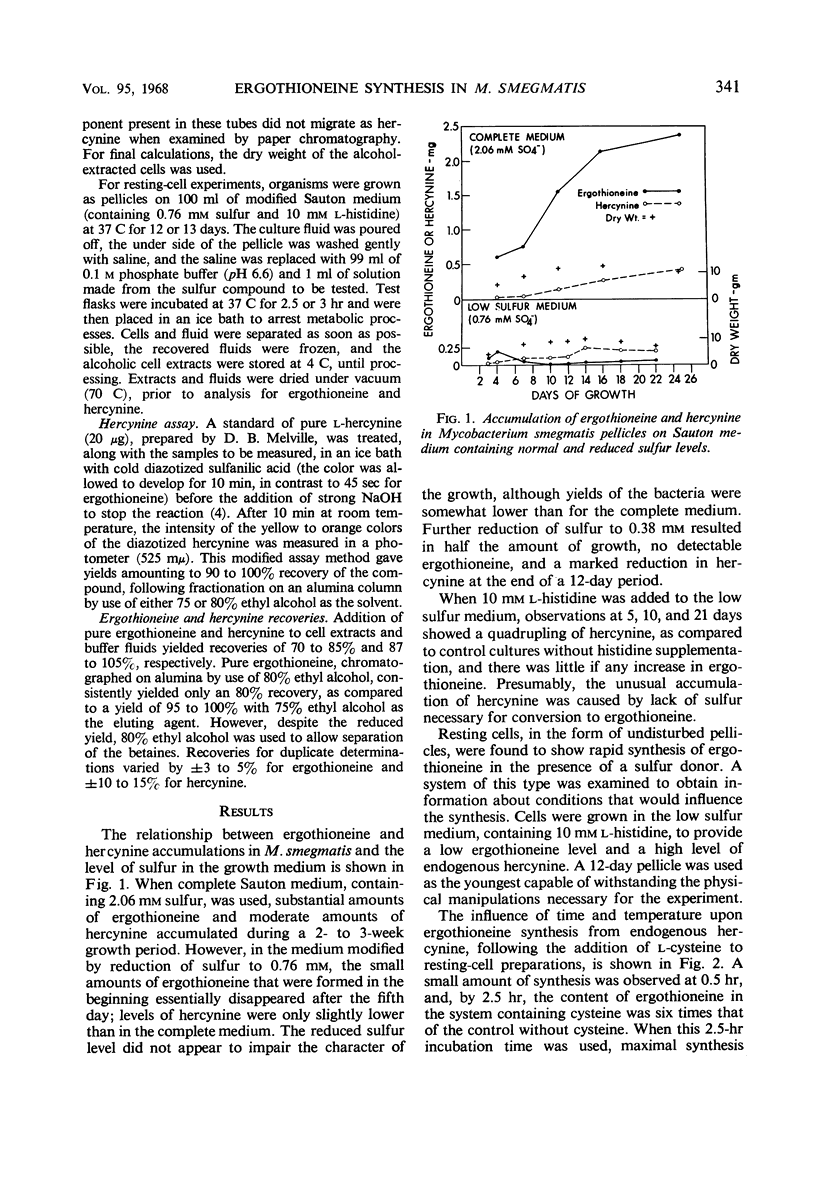
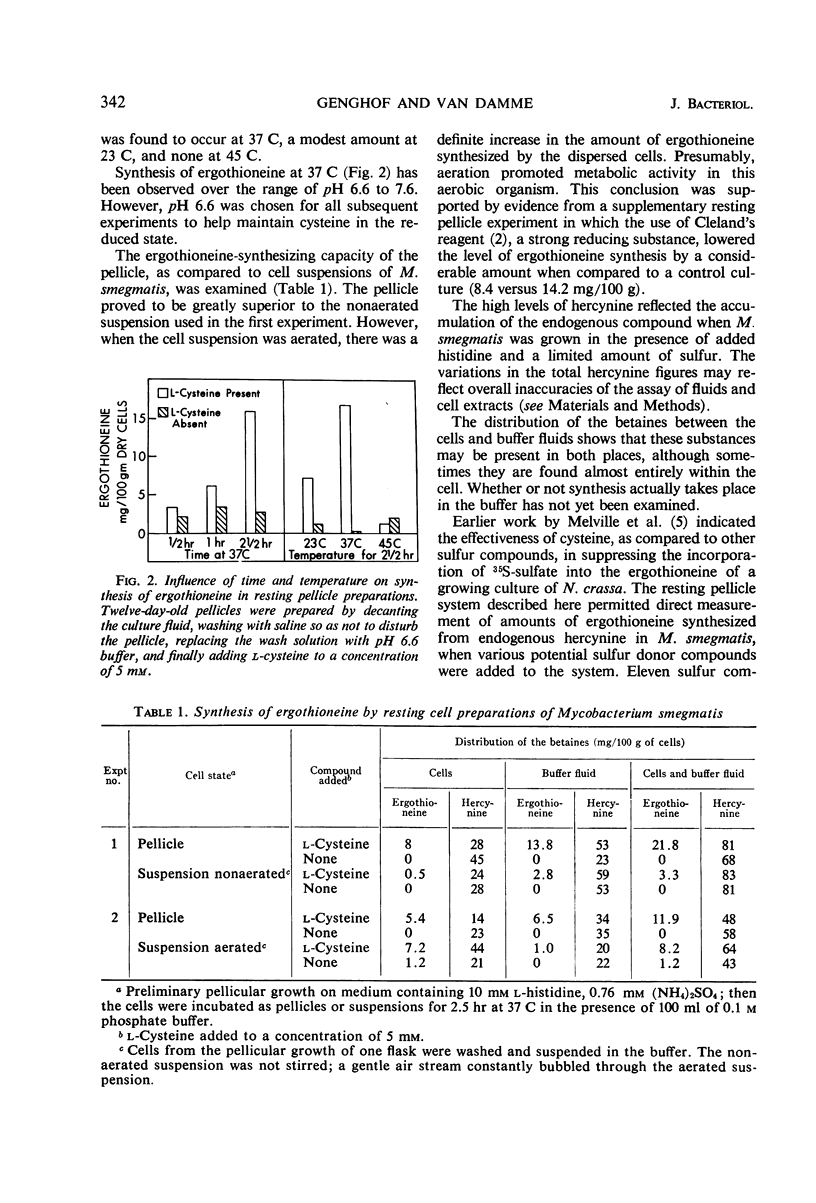
Ergothioneine was synthesized and accumulated in growing cultures of Mycobacterium smegmatis when the medium was adequately supplied with sulfur. In a low sulfur medium, the accumulation was sharply limited although growth of the organism was apparently normal. Synthesis of hercynine, the precursor of ergothioneine, was unaffected by low sulfur levels and was markedly increased by addition of l-histidine, the precursor of hercynine. Resting-cell pellicle experiments, performed with cells grown on the low sulfur high histidine medium, showed that ergothioniene was synthesized from endogenous hercynine, when cysteine or compounds readily converted to cysteine (such as cystine, lanthionine, cystathionine, and thiazolidine carboxylic acid) were added. Homocysteine and djenkolic acid allowed for minimal synthesis of betaine, whereas methionine, S-methylcysteine, sodium sulfate, and sodium thiosulfate were unable to donate sulfur for ergothioniene synthesis under the experimental conditions employed. Addition of cysteine to a resting pellicle preparation caused the formation of 100 to 200 μg of ergothioneine per g of dry cells in 2.5 to 3 hr. A modified procedure for isolating ergothioneine and hercynine, employing a 75% ethyl alcohol extraction of wet organisms, followed by a single alumina column separation of the compounds, is described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKARI A., MELVILLE D. B. The reaction sequence in ergothioneine biosynthesis: hercynine as an intermediate. J Biol Chem. 1962 May;237:1615–1618. [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- GENGHOF D. S. The specificity of the sulfur amino acid requirement of Tetrahymena geleii. Arch Biochem Biophys. 1951 Nov;34(1):112–120. doi: 10.1016/s0003-9861(51)80016-x. [DOI] [PubMed] [Google Scholar]
- GENGHOF D. S., VANDAMME O. BIOSYNTHESIS OF ERGOTHIONEINE AND HERCYNINE BY MYCOBACTERIA. J Bacteriol. 1964 Apr;87:852–862. doi: 10.1128/jb.87.4.852-862.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVERMAN J. L., RAGLAND J. B. S-methyl-L-cysteine as a naturally occurring metabolite in Neurospora crassa. Arch Biochem Biophys. 1956 Dec;65(2):574–576. doi: 10.1016/0003-9861(56)90217-x. [DOI] [PubMed] [Google Scholar]
- MELVILLE D. B., EICH S., LUDWIG M. L. The biosynthesis of ergothioneine. J Biol Chem. 1957 Feb;224(2):871–877. [PubMed] [Google Scholar]


