Abstract
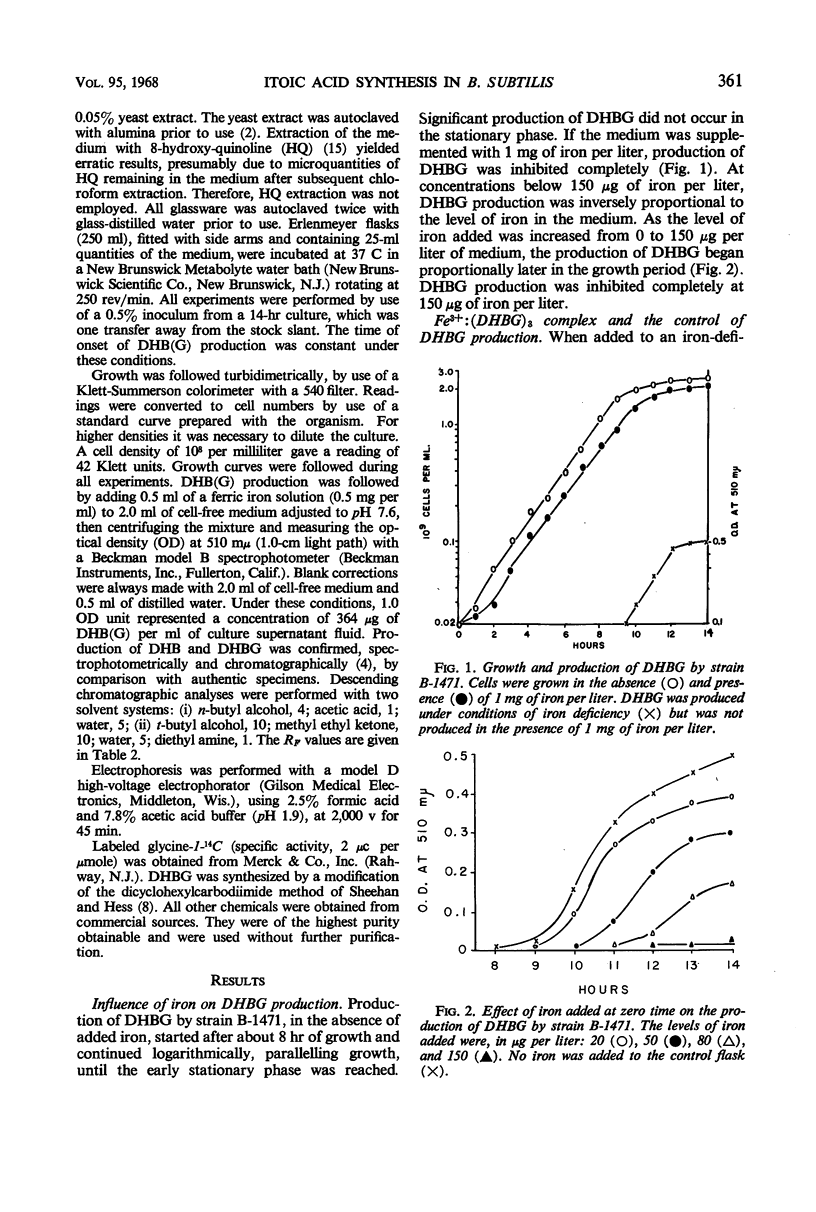
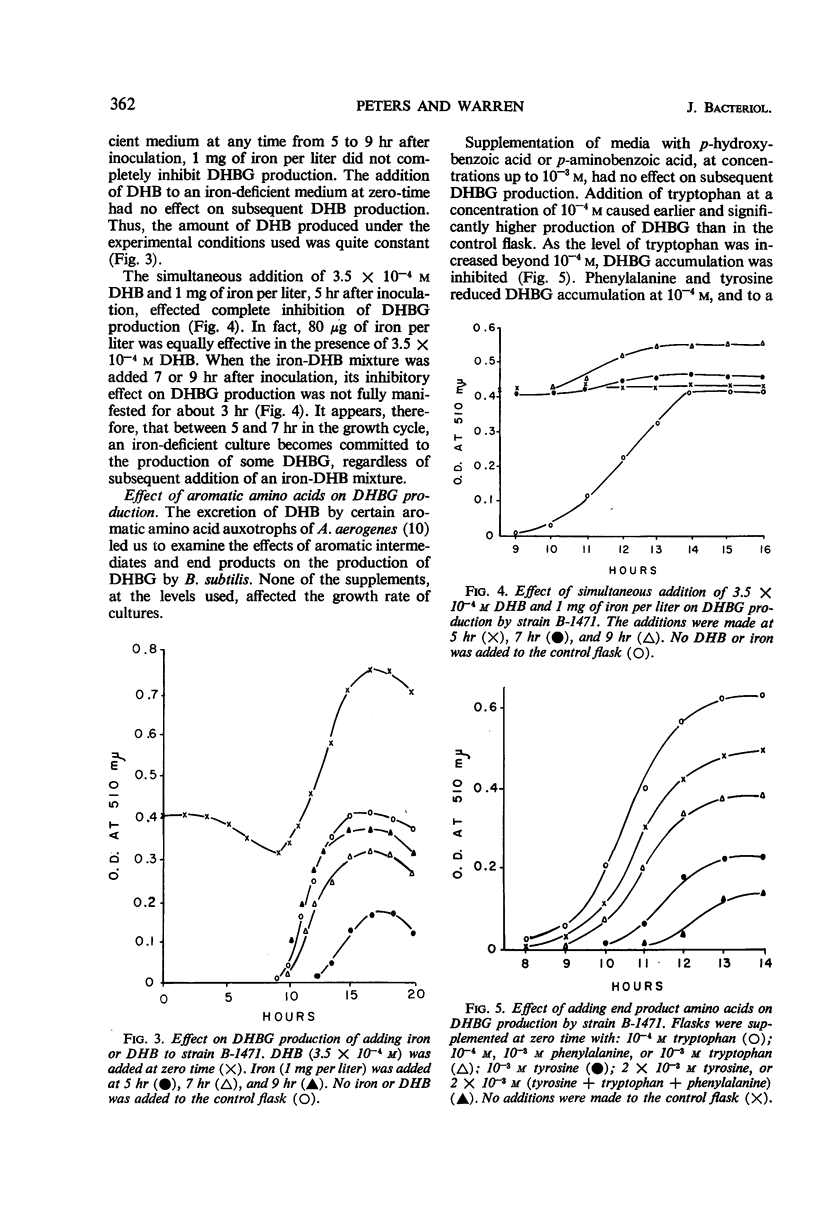
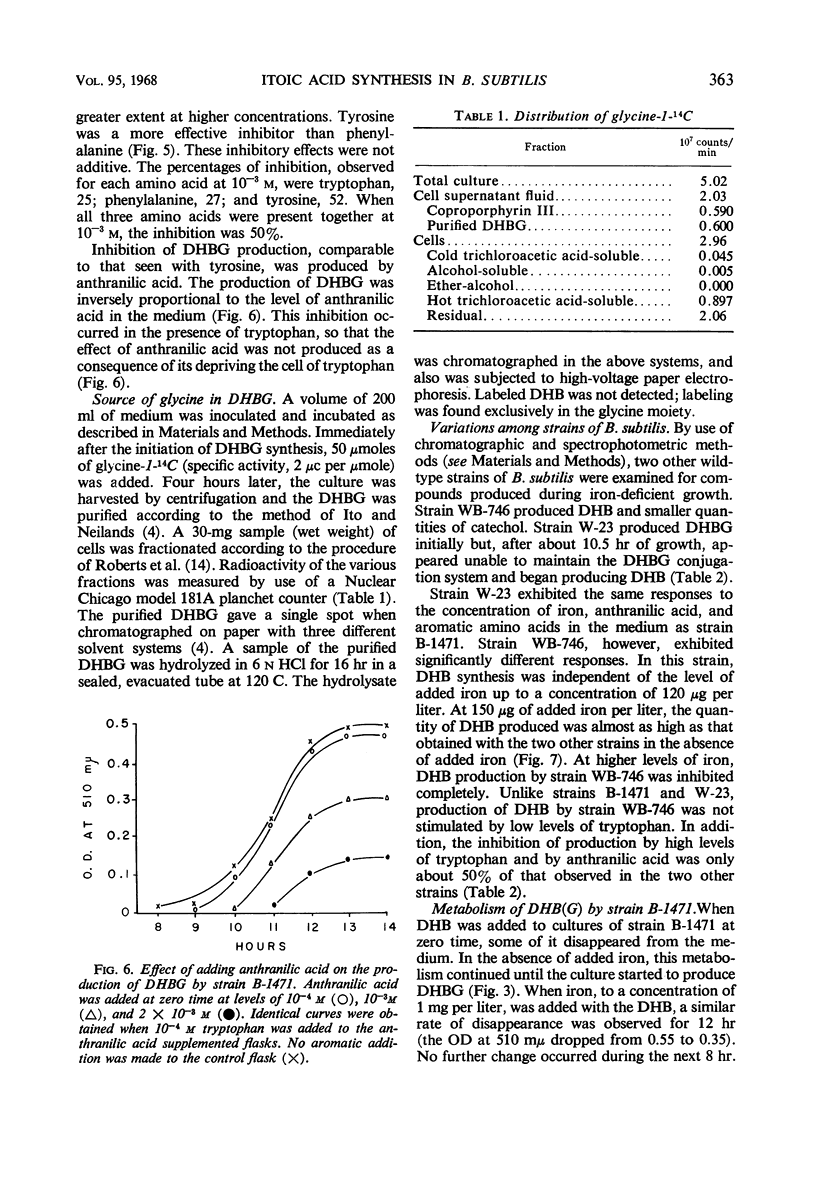
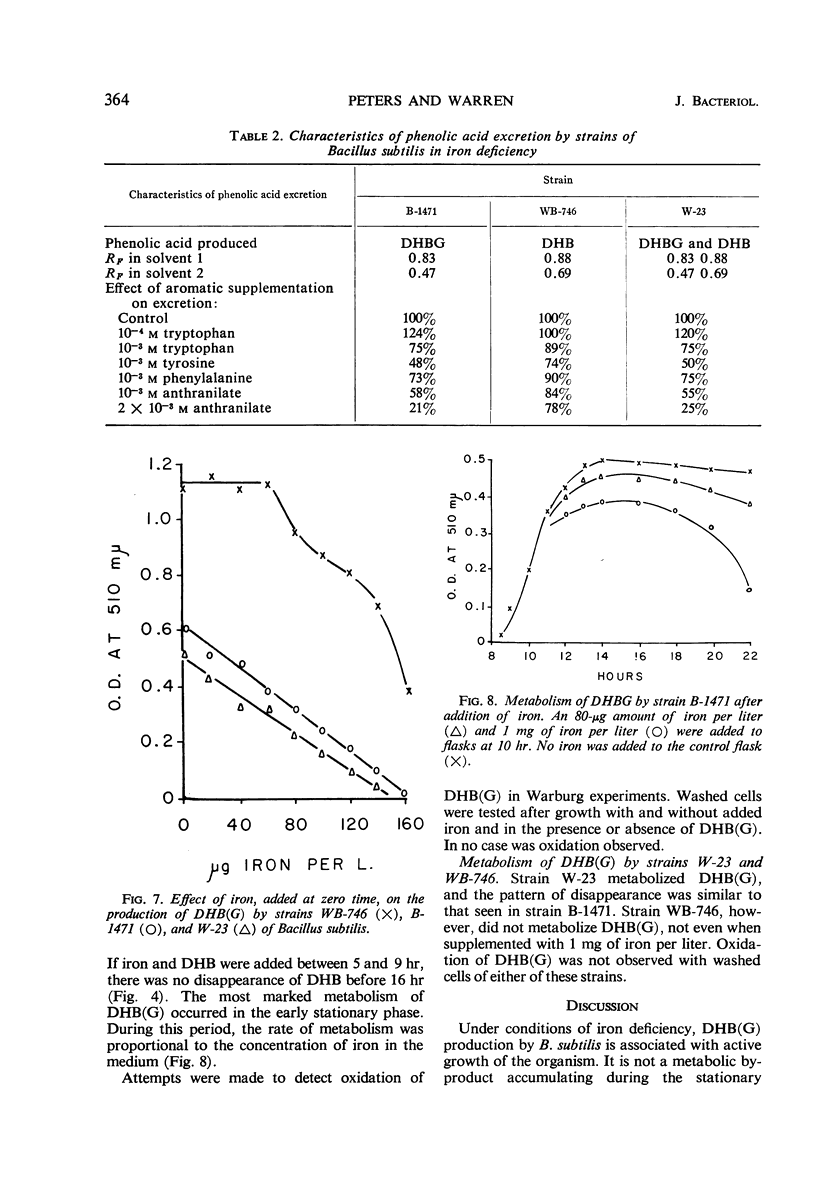
Under conditions of iron deficiency, strains of Bacillus subtilis produced 2,3-dihydroxybenzoic acid (DHB), 2,3-dihydroxybenzolyglycine (DHBG), or both of these compounds. DHB(G) production [production of DHB(G) refers to the production of DHB, or DHBG, or both] was proportional to the amount of iron present and occurred logarithmically, paralleling growth. Supplementation of media with more than 150 μg of iron per liter at zero-time inhibited DHB accumulation completely. In the presence of DHB, lower levels of iron inhibited DHB(G) production, so that the actual inhibitor of synthesis may involve the Fe3+:[DHB(G)]3 complex. The strains producing DHBG also produced coproporphyrin III during iron-deficient growth, whereas a strain producing DHB did not produce coproporphyrin III under these conditions. Accumulation of DHB(G) was influenced by the levels of aromatic amino acids and anthranilic acid in the medium. In vivo experiments with strain B-1471 demonstrated that DHB was coupled to added glycine to form DHBG. Metabolism of DHB(G) was observed in two of the strains studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brot N., Goodwin J., Fales H. In vivo and in vitro formation of 2,3-dihydroxybenzoylserine by Escherichia coli K12. Biochem Biophys Res Commun. 1966 Nov 22;25(4):454–461. doi: 10.1016/0006-291x(66)90227-0. [DOI] [PubMed] [Google Scholar]
- Byers B. R., Powell M. V., Lankford C. E. Iron-chelating hydroxamic acid (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):286–294. doi: 10.1128/jb.93.1.286-294.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARIBALDI J. A., NEILANDS J. B. Formation of iron-binding compounds by micro-organisms. Nature. 1956 Mar 17;177(4507):526–527. doi: 10.1038/177526a0. [DOI] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- PITTARD A. J., GIBSON F., DOY C. H. A possible relationship between the formation of o-dihydric phenols and tryptophan biosynthesis by Aerobacter aerogens. Biochim Biophys Acta. 1962 Feb 26;57:290–298. doi: 10.1016/0006-3002(62)91122-8. [DOI] [PubMed] [Google Scholar]
- PITTARD A. J., GIBSON F., DOY C. H. Phenolic compounds accumulated by washed cell suspensions of a tryptophan auxotroph of Aerobacter aerogenes. Biochim Biophys Acta. 1961 May 27;49:485–494. doi: 10.1016/0006-3002(61)90245-1. [DOI] [PubMed] [Google Scholar]
- RATLEDGE C. RELATIONSHIP BETWEEN THE PRODUCTS OF AROMATIC BIOSYNTHESIS IN MYCOBACTERIUM SMEGMATIS AND AEROBACTER AEROGENES. Nature. 1964 Jul 25;203:428–429. doi: 10.1038/203428a0. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. O-pyrocatechiuc acid carboxy-lyase from Aspergillus niger. Arch Biochem Biophys. 1967 Nov;122(2):466–473. doi: 10.1016/0003-9861(67)90220-2. [DOI] [PubMed] [Google Scholar]
- Ratledge C. The production of an N-acylanthranilic acid from shikimic acid and the effect on iron deficiency on the biosynthesis of other aromatic compounds by Aerobacter aerogenes. Biochim Biophys Acta. 1967 Jun 13;141(1):55–63. doi: 10.1016/0304-4165(67)90244-9. [DOI] [PubMed] [Google Scholar]
- Young I. G., Cox G. B., Gibson F. 2,3-Dihydroxybenzoate as a bacterial growth factor and its route of biosynthesis. Biochim Biophys Acta. 1967 Jul 25;141(2):319–331. doi: 10.1016/0304-4165(67)90106-7. [DOI] [PubMed] [Google Scholar]


