Abstract
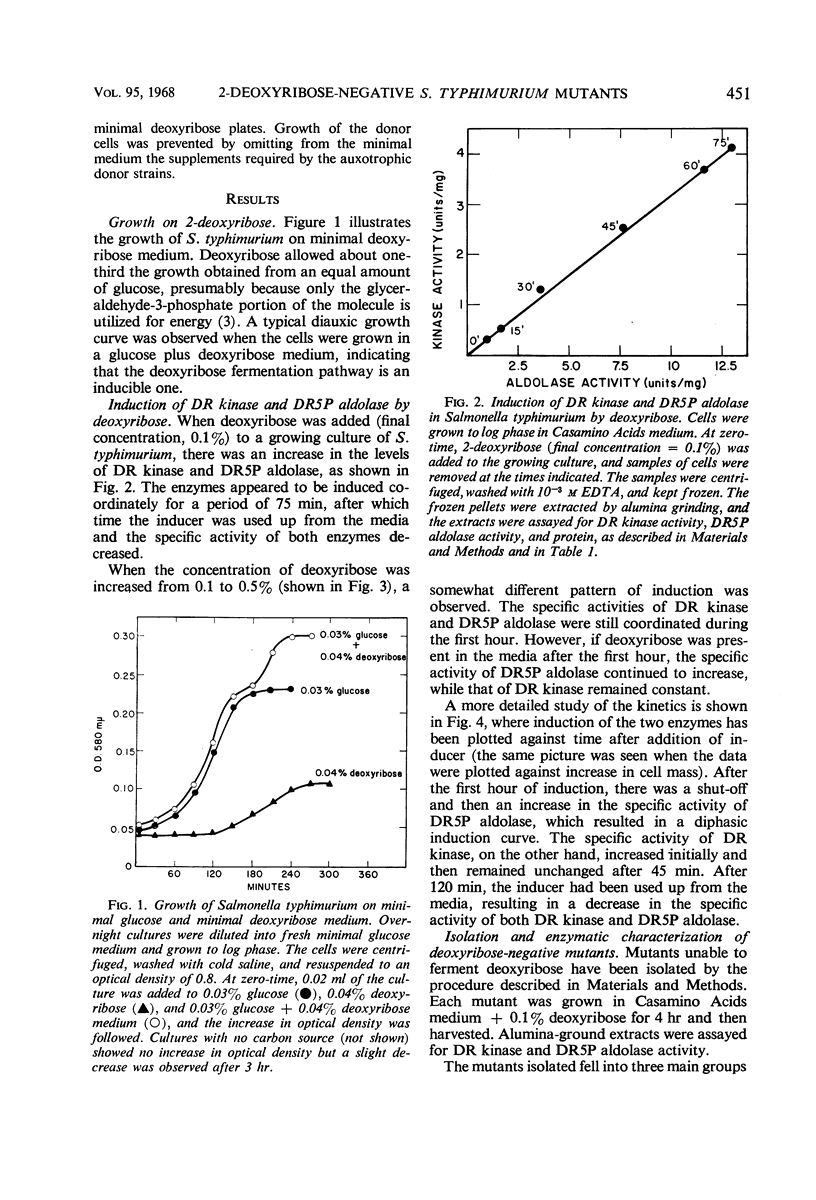
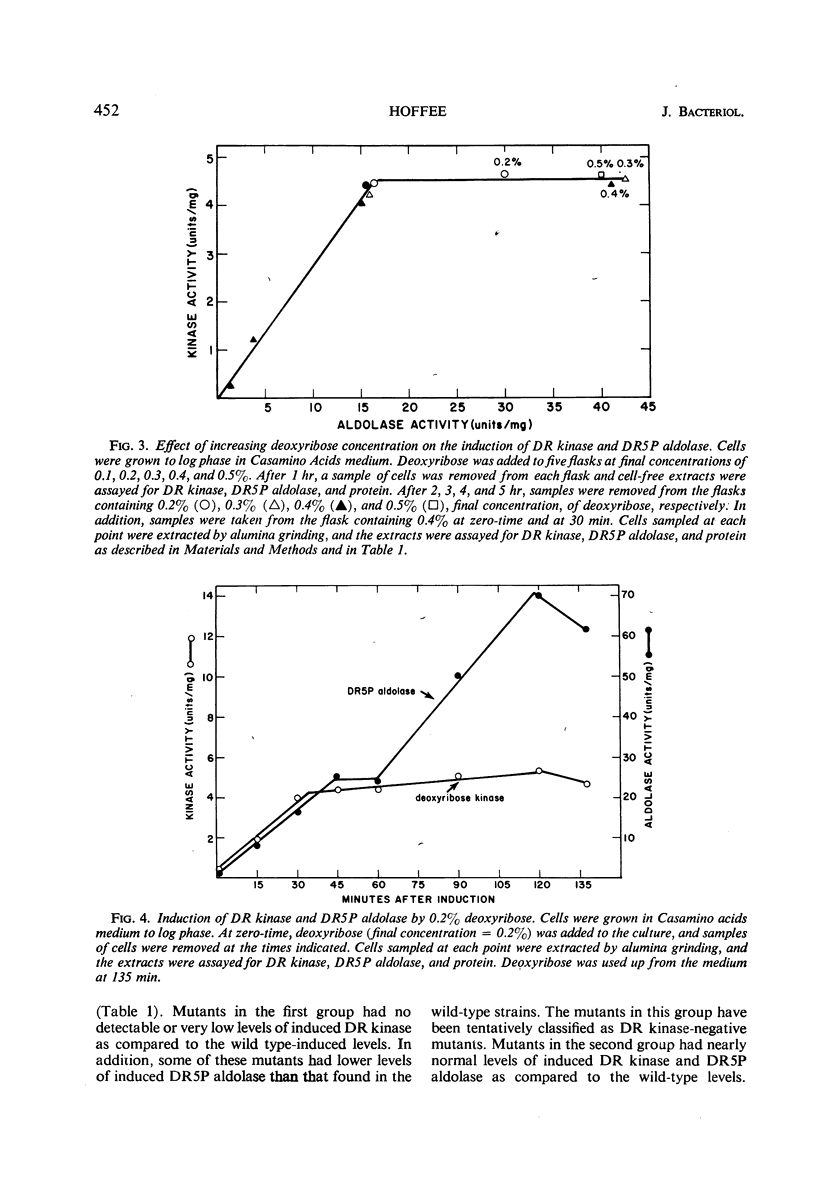
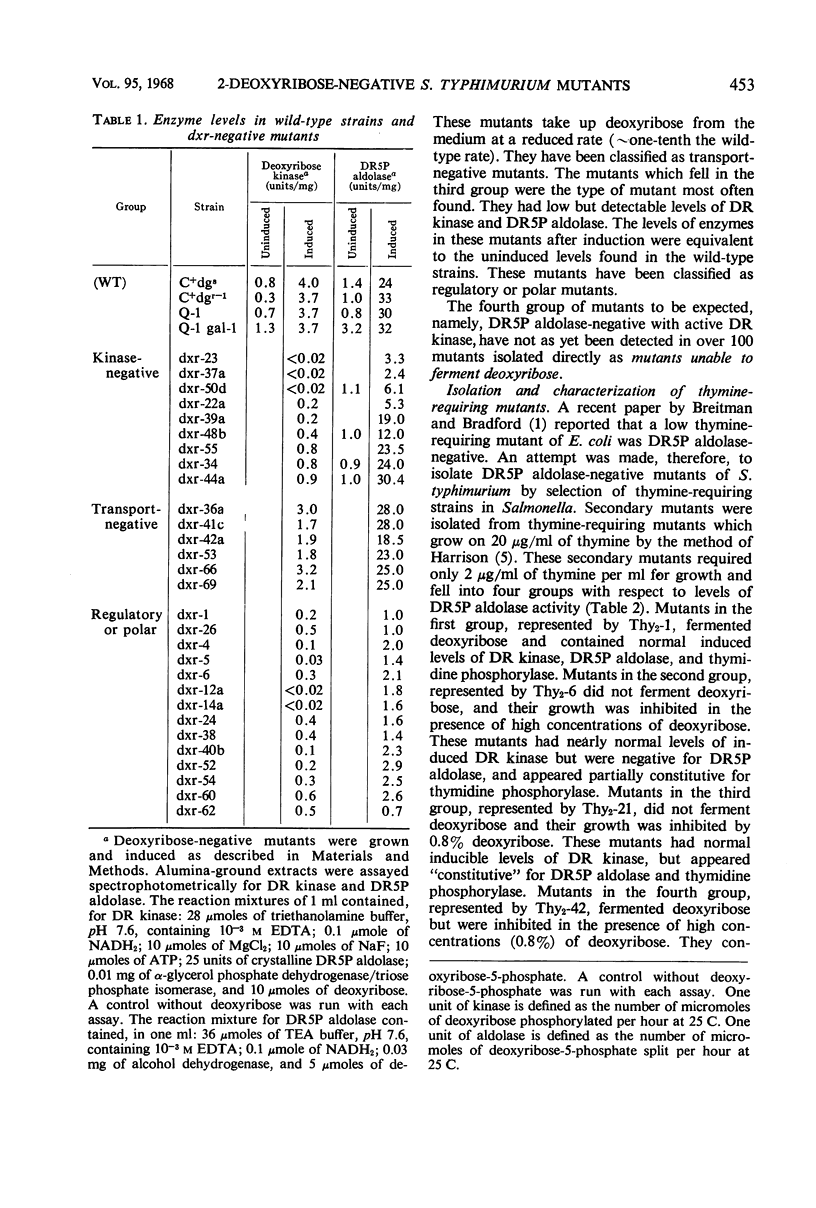
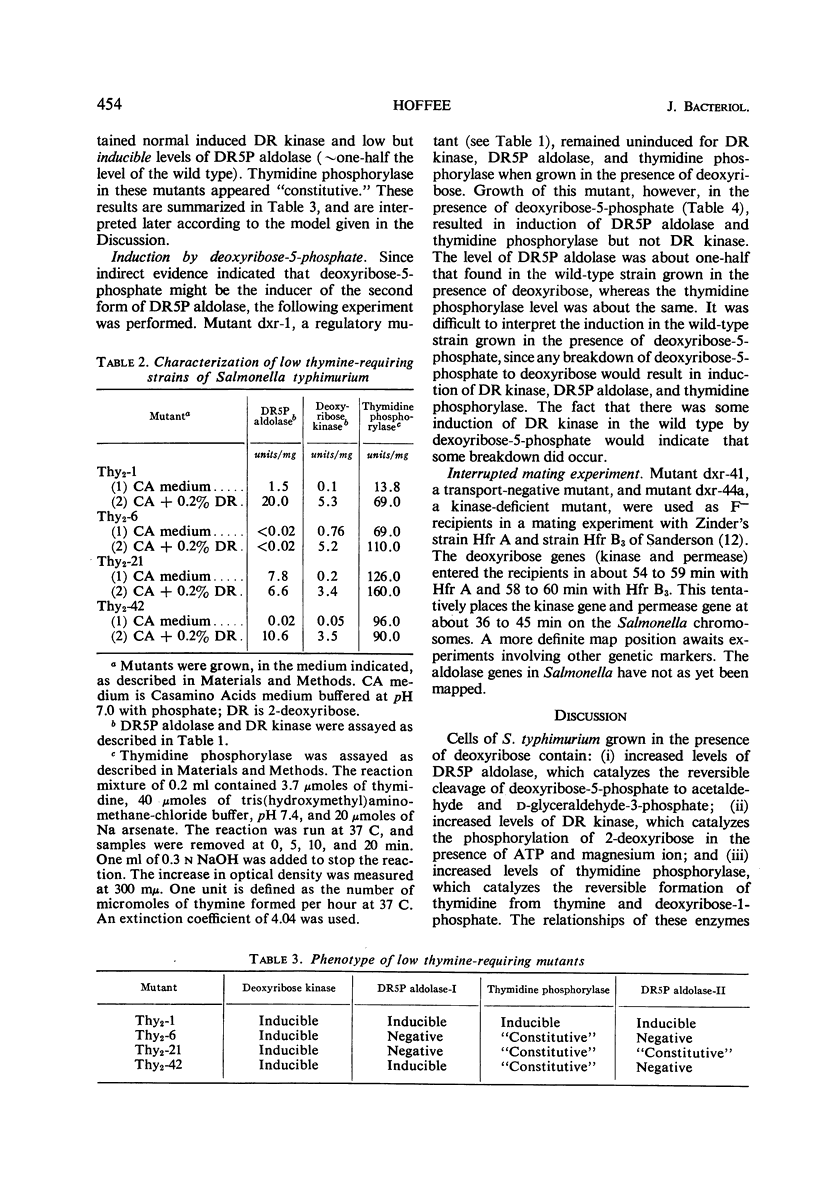
Salmonella typhimurium was found to utilize 2-deoxyribose as a sole carbon and energy source. Cells grown in the presence of deoxyribose contained increased levels of deoxyribose kinase, thymidine phosphorylase, and two forms of deoxyribose-5-phosphate aldolase (DR5P aldolase). One form of DR5P aldolase was induced by deoxyribose and coordinately regulated with deoxyribose kinase. The second form of DR5P aldolase was induced by deoxyribose-5-phosphate and coordinately regulated with thymidine phosphorylase. Mutants unable to ferment deoxyribose have been isolated and shown to be lacking either deoxyribose kinase or deoxyribose permease, but none has been found from which DR5P aldolase is missing. Thymine-requiring mutants which are able to grow on low levels of thymine have been isolated and shown, in some cases, to be lacking one or both DR5P aldolases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman T. R., Bradford R. M. The absence of deoxyriboaldolase activity in a thymineless mutant of Escherichia coli strain 15: a possible explanation for the low thymine requirement of some thymineless strains. Biochim Biophys Acta. 1967 Mar 29;138(1):217–220. doi: 10.1016/0005-2787(67)90610-7. [DOI] [PubMed] [Google Scholar]
- DOMAGK G. F., HORECKER B. L. Pentose fermentation by Lactobacillus plantarum. V. Fermentation of 2-deoxy-D-ribose. J Biol Chem. 1958 Aug;233(2):283–286. [PubMed] [Google Scholar]
- HOFFEE P., ROSEN O. M., HORECKER B. L. THE MECHANISM OF ACTION OF ALDOLASES. VI. CRYSTALLIZATION OF DEOXYRIBOSE 5-PHOSPHATE ALDOLASE AND THE NUMBER OF ACTIVE SITES. J Biol Chem. 1965 Apr;240:1512–1516. [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- JONSEN J., LALAND S., STRAND A. Degradation of deoxyribose by E. coli; studies with cell-free extract and isolation of 2-deoxy-D-ribose 5-phosphate. Biochim Biophys Acta. 1959 Mar;32(1):117–123. doi: 10.1016/0006-3002(59)90559-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICER W. E., Jr, HORECKER B. L. Deoxyribose aldolase from Lactobacillus plantarum. J Biol Chem. 1960 May;235:1292–1298. [PubMed] [Google Scholar]
- RACKER E. Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem. 1952 May;196(1):347–365. [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]


