Abstract
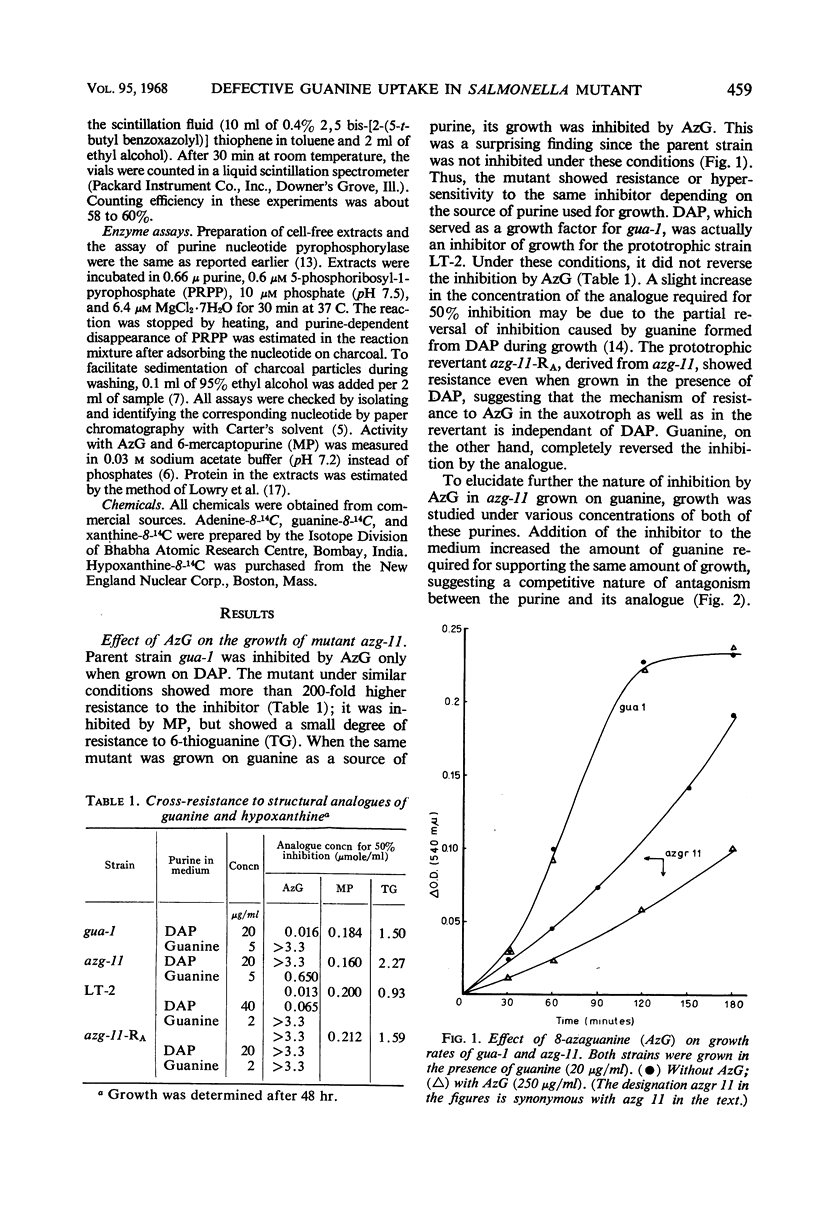
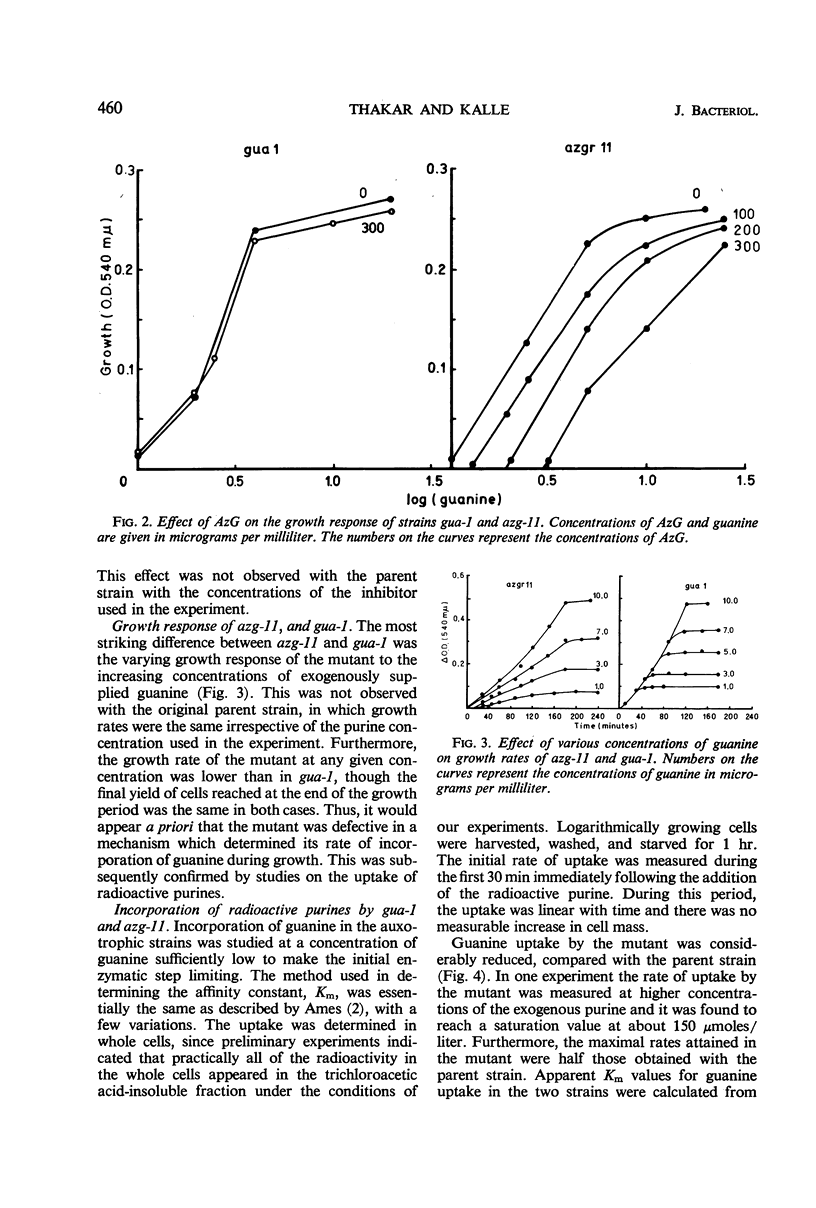
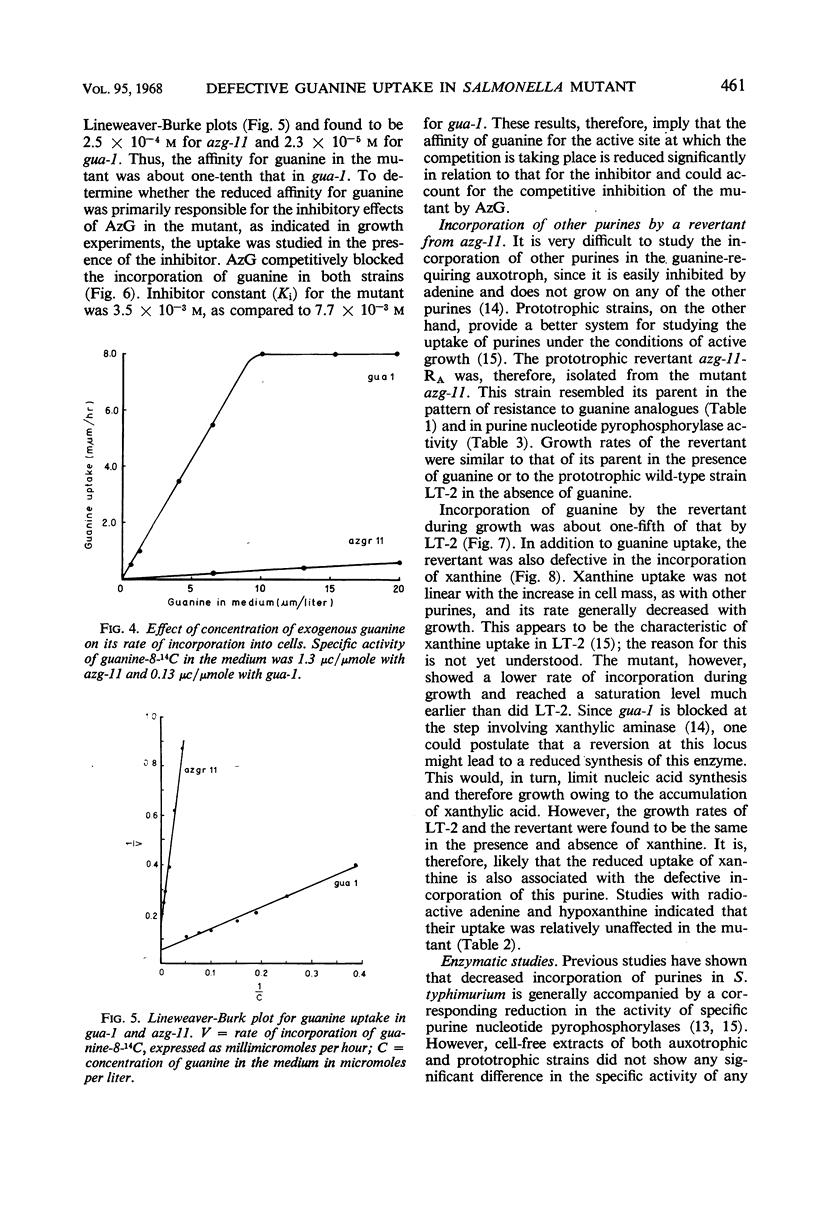
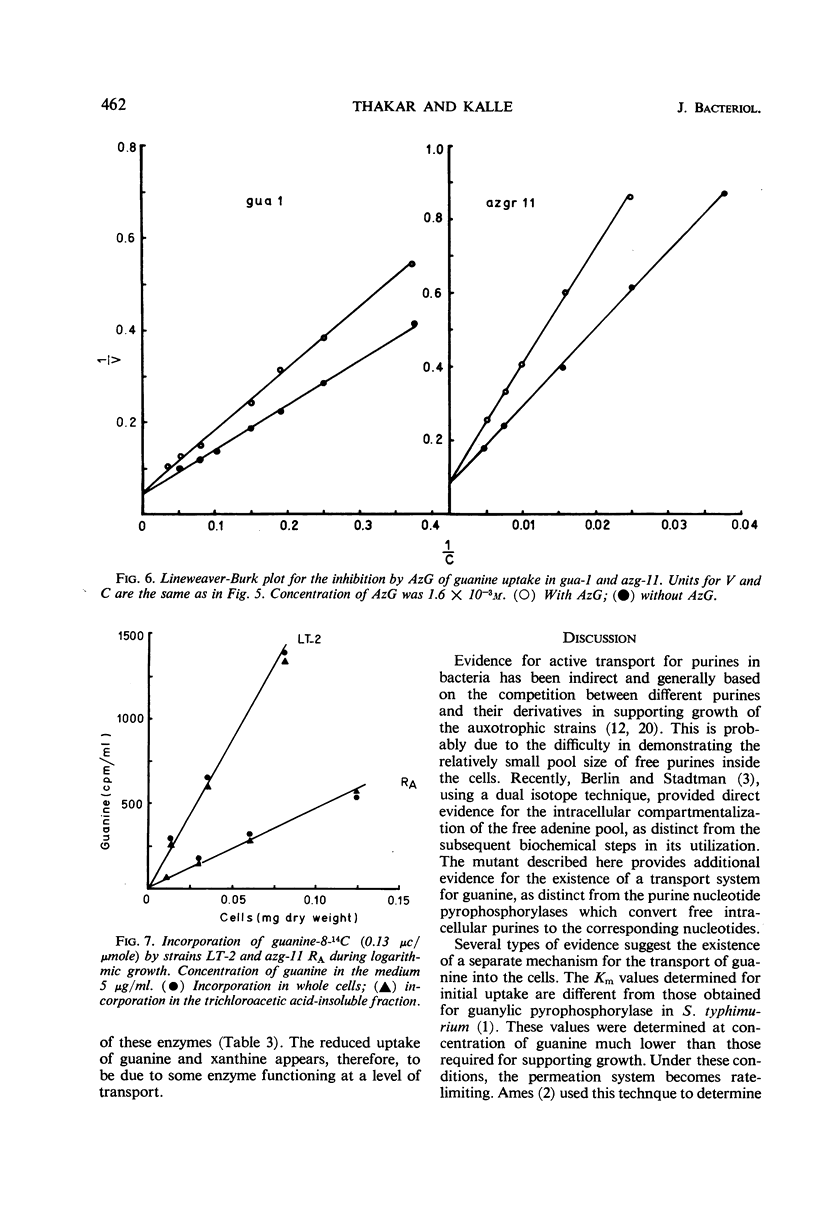
An 8-azaguanine-resistant mutant, azg-11, derived from a guanine auxotroph, gua-1, of Salmonella typhimurium was isolated. This mutant was resistant to the analogue when grown on 2,6-diaminopurine, but showed greater susceptibility than the parent on guanine. Studies with the uptake of radioactive purines revealed that the mutant was defective in a mechanism for incorporation of guanine as well as of xanthine. Initial rates of uptake were determined for guanine at concentrations which were sufficiently low to make permeases limiting. The affinity constant Km for the mutant was found to be 2.5 × 10−4m; that of the parent was 2.3 × 10−5m. Examination of cell-free extracts suggested that the purine nucleotide pyrophosphorylases, responsible for the conversion of free intracellular purines to the corresponding nucleotides, were present and unaltered. The results indicate that the mutant is defective in a mechanism for the active transport for guanine and possibly xanthine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- AMMANN E. C., LYNCH V. H. PURINE METABOLISM BY UNICELLULAR ALGAE. II. ADENINE, HYPOXANTHINE, AND XANTHINE DEGRADATION BY CHORELLA PYRENOIDOSA. Biochim Biophys Acta. 1964 Jul 22;87:370–379. doi: 10.1016/0926-6550(64)90110-0. [DOI] [PubMed] [Google Scholar]
- Adye J. C., Gots J. S. Further studies on genetically altered purine nucleotide pyrophosphorylases of Salmonella. Biochim Biophys Acta. 1966 May 5;118(2):344–350. doi: 10.1016/s0926-6593(66)80043-7. [DOI] [PubMed] [Google Scholar]
- BROCKMAN R. W. MECHANISMS OF RESISTANCE TO ANTICANCER AGENTS. Adv Cancer Res. 1963;7:129–234. doi: 10.1016/s0065-230x(08)60983-5. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- CARTER C. E. Reaction of 6-mercaptopurine with inosine- and guanosine-5'-phosphate pyrophosphorylase purified from E. coli. Biochem Pharmacol. 1959 Aug;2:105–111. doi: 10.1016/0006-2952(59)90077-2. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- DAVIDSON J. D., FEIGELSON P. The inhibition of adenosine deaminase by 8-azaguanine in vitro. J Biol Chem. 1956 Nov;223(1):65–73. [PubMed] [Google Scholar]
- DEMAIN A. L. ANTAGONISM BY PURINES AND DERIVATIVES IN THE NUTRITION OF A BACILLUS SUBTILIS MUTANT. Arch Biochem Biophys. 1964 Dec;108:403–408. doi: 10.1016/0003-9861(64)90420-5. [DOI] [PubMed] [Google Scholar]
- Darlington A. J., Scazzocchio C. Use of analogues and the substrate-sensitivity of mutants in analysis of purine uptake and breakdown in Aspergillus nidulans. J Bacteriol. 1967 Mar;93(3):937–940. doi: 10.1128/jb.93.3.937-940.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE R., LU W. C. ANTAGONISM BETWEEN PURINES IN PURINE-REQUIRING BACILLUS SUBTILIS MUTANTS. Arch Biochem Biophys. 1964 Dec;108:398–402. doi: 10.1016/0003-9861(64)90419-9. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Alterations in purine nucleotide pyrophosphorylases and resistance to purine analogues. Biochim Biophys Acta. 1961 Oct 14;53:166–173. doi: 10.1016/0006-3002(61)90803-4. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Antagonisms between purines and purine analogues in auxotrophs of Salmonella typhimurium. J Bacteriol. 1961 Mar;81:331–337. doi: 10.1128/jb.81.3.331-337.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Mechanism of resistance to 2,6-diaminopurine in Salmonella typhimurium. Biochim Biophys Acta. 1961 Jul 22;51:130–137. doi: 10.1016/0006-3002(61)91023-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUBIN M., KESSEL D. H., BUDREAU A., GROSS J. D. The isolation of bacterial mutants defective in amino acid transport. Biochim Biophys Acta. 1960 Aug 26;42:535–538. doi: 10.1016/0006-3002(60)90836-2. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. BIOCHEMICAL MECHANISMS OF DRUG RESISTANCE. Annu Rev Microbiol. 1964;18:347–366. doi: 10.1146/annurev.mi.18.100164.002023. [DOI] [PubMed] [Google Scholar]
- Peterson R. N., Koch A. L. The relationship of adenosine and inosine transport in Escherichia coli. Biochim Biophys Acta. 1966 Sep 5;126(1):129–145. doi: 10.1016/0926-6585(66)90043-4. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K., SIMON E. J. An impaired concentrating mechanism for amino acids in mutants of Escherichia coli resistant to L-canavanine and D-serine. Biochim Biophys Acta. 1959 Apr;32:582–583. doi: 10.1016/0006-3002(59)90650-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


