Abstract
Mutations in the hook gene alter intracellular trafficking of internalized ligands in Drosophila. To dissect this defect in more detail, we developed a new approach to visualize the pathway taken by the Bride of Sevenless (Boss) ligand after its internalization into R7 cells. A chimeric protein consisting of HRP fused to Boss (HRP-Boss) was expressed in R8 cells. This chimera was fully functional: it rescued the boss mutant phenotype, and its trafficking was indistinguishable from that of the wild-type Boss protein. The HRP activity of the chimera was used to follow HRP-Boss trafficking on the ultrastructural level through early and late endosomes in R7 cells. In both wild-type and hook mutant eye disks, HRP-Boss was internalized into R7 cells. In wild-type tissue, Boss accumulated in mature multivesicular bodies (MVBs) within R7 cells; such accumulation was not observed in hook eye disks, however. Quantitative electron microscopy revealed a loss of mature MVBs in hook mutant tissue compared with wild type, whereas more than twice as many multilammelar late endosomes were detected. Our genetic analysis indicates that Hook is required late in endocytic trafficking to negatively regulate delivery from mature MVBs to multilammelar late endosomes and lysosomes.
INTRODUCTION
Eukaryotic cells carefully regulate trafficking of internalized proteins (reviewed in Mukherjee et al., 1997). In most cells, endocytic vesicles deliver internalized cargo to early endosomes from which many proteins are shuttled back to the plasma membrane through a recycling compartment (Ghosh et al., 1994). Other proteins accumulate in vacuolar subcompartments of early endosomes before their transformation into mature multivesicular bodies (MVBs)1 named for their characteristic morphology (Stoorvogel et al., 1991; Dunn and Maxfield, 1992; van Deurs et al., 1993; Futter et al., 1996). From MVBs, endocytic cargo is delivered to morphologically distinct prelysosomal structures, the multilammelar late endosomes (Gruenberg and Maxfield, 1995; van Deurs et al., 1995; Futter et al., 1996; Mullock et al., 1998). The biochemical mechanisms that regulate these trafficking events late in the endocytic pathway are not well understood (Mukherjee et al., 1997).
The genetic dissection of endocytosis and vacuolar delivery in yeast has contributed much to our understanding of the underlying biochemical mechanisms (Wendland et al., 1998). Many genes have been identified that are required in the early and late phases of trafficking from the yeast cell surface to the vacuole (Riezman, 1993; Stack et al., 1995). Different subclasses of these genes affect specific steps along the endocytic and biosynthetic pathways, and their conservation suggests similar roles for their mammalian counterparts (Horazdovsky et al., 1994; Odorizzi et al., 1998; Sato et al., 1998).
Mutations in Drosophila constitute a resource for the genetic dissection of endocytic trafficking in multicellular organisms (Lloyd et al., 1998). Notably, the discovery that the shibire gene encodes the Drosophila homologue of Dynamin was important for revealing Dynamin function (Chen et al., 1991; van der Bliek and Meyerowitz, 1991). Early work on the shibire gene suggested its role in pinching endocytic vesicles from the plasma membrane (Kosaka and Ikeda, 1983a,b), a hypothesis recently confirmed by detailed biochemical analysis (Damke et al., 1994; Sweitzer and Hinshaw, 1998; Takei et al., 1998).
A direct effect of the shibire mutation on receptor-mediated endocytosis was first demonstrated for the Bride of Sevenless (Boss) ligand (Krämer et al., 1991). A search for additional mutations that altered internalization of Boss revealed a role for the Drosophila hook gene in endocytic trafficking (Krämer and Phistry, 1996). At the light microscopy level, the effects of hook and shibire mutations appeared similar: the amount of detectable Boss protein in R7 cells was reduced compared with wild type. An analysis of a viable hook null allele revealed considerable differences in the functional consequences of hook and shibire mutations, however. For example, the shibire mutation caused paralysis and defects in cell–cell communication during development (Ramaswami et al., 1993; Seugnet et al., 1997), processes unaffected by a complete loss of hook function (Krämer and Phistry, 1999).
Initial insights into the function of Hook were provided by molecular analysis. The hook gene encodes a cytoplasmic dimeric protein of 679 amino acids with an extended central coiled coil domain, which is conserved in two human homologues (Krämer and Phistry, 1996, 1999). Immunohistochemical studies revealed that Hook localizes to endocytic vesicles and large vacuoles that are distinct from lysosomes (Krämer and Phistry, 1996). This localization indicates that Hook may function late in endocytic trafficking. To better understand the role of the Hook, we sought to determine the specific step in endocytosis altered by the hook mutation.
The Boss ligand serves as a convenient marker for analyzing endocytic trafficking in Drosophila. Boss is a transmembrane protein with seven membrane-spanning segments and a large extracellular domain (Hart et al., 1990). In eye imaginal disks, Boss is expressed only on the surface of R8 photoreceptor cells (Krämer et al., 1991). Upon binding to the Sevenless receptor on the neighboring R7 cell, the entire Boss transmembrane ligand is internalized into R7 cells by receptor-mediated endocytosis (Cagan et al., 1992). The term “trans-endocytosis” has been coined to describe such internalization of transmembrane ligands across cell boundaries (Klueg et al., 1998).
To characterize trafficking of Boss through the endocytic compartments of R7 cells in more detail, we modified a method first introduced by Connolly et al. (1994) and Stinchcombe et al. (1995) to analyze Golgi protein trafficking. They fused the enzyme HRP to proteins and then followed these tagged proteins through the Golgi complex by electron microscopy (EM). This method provides two important benefits for the analysis of late endocytic trafficking. First, ultrastructural detection of HRP activity is straightforward using 3,3′-diaminobenzidine (DAB) as a substrate. Second, whereas most ligands are quickly degraded in the destructive environment of late endosomes and lysosomes, the HRP enzyme remains stable at low pH and in the presence of lysosomal enzymes.
We fashioned a chimera between HRP and Boss and then followed its movement through the endocytic compartments of wild-type Drosophila and mutants affecting endocytic trafficking
MATERIALS AND METHODS
Fly Stocks and Transgenic Flies
The shits1 and w1118; cu boss1 stocks were obtained from Mani Ramaswami (University of Arizona, Tucson, AZ) and Larry Zipursky (University of California, Los Angeles, CA), respectively, and have been described (Lindsley and Zimm, 1992). Recombining the hk11 null mutation with the P[w+, gen.HRP-Boss]17 transgene (see below) simultaneously removed the cn and bw mutations and at least one unknown lethal mutation from the original hk11 chromosome (Krämer and Phistry, 1999).
The HRP-Boss Chimera
DNA encoding HRP (Hartmann and Ortiz de Montellano, 1992; Connolly et al., 1994) was inserted into an 8-kb genomic fragment containing the entire boss gene (Hart et al., 1993a) using standard PCR-based cloning. The insertion site was just 3′ to the DNA encoding the signal sequence of Boss after histidine 33 (Hart et al., 1990). The resulting sequence was V L E C H(33) a g MQLTPT . . . . . SNSGG H(33) G A D L T S P T K K S A P. Bold letters indicate sequences derived from Boss, and italic letters represent the inserted full-length HRP enzyme. Two amino acids indicated in lower case were introduced as spacers. Histidine 33 was repeated in the Boss sequence 3′ to the inserted HRP sequences. This region of Boss is most divergent between the Drosophila melanogaster and virilis Boss proteins, suggesting that it is not directly involved in binding to the Sevenless receptor (Hart et al., 1993b). After cleavage of the signal peptide, the HRP enzyme constituted the new N terminus of the mature chimeric protein (Figure 1A).
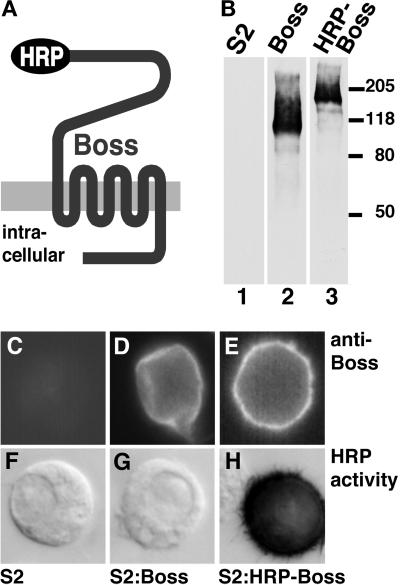
Figure 1.
The HRP-Boss chimera exhibits HRP activity. (A) Schematic diagram indicates the site of fusion between the HRP protein and the extracellular N terminus of the mature Boss protein. (B) Western analysis confirmed that the chimeric protein (HRP-Boss, lane 3) was expressed as a single protein with the expected increase in size compared with the Boss protein (Boss, lane 2). The diffuse appearance of Boss and HRP-Boss are due to the high degree of glycosylation of Boss (Hart et al., 1993b). Untransfected S2 cells (S2, lane 1) were used as a control. Immunofluorescence staining (anti-Boss NN1 antibodies) of untransfected S2 cells (C) and cells expressing either Boss (D) or HRP-Boss (E) confirmed that the chimeric protein was transported to the cell surface like wild-type Boss. HRP activity stain of these cells indicated that HRP-Boss expressed on the cell surface retained its activity (H), whereas no HRP activity was detected in S2 (F) or S2:Boss control cells (G).
The boss gene containing the HRP sequences was cloned into the pCaSpeR transformation vector yielding the construct pCapser-gen.HRP-Boss (Thummel and Pirrotta, 1992), and transgenic flies were established by injecting it into w1118; boss1 cu flies using standard techniques (Rubin and Spradling, 1982). From four transgenic lines that expressed Boss in the wild-type Boss pattern, we chose a line carrying the P element expressing HRP-Boss, P[w+, gen.HRP-Boss]17, on the second chromosome for all further experiments, because its level of expression was indistinguishable from that of wild-type Boss.
The HRP-coding sequences were also introduced into a boss cDNA at the same position relative to the Boss protein (3′ to histidine 33). This cDNA construct was introduced in the pCaSpeR-HS vector (Thummel and Pirrotta, 1992) for heat-inducible expression in tissue culture cells.
Light Microscopy
Cell surface expression of Boss and HRP-Boss in S2 tissue culture cells was detected by anti-Boss antibodies (anti-Boss NN1, at a dilution of 1:3000) and FITC-labeled secondary antibodies as previously described (Krämer et al., 1991). In tissue culture cells, intrinsic HRP activity of HRP-Boss was visualized using DAB (Connolly et al., 1994). In eye disks, Boss proteins were detected by light microscopy using primary anti-Boss antibodies (anti-Boss NN1, 1:3000), HRP-conjugated secondary antibodies, and the Ni2+-enhanced DAB method previously described (Sevrioukov et al., 1998). Two fixation steps in the eye disk staining protocol abolished the intrinsic HRP activity of HRP-Boss (our unpublished results). For quantitative assays, we counted R7 cells with detectable internalized Boss protein. Boss staining in R7 cells was judged by examining different focal planes of stained eye disks (Sevrioukov et al., 1998). To score for the presence of R7 cells, adult eyes were fixed, embedded in plastic, and sectioned as described (Van Vactor et al., 1991).
EM
Eye imaginal disks were fixed and embedded as described by Van Vactor et al. (1991). Sections (30–50 nm) were poststained in 5% uranyl acetate in 50% methanol/water and Reynold’s lead citrate and examined on a Jeol (Tokyo, Japan) 1200 transmission electron microscope. For quantitative analysis of the hook mutant phenotype, we assessed the endocytic pathway in eye disks from wild-type, hk11, and hk8 larvae. For each genotype we analyzed ∼85 ommatidia (∼1500 cells) in each of three eye disks, totaling at least 250 ommatidia. Within an ommatidium, vesicles recognized as part of the endocytic pathway were counted at 30,000-fold magnification and categorized into three groups according to the criteria of Futter et al. (1996): 1) immature MVBs (vacuoles containing one to five internal vesicles), 2) mature MVBs (large vacuoles containing more than five internal vesicles), and 3) multilammelar late endosomes or lysosomes. Statistical analysis of the number of vesicles from each category of wild type versus hk11 or hk8 was performed using a two-tailed Student’s t test assuming equal variance.
To visualize the HRP-Boss chimera by EM, eye imaginal disks were dissected in PBS and incubated in the membrane-permeable substrate DAB (0.5 mg/ml) for 10 min, and then H2O2 was added to a final concentration of 0.003%. Note that this HRP development step acts as an initial fixation step (Futter et al., 1996). After 20 min, stained eye disks were washed in PBS, further fixed for 30 min in 2% paraformaldehyde, 0.075 M lysine, 0.01 M NaIO, 0.037 M phosphate buffer, pH 7.4, and 1 h in 2% glutaraldehyde, and then processed for EM as described above. By contrast to HRP expressed in mammalian tissue culture cells (e.g., Stinchcombe et al., 1995), even modest fixation with 2% paraformaldhyde considerably reduced intrinsic HRP activity of the chimeric protein expressed in transgenic flies.
The shits1 mutation was used to block internalization of the HRP-Boss chimera into R7 cells. Third instar larvae of the genotype shits1 w; P[w+, gen.HRP-boss]17 or wild-type flies carrying the P[w+, gen.HRP-boss]17 transgene were incubated at the nonpermissive temperature (30°C) for 1 or 2 h. At 30°C, immobilized larvae were dissected in prewarmed (37°C) PBS and incubated in 0.5 mg/ml DAB in PBS (30°C) for 10 min. H2O2 was added to a final concentration of 0.003%. After 20 min, eye disks were fixed as described above and processed for EM.
To capture early events in endocytosis, shits1 w; P[w+, gen.HRP-boss]17 third instar larvae were incubated at 30°C for 1 h and then placed at room temperature for 5 min. Eye disks were dissected and incubated in 0.5 mg/ml DAB for 10 min before HRP activity was visualized as described above.
Molecular Biology Techniques
Standard molecular biology techniques were used as described (Ausubel et al., 1994). To test expression of HRP-Boss in tissue culture cells, S2 cells were transfected with the pCaSpeR-HS-HRP-Boss vector using previously described methods (Krämer et al., 1991). For Western analysis, extracts from 106 S2 cells expressing wild-type Boss, HRP-Boss, or no Boss proteins were prepared in 1× Laemmli loading buffer and separated by SDS-PAGE. After transfer to nitrocellulose membranes, Boss proteins were detected using anti-Boss NN1 antibodies (1:3000) and enhanced chemiluminescence (Pierce Chemical, Rockford, IL). Molecular weight markers were prestained proteins (Life Technologies, Grand Island, NY).
RESULTS
The HRP-Boss Chimera
To follow the pathway of internalized Boss in R7 cells, we fused HRP to the N terminus of the mature Boss protein (Figure 1A). We first assessed whether we had created a functional transmembrane protein by expression in S2 Drosophila tissue culture cells. The chimeric HRP-Boss protein was detected at the expected mass of ∼150 kDa (Figure 1B). Addition of the HRP moiety did not affect the transport of Boss to the cell surface, as determined by its unchanged accessibility to anti-Boss antibodies (Figure 1, C–E). Finally, the chimeric HRP-Boss protein had substantial enzymatic activity, as demonstrated by the DAB substrate (Figure 1, F–H). No secreted soluble HRP activity was detected in media of cells expressing HRP-Boss (our unpublished results).
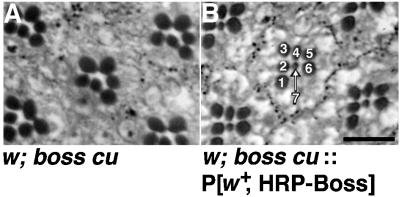
HRP-Boss also retained the R7-inducing activity of Boss. A genomic 8-kb fragment of the boss gene is sufficient to direct R8-specific expression and to rescue the boss mutant phenotype (Hart et al., 1993a). When HRP-Boss was expressed in transgenic flies under control of this boss genomic fragment, the HRP moiety did not perturb the inductive activity of Boss. The HRP-Boss transgene, P[w+, gen.HRP-boss], rescued the boss mutant phenotype and restored normal development of R7 cells, which are missing in boss mutants (Figure 2). Together, these assays confirmed that we had successfully produced a fully functional HRP-Boss transmembrane ligand.
Figure 2.
The HRP-Boss chimera rescues the boss phenotype. Light micrographs of plastic sections of adult eyes from either a boss1 mutant (A) or a boss1 mutant expressing the HRP-Boss transgene (B) revealed that the HRP-Boss transgene rescued the boss phenotype and restored normal development of R7 cells, which can be recognized by their small, centrally located rhabdomeres in each ommatidium in these apical sections. The relevant genetic backgrounds are indicated. Bar, 10 μm.
HRP-Boss Expression in Eye Imaginal Disks
Boss function is required for neural induction of R7 cells early in larval development. Cellular differentiation in the Drosophila eye is initiated at the posterior rim of the larval eye imaginal disk (Wolff and Ready, 1993). The morphogenetic furrow, which can be recognized as an indentation of the apical surface, marks the leading edge of development as it moves from posterior to anterior across the eye disk. As a result, development occurs in a graded manner; each row of ommatidia, numbered from the furrow in the posterior direction, is ∼2 h less mature than the neighboring posterior row. Within this gradient of development, Boss expression in R8 cells is initiated at row 3, and its internalization into R7 cells occurs in rows 5–12 (Krämer et al., 1991). Most of the following analysis was restricted to this window in development. In the developing ommatidia, different cell types can be recognized based on their stereotyped positions, shapes, and contacts to neighboring cells (Wolff and Ready, 1993).
First, we compared the localization of the HRP-Boss protein to that of the endogenous Boss protein. When evaluated by antibody staining and light microscopy, both the endogenous Boss protein (Figure 3A) and the HRP-Boss chimera (Figure 3C) were detected on the apical surface of R8 cells and internalized into R7 cells. Internalization of HRP-Boss into R7 cells was dependent on the Sevenless receptor (Figure 3E) as previously described for the wild-type Boss protein (Krämer et al., 1991; Cagan et al., 1992). We concluded that the expression pattern and trafficking of the HRP-Boss chimera was indistinguishable from that of endogenous Boss at this level of analysis.
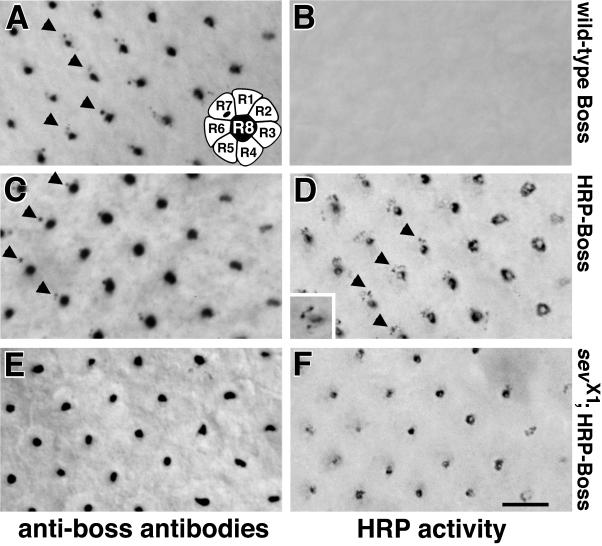
Figure 3.
The HRP-Boss chimera is internalized into R7 cells by receptor-mediated endocytosis. Eye imaginal disks expressing either the endogenous Boss protein (A and B) or the HRP-Boss chimera in the absence of wild-type Boss (C–F) were either stained with anti-Boss antibodies (A, C, and E) or developed for HRP activity intrinsic to the chimera (B, D, and F). Antibodies detected endogenous Boss (A) and HRP-Boss (C) on the apical R8 cell surface (large dots of staining) and internalized into R7 cells (arrowheads). Recognition of R7 cells in the eye disk is based on their stereotyped positioning relative to the R8 cell in each ommatidium (A, inset). As described for wild-type Boss (Krämer et al., 1991), uptake of the HRP-Boss chimera into R7 cells was dependent on the presence of the Sevenless receptor, because it was not observed in sevX1 mutant eye disks (E). Similar results were obtained when the HRP-Boss chimera was visualized by its HRP activity (D and F), whereas no HRP activity stain was detected in wild-type eye disks (B). In HRP-Boss–expressing eye disks, HRP activity was detected in R7 cells up to the posterior edge of the disk >16 h after Boss internalization into R7 cells (D, inset). Whereas some vesicles in other cells next to R8 cells might also exhibit weak labeling, EM analysis revealed that the vast majority of HRP activity is restricted to R8 and R7 cells (see Figures 4–8). For these panels and similar images of eye imaginal disks in Figures 6 and 8, posterior is to the left, and the right edge of each panel corresponds to row 3, the onset of Boss expression (Krämer et al., 1991). The relevant genotypes were Oregon R (A and B), w1118; P[w+, gen.HRP-Boss]17; boss1 (C and D), and sevX1; P[w+, gen.HRP-Boss]17; boss1 (E and F). Bar, 5 μm.
Next, we compared the detection of HRP-Boss by its intrinsic HRP activity to its detection by antibodies. HRP activity was specific for HRP-Boss, because no endogenous HRP activity was observed in wild-type eye disks (Figure 3B). In transgenic lines expressing the chimera, the typical pattern of apical surface staining on R8 cells and the staining of internalized Boss in R7 cells emerged (Figure 3D). HRP activity in R7 cells was dependent on the presence of Sevenless receptor (Figure 3F), indicating that it entered R7 cells by receptor-mediated endocytosis.
Two instructive differences were evident between the detection of HRP-Boss using antibodies and using its intrinsic HRP activity. First, a larger number of stained internal vesicles were detected in R8 and R7 cells by HRP activity (Figure 3, compare C and D). Second, Boss antibody staining was not detectable in the R7 cells of ommatidia in which expression of the Sevenless receptor on the R7 cell surface had ceased (Figure 3A, posterior to row 11; also see Tomlinson et al., 1987). This indicates rapid degradation of the Boss protein after its internalization into R7 cells. However, HRP activity in R7 cells of transgenic flies persisted to the posterior edge of the eye imaginal disk, often >20 rows after the passing of the morphogenetic furrow (Figure 3D, inset). This corresponds to a time of >16 h after the cessation of Sevenless receptor expression on R7 cells. These findings are consistent with previous reports that, in mammalian cells, the HRP enzyme remains active after its internalization into lysosomes (Futter et al., 1996). Persistent HRP activity in the absence of anti-Boss antibody staining is therefore likely to reflect the survival of the HRP moiety in late endosomes and lysosomes long after the Boss component of the chimeric protein has vanished.
Endocytic Trafficking of Boss in Wild-Type R7 Cells
To analyze Boss trafficking in more detail, we visualized HRP activity by EM. In R8 cells, localization of the HRP-Boss fusion protein was similar to that of the wild-type Boss protein by immuno-EM (Krämer et al., 1991). The intrinsic activity of HRP-Boss was first detected in the Golgi and in vesicles in R8 cells (Figure 4B). We did not detect Golgi labeling in other cell types, consistent with the R8-specific expression of the HRP-Boss chimera. On the R8 cell plasma membrane, no staining was detectable at the basolateral surface, whereas strong staining decorated the microvilli and the cell surface just basal to the microvilli (Figure 4, C and D). In sections ∼5 μm below the apical surface of R7 cells, we detected the dramatic accumulation of HRP activity in endocytic structures (Figure 4E). These images confirm that the HRP-Boss chimera is a powerful tool for visualizing the endocytic compartment in Drosophila.
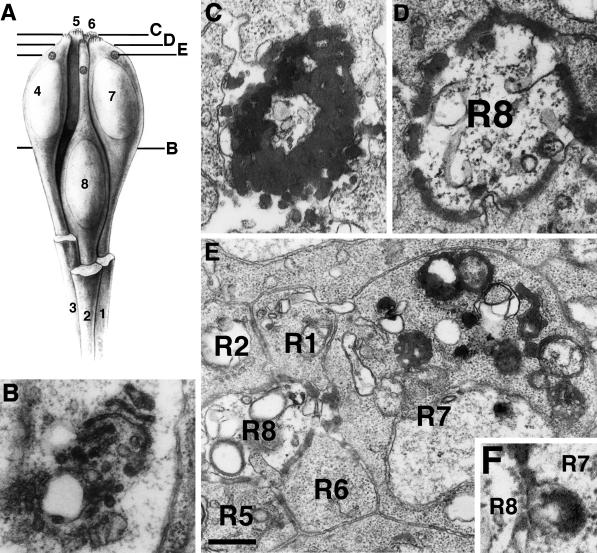
Figure 4.
The HRP-Boss chimera reveals Boss trafficking on the ultrastructural level. (A) Schematic drawing of a developing ommatidium indicates the relative position of the eight photoreceptor cell bodies (1–8), which can be identified based on their shapes and their specific contacts to neighboring cells (Wolff and Ready, 1993). Photoreceptor cells R1–R3 in the front are broken away to reveal the central R8 cell and the neighboring R7 cell. The levels of EM sections in B–E are indicated in the diagram. At the R8 perinuclear level (B), HRP activity was detected in the stacks of the Golgi complex of R8 cells. No labeling was detected on the basolateral R8 cell surfaces. From the Golgi complex, HRP-Boss was transported to the apical microvilli of R8 cells (C). In sections just below the microvilli (D), the apical R8 cell surface was specifically labeled by HRP activity. At a slightly more basal level (E), the specific uptake of HRP-Boss into R7 cells was revealed. Whereas weak HRP staining was sometimes observed in cells other than R7, especially R3, R4, and cone cells, only R7 cells consistently exhibited the dramatic staining of the multitude of vesicular and vacuolar structures that constitute the endocytic compartment. (F) Occasionally, we observed patches of stained R8 cell surface pulled into the R7 cell. A was reprinted with modifications from (Krämer et al., 1991) by permission from Nature (copyright 1991 Macmillan Magazines Ltd.). Relevant genotypes were w1118; P[w+, gen.HRP-Boss]17; boss1. Bar: B and D, 0.25 μm; C, 0.3 μm; E, 0.4 μm; F, 0.16 μm.
HRP staining on the R8 cell surface often appeared in patches (Figure 4, D–F), which is likely to reflect oligomeric ligand–receptor complexes. Such complexes seemed to be pulled into R7 cells (e.g., Figure 4F). Such trans-endocytosis of membrane-bound Boss should produce in R7 cells HRP-stained vesicles with internal vesicles. Such structures were observed next to the R8–R7 interface in R7 cells (Figure 5A). This endocytic stage appeared short lived, however, because it was only rarely captured in electron micrographs, even when endocytosis was blocked using the shibire mutation (see below). Only two of several hundred R7 cells exhibited the type of endocytic structure shown in Figure 5A.
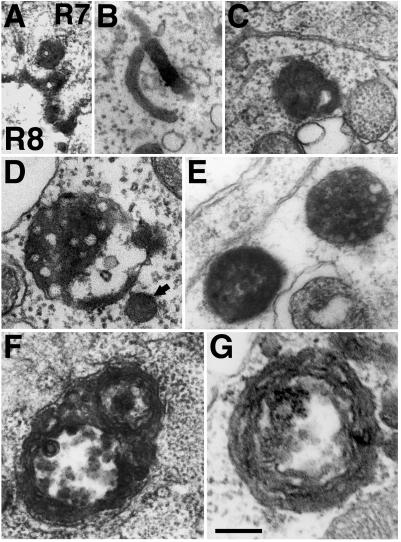
Figure 5.
The internalized HRP-Boss chimera labels typical early and late endocytic structures in R7 cells. Internalized HRP-Boss was identified in electron micrographs by its HRP activity. Adjacent to the R8–R7 cell interface, HRP activity could be detected in vesicles with an internal vesicle (A), likely to represent patches of R8 cell surface pulled into R7 cells by the Boss–Sevenless interaction. Inside R7 cells, HRP-Boss was detected in all the typical endocytic structures: tubular (B) and tubular-vacuolar (C) large, mature MVBs (D and E), and multilammelar late endosomes or lysosomes (G). Structures of character intermediate to those of MVBs and multilammelar endosomes (F) were consistent with the previously described direct fusion between these compartments (Futter et al., 1996). Bar: F, 120 nm; A–E and G, 200 nm.
After internalization into R7 cells, HRP-Boss was detected in all classically described endocytic structures. It could be found in tubular and tubular-vesicular early endosomes (Figures 4E and 5, B and C) and in mature MVBs larger than 300 nm with many internal vesicles (Figure 5, D and E). We also noted smaller vacuoles of a size of 120–150 nm, which appeared to correspond to immature MVBs (Futter et al., 1996). These small MVBs (Figure 5D, arrow) were often intensely stained, and it was rarely possible to unequivocally outline the few internal vesicles. And finally, HRP activity was detected in multilammelar structures that are late endosomes or lysosomes (Figure 5G). The presence of large vacuolar structures composed of both multilammelar and multivesicular sections (Figures 4E and 5F) was consistent with previous findings that these structures can fuse directly (Futter et al., 1996). The endocytic compartments labeled by the HRP-Boss chimera were morphologically indistinguishable from those described in mammalian tissue culture cells (reviewed in Mukherjee et al., 1997).
Blocking Endocytosis Using shibire
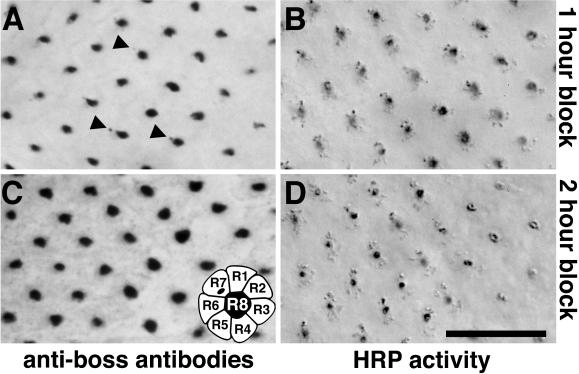
To establish the temporal order in which Boss is transported through these endocytic structures, we blocked endocytosis using the shits1 mutation, a temperature-sensitive allele of the shibire gene, which encodes the Drosophila homologus of the mammalian Dynamin protein (Chen et al., 1991; van der Bliek and Meyerowitz, 1991). At the permissive temperature, shits1 eye disks were indistinguishable from wild type. At the nonpermissive temperature, Boss internalization was blocked in shits1 mutants (Krämer et al., 1991). In accordance with this previous finding, staining with anti-Boss antibodies detected a dramatic reduction of HRP-Boss in R7 cells after 1 h at the nonpermissive temperature (compare Figures 3A and 6A). Under these conditions, only 6% of R7 cells between rows 5 and 12 exhibited anti-Boss staining (Figure 6A, arrowheads; n = 5 eye disks), whereas antibodies detected Boss in 63% of corresponding wild-type R7 cells (n = 5). After 2 h at the nonpermissive temperature, HRP-Boss could no longer be detected with anti-Boss antibodies in R7 cells (Figure 6C). By contrast, the detection of HRP activity in R7 cells was not substantially changed at the light microscopy level when the endocytosis of HRP-Boss was blocked for 1 or 2 h by the shits1 mutation (Figure 6, B and D).
Figure 6.
The shits1 mutation blocks Boss internalization. Light micrographs of eye disks reveal the block of Boss endocytosis in shits1 mutant larvae after 1 h (A and B) or 2 h (C and D) at the nonpermissive temperature (30°C). The reduced level of Boss internalized into R7 cells was visualized with anti-Boss antibody staining (compare A and C with Figure 3A). However, no change could be detected in HRP activity in R7 cells (B and D). The relevant genotypes of the larvae were w1118 shits1; P[w+, gen.HRP-Boss]17. Bar, 12 μm.
The basis for this differential behavior of the HRP activity stain compared with antibody detection became obvious after analysis of shibire eye disks at the EM level. After a 1-h block of endocytosis at the nonpermissive temperature, HRP activity was dramatically reduced in early endocytic structures and mature MVBs and had been chased into multilammelar late endosomes and lysosomes (Figure 7A). After a 2-h block, this shift was complete; no stained MVBs were observed in >60 R7 cells examined (n = 3 eye disks), whereas intense staining was still observed in multilammelar late endosomes or lysosomes (Figure 7, C–E).
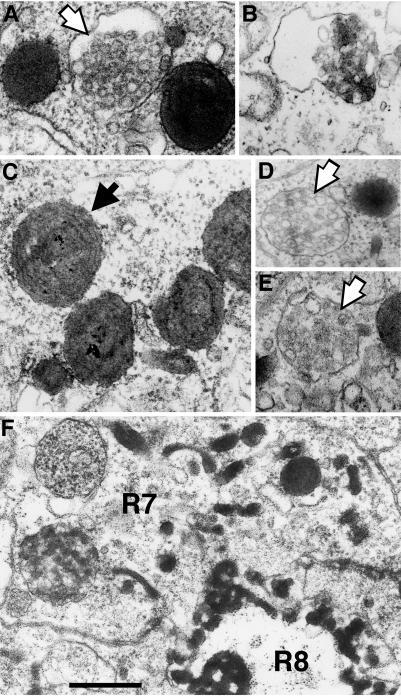
Figure 7.
Endocytic compartments can be ordered using the shits1 mutation. HRP-Boss was localized by EM in shits1 eye disks after blocking endocytosis at 30°C. After 1 h, the majority of HRP activity was detected in multilammelar late endosomes or lysosomes (A). Only rarely did we detect remnants of HRP activity in MVBs (B). After 2 h at the nonpermissive temperature, strong staining in multilammelar late endosomes and lysosomes persisted (C–E), whereas labeled MVBs could not no longer be detected. Strikingly, after blocking shibire function, unstained MVBs were detected in R7 cells (A, D, and E, open arrows). A synchronized wave of endocytosis was released by holding shits1 larvae at 30°C for 1 h and than returning them to 22°C for 15 min before visualizing HRP activity (F). The relevant genotypes of the larvae were w1118 shits1; P[w+, gen.HRP-Boss]17. Bar: A, C, and E, 250 nm; B, D, and F, 400 nm.
Strikingly, we did observe unstained mature MVBs in R7 cells after a 2-h block of endocytosis (Figure 7, D and E, open arrows) and occasionally even after a 1-h block (Figure 7A, open arrow). Such unstained mature MVBs were never observed in >200 wild-type R7 cells examined (n = 7 eye disks). Together, these data indicate that anti-Boss antibody staining in R7 cells reflected Boss present in MVBs and that HRP activity in the absence of accompanying Boss staining reflected HRP-Boss after degradation of its Boss moiety in the proteolytic environment of the multilammelar late endosomes and lysosomes (see DISCUSSION).
The shibire mutation was also useful to define early endocytic structures after the trans-endocytosis of Boss. To release a synchronized pulse of Boss internalization, shits1 eye disks were returned to 22°C after a 1-h block of endocytosis at the nonpermissive temperature (Tsuruhara et al., 1990). After 15 min at the permissive temperature, eye disks were processed for the ultrastructural detection of HRP activity. Under these conditions, a majority of HRP activity was detected in small vesicles and tubular early endosomes (Figure 7F). These tubular structures were reminiscent of tubular early endocytic structures described in mammalian tissue culture cells (Mukherjee et al., 1997) and Drosophila oocytes (Tsuruhara et al., 1990). We concluded that the pathway through the endocytic compartment is highly conserved between Drosophila and mammalian cells. Furthermore, although the trans-endocytosis of the Boss transmembrane ligand into R7 cells may be unusual, these results indicated that its pathway through the endocytic compartments was similar to the pathway of internalized ligands in mammalian tissue culture cells.
Endocytic Trafficking of Boss in R7 Cells Mutant for hook
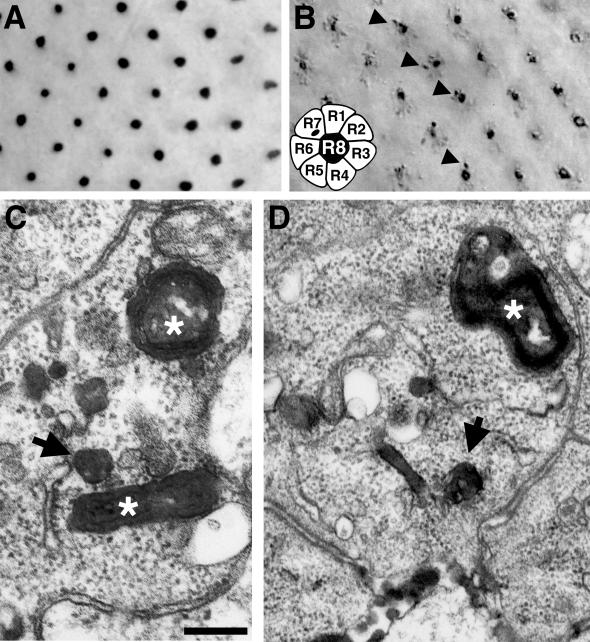
The HRP-Boss transgene was used to determine the effect of hook mutations on endocytic trafficking. When analyzed by light microscopy after antibody staining, the hk11 null mutation appeared to block Boss accumulation similar to the shibire mutation (compare Figures 6, A and C, and 8A). When detected by HRP activity, however, there appeared to be no reduction in the uptake of HRP-Boss into hk11 mutant R7 cells (Figure 8B) when compared with wild-type R7 cells (Figure 3D). By EM the strikingly different subcellular localization of HRP-Boss in hk11 R7 cells was revealed. In wild-type eye disks, a large fraction of HRP activity appeared in mature MVBs (e.g., Figures 4E and 5). In hk11 mutant R7 cells, HRP activity was evident in small, immature MVBs (Figure 8, C and D, arrows); however, no stained mature MVBs were detected. Finally, multilammelar late endosomes or lysosomes were prominently labeled (Figure 8, C and D, stars).
Figure 8.
The hook mutation alters late endocytic trafficking. HRP-Boss was not detected by antibody staining in R7 cells of hk11 eye disks (A). However, HRP staining revealed that the chimeric protein was internalized into R7 cells (B). By EM (C and D), HRP activity in hk11 R7 cells was detected in tubular early endosomes, small MVBs (arrows), and multilammelar late endosomes or lysosomes (white stars). However, the large mature MVBs, which are a dominant feature of stained wild-type R7 cells, were absent. The relevant genotypes of the larvae were w1118; hk11 P[w+, gen.HRP-Boss]17. Bar: A and B, 7.5 μm; C, 200 nm; D, 270 nm.
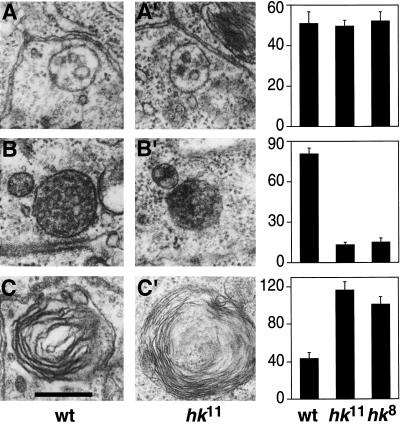
To confirm that this change in the endocytic compartment of hk11 mutant cells was not induced by the expression of HRP-Boss, we analyzed hk11 mutant tissue by quantitative EM in the absence of the transgene. Three components of the endocytic pathway could be easily identified based on their morphology (Futter et al., 1996): 1) small, immature MVBs with one to five internal vesicles, 2) large, mature MVBs that contained more than five internal vesicles, and 3) multilammelar late endosomes or lysosomes. These structures were counted in apical sections through at least 250 ommatidia in eye disks from wild-type and hk11 larvae. Consistent with the results obtained in eye disks expressing HRP-Boss, we found no difference in the number of small, immature MVBs between wild-type and hk11 tissue (Figure 9A; p > 0.5). By contrast, the number of large mature MVBs in hk11 tissue was dramatically reduced to a level one-sixth that of wild-type tissue (Figure 9B; p < 0.01). Furthermore, the number of multilammelar late endosomes or lysosomes was significantly increased in hk11 mutant tissue (Figure 9C; p < 0.01). To confirm that these changes were caused by the loss of hook function and were not due to a different mutation in the genetic background, we analyzed a second, strong loss of function hook allele, hk8 (Krämer and Phistry, 1999). Ultrastructural analysis of the hk8 allele revealed a loss of mature MVBs and an increase in multilammelar late endosomes or lysosomes, indistinguishable from the changes observed in the hk11 null allele (Figure 9).
Figure 9.
In hook mutants, mature MVBs are drastically reduced in number. For quantification of ultrastructural defects in hook mutant tissue, sections through wild-type (wt; A–C), hk11 (A′–C′), or hk8 mutant ommatidia were inspected. From three eye disks, we analyzed a total of 250 ommatidia for each genotype. We counted the occurrence of immature MVBs (vacuoles with one to five internal vesicles; A), mature MVBs (large vacuoles with more than five internal vesicles; B), and multilammelar late endosomes and lysosomes (C). The difference between the multilammelar late endosomes in C and C′ is not representative of the mutant phenotype; rather, they represented the wide spectrum of encountered morphologies. The average numbers of counted structures per eye disk are displayed in the bar graph; error bars indicate SEM. In hk11 and hk8 mutant tissue, the number of mature MVBs was less than one-sixth that of wild-type (p < 0.01), whereas multilammelar structures that represent late endosomes and lysosomes were significantly more abundant than in wild-type tissue (∼2.5-fold; p < 0.01). Bar: A, A′, B, and B′, 300 nm; C, 260 nm; C′, 340 nm.
DISCUSSION
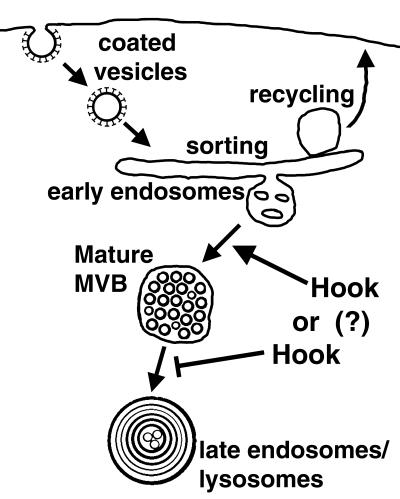
To explore the effect of the hook mutation on endocytic trafficking, we have established a new tool, the HRP-Boss transgene, to follow the pathway of the endocytosed Boss ligand on the ultrastructural level. We found that the most drastic change in the endocytic pathway of hook mutant cells was the lack of mature MVBs and the overabundance of multilammelar late endosomes or lysosomes. These results indicate that hook encodes a novel activity, which stabilizes mature MVBs and negatively regulates transport to late endosomes (Figure 10).
Figure 10.
Model for Hook function. HRP-Boss chimera was detected in early endosomes, mature MVBs, late multilammelar endosomes, or lysosomes. Our results indicate that Hook is required for proper maturation of MVBs. This could reflect a positive role in the genesis of mature MVBs. We favor a second model, however, which predicts that Hook negatively regulates the fusion between mature MVBs and multilammelar late endosomes or lysosomes.
The relationship of these late endocytic structures had previously not been explored in Drosophila. Our analysis established that the journey of the Boss transmembrane protein through the endocytic pathway of R7 cells is very similar to that of many endocytosed ligands in mammalian cells. HRP activity of HRP-Boss highlighted tubular and tubular-vesicular early endosomes, MVBs, and multilammelar late endosomes, which are the classic intermediate compartments through which endocytosed ligands are transported to lysosomes (Figure 10; Mukherjee et al., 1997). Although we have not directly demonstrated transport of internalized cargo from MVBs to multilammelar structures, the time course of the endocytic pathway established using the shibire mutation is consistent with such models previously proposed for mammalian cells (e.g., Felder et al., 1990; Gruenberg and Maxfield, 1995; Futter et al., 1996; Mullock et al., 1998).
In mammalian cells, MVBs are thought to be dynamic structures that are generated from early endosomes and consumed by their fusion to late multilammelar endosomes or lysosomes (Gruenberg and Maxfield, 1995; Futter et al., 1996). Ligands found in mature MVBs of mammalian cells have progressed beyond the early endocytic compartment, which is in fast exchange with the recycling compartment (Mayor et al., 1993); however, they have not yet been exposed to lysosomal hydrolases. Our observations of mature MVBs in Drosophila cells are fully consistent with these properties. Whenever antibody staining detected internalized HRP-Boss in R7 cells, EM demonstrated the accumulation of HRP activity in MVBs.
Similarly, our results were consistent with previous descriptions of large multilammelar structures as late endosomes or lysosomes, the compartments in which endocytosed proteins are degraded (Mukherjee et al., 1997). Under several conditions, we could detect internalized HRP-Boss by HRP activity but not by antibody staining. In such cases, EM revealed that HRP activity was present in multilammelar late endosomes or lysosomes but not in mature MVBs. In this context, it is important to note that the HRP enzyme is resistant to lysosomal hydrolases and remains active for many hours after its internalization into lysosomes (e.g., Figure 3; Futter et al., 1996). Thus, a straightforward explanation for the discrepancy between the presence of HRP-Boss by HRP activity and its absence according to anti-Boss antibody staining is the presence of active lysosomal hydrolases in multilammelar late endosomes that degrade the Boss portion of the chimera but not HRP activity.
Multiple mechanisms could explain the effect of hook mutations on mature MVBs and late endosomes. We favor a model in which the wild-type Hook protein is directly required for the stability of mature MVBs by inhibiting their fusion to multilammelar late endosomes or lysosomes (Figure 10). We propose that hook mutations result in an increased rate of transport of internalized ligands to multilammelar late endosomes causing premature degradation of ligands. This model is consistent with the following observations. First, Hook was detected on endocytic vesicles and vacuoles but not lysosomes by indirect immunofluorescence (Krämer and Phistry, 1996). Second, internalized Boss is not detected in hook mutant R7 cells, although no change is detected in the internalization of HRP-Boss as determined by its HRP activity (Figure 8). Third, hook mutations alter endocytic trafficking for all internalized ligands tested (Krämer and Phistry, 1999). Finally, the increase of multilammelar late endosomes or lysosomes in the absence of mature MVBs in hook mutant tissue points to increased lysosomal transport (Figures 8 and 9). The hook mutant phenotype is reminiscent of the cellular defects in cells derived from mucolipidosis type IV patients that exhibit a dramatic increase in multilammelar endocytic structures and an increased transport rate of internalized lipids to lysosomes (Chen et al., 1998).
An alternative explanation for the loss of mature MVBs in hook mutants could be a defect early in the endocytic pathway. For example, in Hep2 cells a decreased rate of endocytosis induced by serum starvation can lower the ratio of mature to immature MVBs (Futter et al., 1996). However, several observations argue against this interpretation. First, HRP activity of HRP-Boss is internalized into multilammelar late endosomes in hook mutant R7 cells (Figure 8), arguing against a block early in the endocytic pathway. Consistent with these observations, Boss immunoreactivity accumulates in R7 cells even in the absence of hook function when delivery of proteins to lysosomes is blocked by the deep orange mutation (Sevrioukov and Krämer, unpublished results), arguing against the possibility that Boss is degraded in early endosomes in hook mutant R7 cells because of mislocalized lysosomal hydrolases. Second, even a complete block of endocytosis by the shibire mutation does not abolish the generation of mature MVBs, because we observe it in hook mutants. In R7 cells, we could recognize such newly generated MVBs because they no longer contained HRP activity once endocytosis was blocked by the shibire mutation (Figure 7).
Two alternative scenarios might explain why the genesis of new MVBs can be observed even when receptor-mediated endocytosis is blocked. In mammalian tissue culture cells, expression of a dominant negative Dynamin I protein results in a lasting block of receptor-mediated endocytosis but only a transient reduction of fluid phase endocytosis (Damke et al., 1994; Damke et al., 1995). It is not known whether the shibire mutation exhibits a similar biphasic response after the initial block of fluid phase endocytosis (Kosaka and Ikeda, 1983b). A second potential source of membrane traffic fueling the genesis of new MVBs is the Golgi-derived biosynthetic traffic that is targeted for the endocytic pathway (Hirst et al., 1998; Press et al., 1998). In this context it will be interesting to see how the hook mutation effects the biosynthetic pathway of lysosomal enzymes, once markers are available to follow this pathway in Drosophila.
Although the HRP-Boss transgene is a useful tool to study late stages in Boss endocytosis, it also provided us with information about the uptake of the Boss transmembrane ligand across cell boundaries. Our observations argue that Boss trans-endocytosis occurs through the uptake of small patches of R8 membrane into R7 cells (Figures 4F and 5A), rather than a phagocytic process (Cagan et al., 1992). Internalization of Boss is preceded by its clustering on the R8 cell membrane, visualized by patches of membrane stained with HRP activity. Oligomerization of Boss–Sevenless complexes into higher-order complexes has previously been evoked to explain the lack of Sevenless activation by monomeric and dimeric Boss ligands (Hart et al., 1993b; Sevrioukov et al., 1998). Such clustering might effectively exclude other transmembrane proteins from the small membrane patches that are being pulled into the R7 cell by receptor-mediated endocytosis (Klueg et al., 1998).
Our results with Boss are consistent with the observations of trans-endocytosis of other transmembrane proteins. The Delta, Serrate, and Lag-2 transmembrane ligands are trans-endocytosed after binding to receptors of the Notch class (Henderson et al., 1994; Couso et al., 1995; Klueg et al., 1998). For Delta trans-endocytosis, phagocytosis could be ruled out, because independent cell surface markers were not cointernalized (Klueg et al., 1998). A similar mechanism of trans-endocytosis might also explain the internalization of the homotypic cell adhesion protein apCam. Bailey et al. (1992) detected internalized apCam by immuno-EM in endocytic vesicles with small internal vesicles, likely to be derived from neighboring cells.
In summary, we have established a model system to dissect the effects of Drosophila mutations that disturb endocytic trafficking. Using the HRP-Boss chimera, we demonstrated that hook is required late in endocytic trafficking for a novel activity that negatively regulates transport to late endosomes. To elucidate the specific mechanism of this inhibitory effect, direct measurements of fusion between different endocytic compartments in wild-type and hook mutant cells will be necessary.
ACKNOWLEDGMENTS
We thank Colin Hopkins and P.R. Ortiz de Montellano for HRP cDNAs, Mani Ramaswami, Larry Zipursky, and the Bloomington Stock center for fly stocks. Technical advice from Colin Hopkins, Dennis Belotto, and George Lawton was crucial for this work. We are grateful to Richard Anderson, Bruce Horazdovsky, Ellen Lumpkin, Mani Ramaswami, Mike Roth, and the members of the Krämer laboratory for their critical comments to the manuscript. This work was supported by National Eye Institute grant EY10199, Welch foundation grant I-1300, and March of Dimes Foundation grant 97-0475.
Abbreviations used:
- boss
bride of sevenless
- DAB
3,3′-diaminobenzidine
- EM
electron microscopy
- MVB
multivesicular body
REFERENCES
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1994. [Google Scholar]
- Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Krämer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992;69:393–399. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc Natl Acad Sci USA. 1998;95:6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Futter CE, Gibson A, Hopkins CR, Cutler DF. Transport into and out of the Golgi complex studied by transfecting cells with cDNAs encoding horseradish peroxidase. J Cell Biol. 1994;127:641–652. doi: 10.1083/jcb.127.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso JP, Knust E, Arias AM. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr Biol. 1995;5:1437–1448. doi: 10.1016/s0960-9822(95)00281-8. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Maxfield FR. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J Cell Biol. 1992;117:301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1123. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh RN, Gelman DL, Maxfield FR. Quantification of low density lipoprotein and transferrin endocytic sorting HEp2 cells using confocal microscopy. J Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hart AC, Harrison SD, Van Vactor DLJ, Rubin GM, Zipursky SL. The interaction of bride of sevenless with sevenless is conserved between Drosophila virilis and Drosophila melanogaster. Proc Natl Acad Sci USA. 1993a;90:5047–5051. doi: 10.1073/pnas.90.11.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Krämer H, Van Vactor DLJ, Paidhungat M, Zipursky SL. Induction of cell fate in the Drosophila retina: the bride of sevenless protein is predicted to contain a large extracellular domain and seven transmembrane segments. Genes & Dev. 1990;4:1835–1847. doi: 10.1101/gad.4.11.1835. [DOI] [PubMed] [Google Scholar]
- Hart AC, Krämer H, Zipursky SL. Extracellular domain of the boss transmembrane ligand acts as an antagonist of the sev receptor. Nature. 1993b;361:732–736. doi: 10.1038/361732a0. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Ortiz de Montellano PR. Baculovirus expression and characterization of catalytically active horseradish peroxidase. Arch Biochem Biophys. 1992;297:61–72. doi: 10.1016/0003-9861(92)90641-9. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–2924. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- Hirst J, Futter CE, Hopkins CR. The kinetics of mannose 6-phosphate receptor trafficking in the endocytic pathway in HEp-2 cells: the receptor enters and rapidly leaves multivesicular endosomes without accumulating in a prelysosomal compartment. Mol Biol Cell. 1998;9:809–816. doi: 10.1091/mbc.9.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky BF, Busch GR, Emr SD. VPS21 encodes a rab5-like GTP binding protein that is required for the sorting of yeast vacuolar proteins. EMBO J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueg KM, Parody TR, Muskavitch MAT. Complex proteolytic processing acts on δ, a transmembrane ligand for Notch, during Drosophila development. Mol Biol Cell. 1998;9:1709–1723. doi: 10.1091/mbc.9.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983a;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol. 1983b;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H, Cagan RL, Zipursky SL. Interaction of bride of sevenless membrane-bound ligand and the sevenless tyrosine-kinase receptor. Nature. 1991;352:207–212. doi: 10.1038/352207a0. [DOI] [PubMed] [Google Scholar]
- Krämer H, Phistry M. Mutations in the Drosophila hook gene inhibit endocytosis of the Boss transmembrane ligand into multivesicular bodies. J Cell Biol. 1996;133:1205–1216. doi: 10.1083/jcb.133.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H, Phistry M. Genetic analysis of hook, a gene required for endocytic trafficking in Drosophila. Genetics. 1999;151:675–684. doi: 10.1093/genetics/151.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The Genome of Drosophila melanogaster. San Diego: Academic Press; 1992. [Google Scholar]
- Lloyd V, Ramaswami M, Krämer H. Not just pretty eyes: Drosophila eye-color mutations and lysosomal delivery. Trends Cell Biol. 1998;8:257–259. doi: 10.1016/s0962-8924(98)01270-7. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol. 1998;140:591–601. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Rao S, van der Bliek A, Kelly RB, Krishnan KS. Genetic studies on dynamin function in Drosophila. J Neurogenet. 1993;9:73–87. doi: 10.3109/01677069309083451. [DOI] [PubMed] [Google Scholar]
- Riezman H. Yeast endocytosis. Trends Cell Biol. 1993;3:273–277. doi: 10.1016/0962-8924(93)90056-7. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sato TK, Darsow T, Emr SD. Vam7p, a SNAP-25-like molecule, and vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol Cell Biol. 1998;18:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- Sevrioukov E, Walenta JH, Phistry M, Sunio A, Krämer H. Oligomerization of the extracellular domain of Boss enhances its binding to the Sevenless receptor and its antagonistic effect on R7 induction. J Cell Sci. 1998;111:737–747. doi: 10.1242/jcs.111.6.737. [DOI] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Nomoto H, Cutler DF, Hopkins CR. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65:417–427. doi: 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- Thummel CS, Pirrotta V. New pCaSpeR P element vectors. Dros Info Service. 1992;71:150. [Google Scholar]
- Tomlinson A, Bowtell DD, Hafen E, Rubin GM. Localization of the sevenless protein, a putative receptor for positional information, in the eye imaginal disk of Drosophila. Cell. 1987;51:143–150. doi: 10.1016/0092-8674(87)90019-5. [DOI] [PubMed] [Google Scholar]
- Tsuruhara T, Koenig JH, Ikeda K. Synchronized endocytosis studied in the oocyte of a temperature-sensitive mutant of Drosophila melanogaster. Cell Tissue Res. 1990;259:199–207. doi: 10.1007/BF00318441. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol. 1995;66:309–323. [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]
- Van Vactor DLJ, Cagan RL, Krämer H, Zipursky SL. Induction in the developing compound eye of Drosophila: multiple mechanisms restrict R7 induction to a single retinal precursor cell. Cell. 1991;67:1145–1155. doi: 10.1016/0092-8674(91)90291-6. [DOI] [PubMed] [Google Scholar]
- Wendland B, Emr SD, Riezman H. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr Opin Cell Biol. 1998;10:513–522. doi: 10.1016/s0955-0674(98)80067-7. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]