Abstract
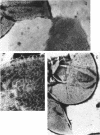
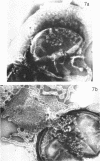
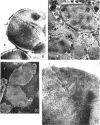
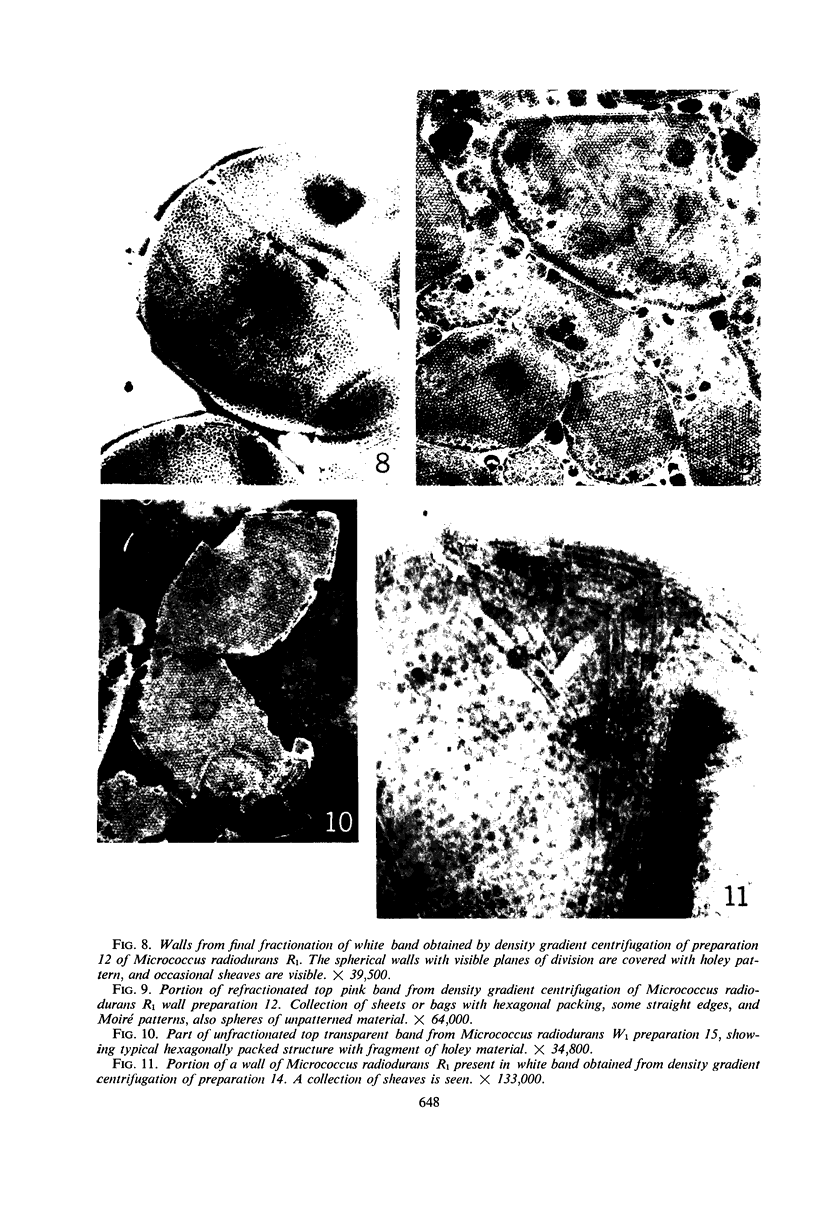
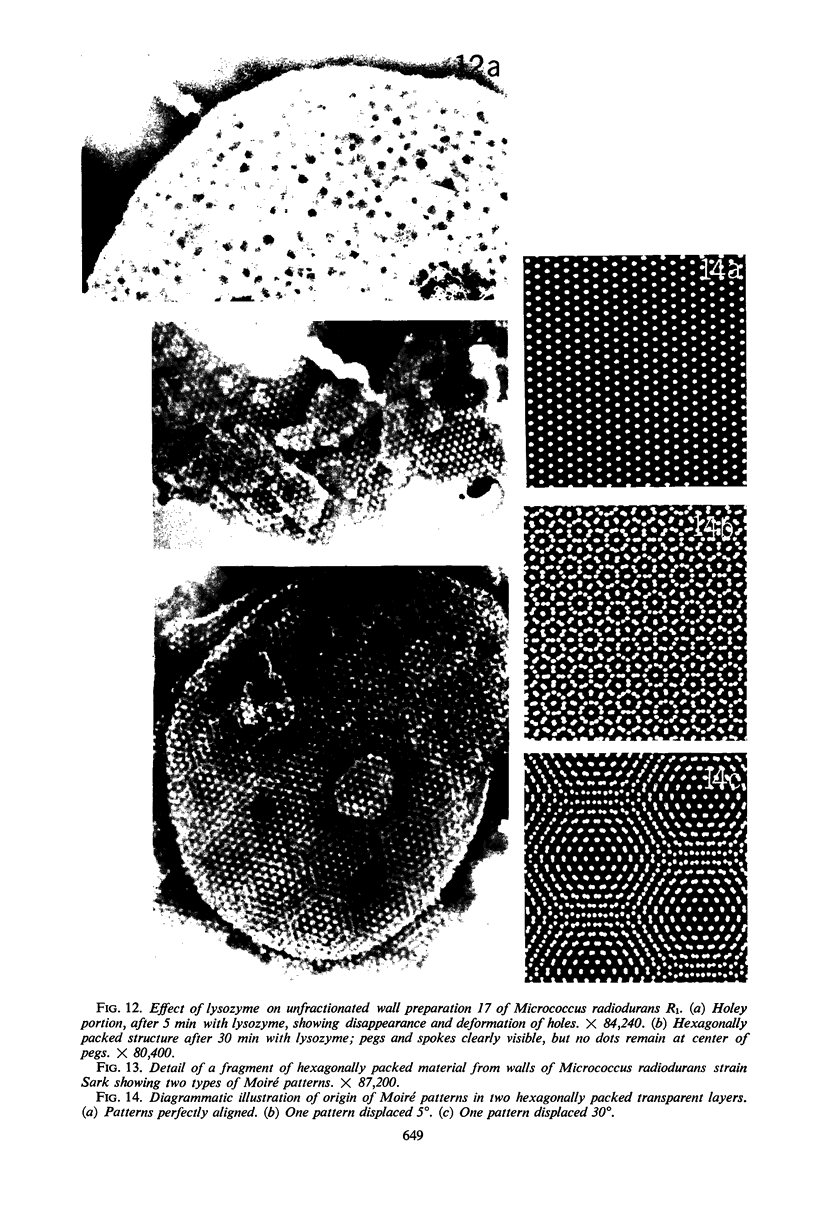
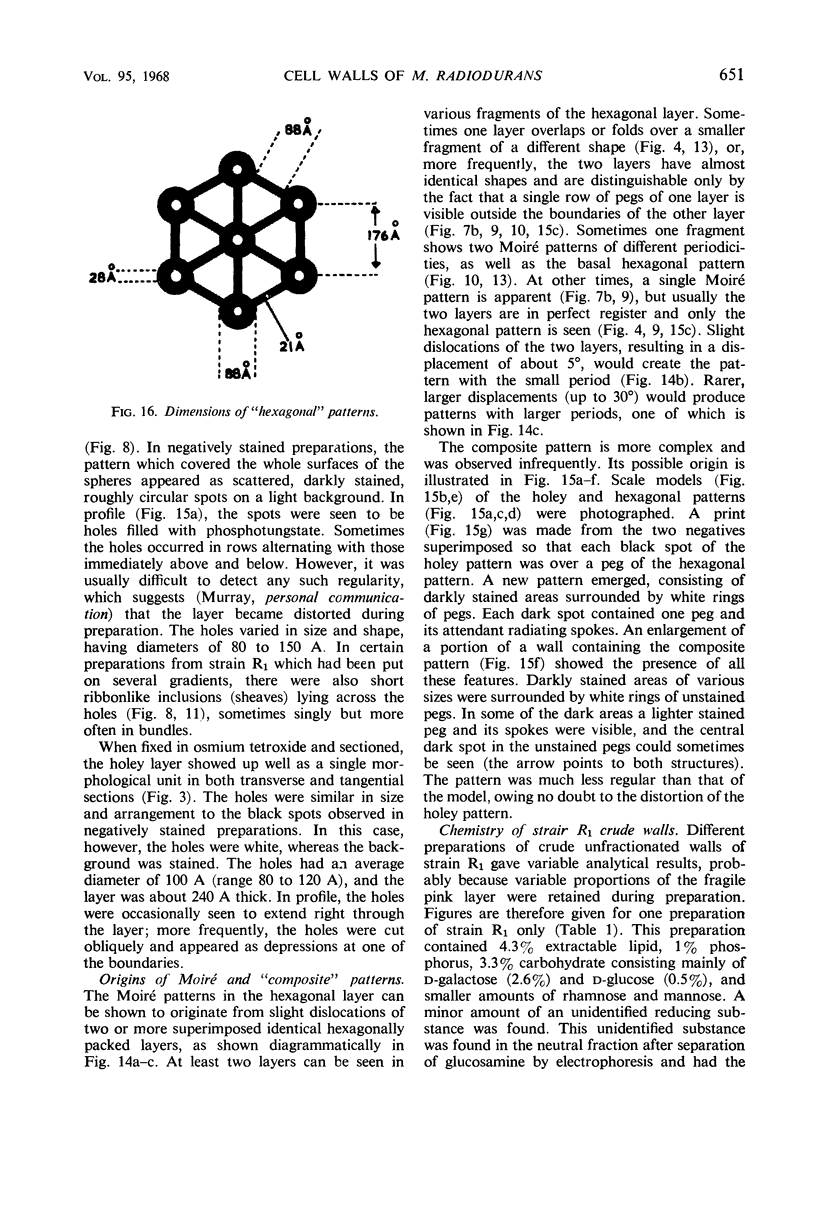
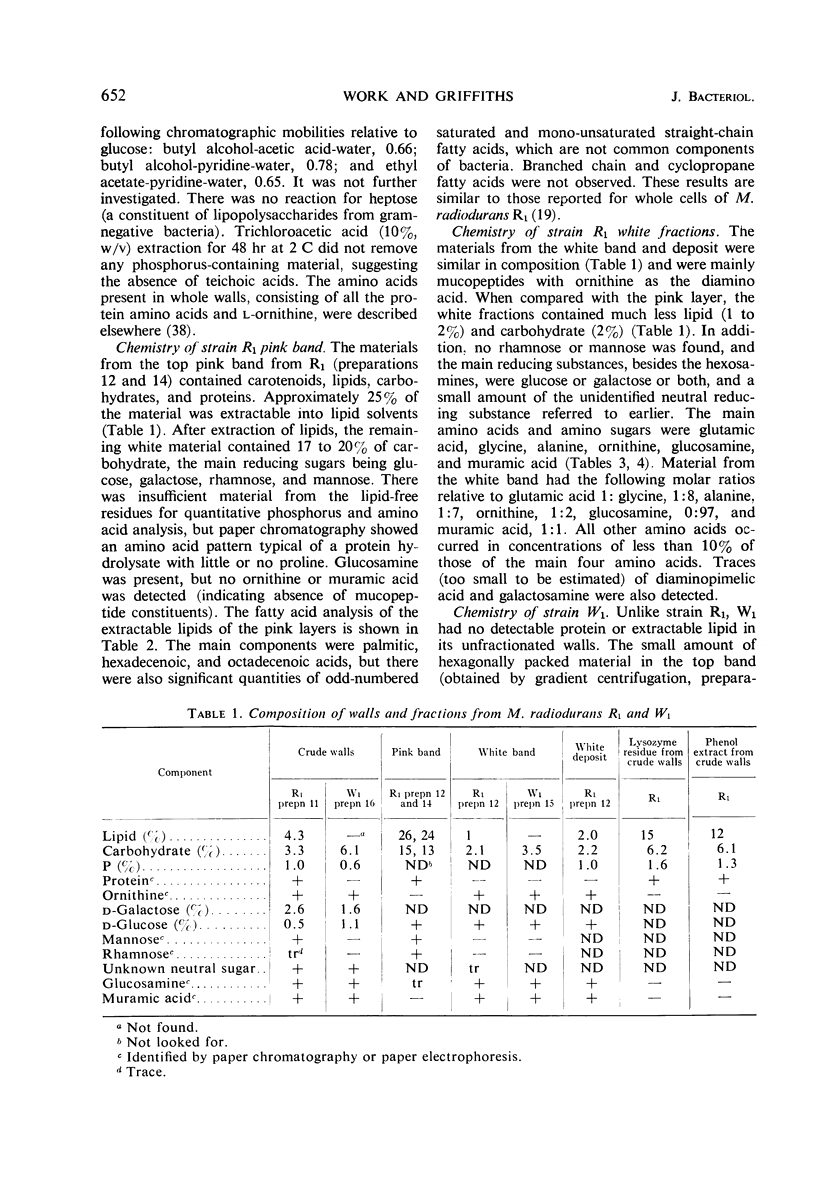
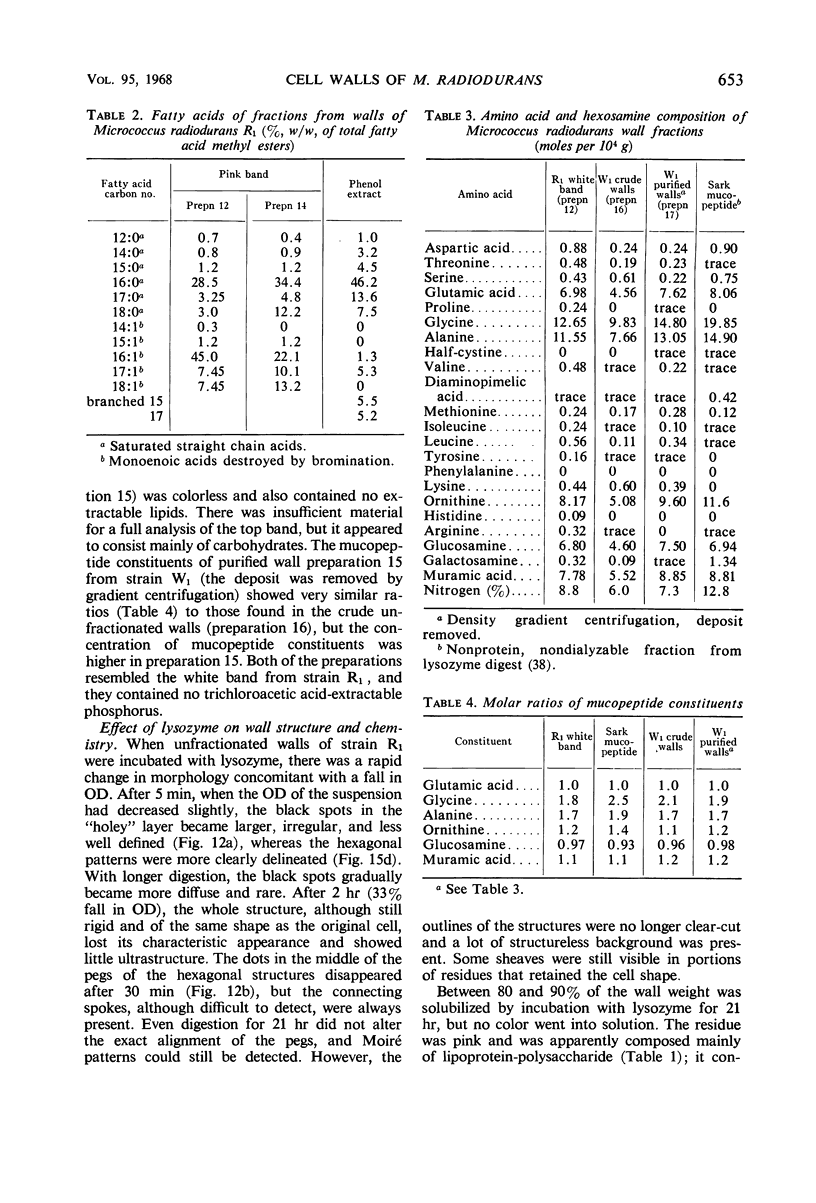
Walls of the pigmented strain of Micrococcus radiodurans showed several layers in the electron microscope. These layers include an outermost network structure removed by trypsin, a fragile soft layer containing hexagonally packed subunits, and a rigid layer penetrated by numerous holes. The two inner layers were separated by a process of autolysis, trypsin treatment, and gradient centrifugation. The hexagonally packed layer was less dense, pink in color, and it contained carotenoids, lipid, protein, and polysaccharide. The lipid consisted of odd-numbered as well as even-numbered fatty acids, and the polysaccharide contained rhamnose and mannose, but it did not contain heptose. The “holey” layer was white and was composed of a mucopeptide containing glucosamine, muramic acid, and four main amino acids (glutamic acid, alanine, glycine, and l-ornithine, in the ratios of 1:1.7:1.8:1.2, respectively). This layer also contained phosphorus, glucose, and a trace of meso- and ll-diaminopimelic acid. A white mutant, W1, of M. radiodurans had no pigment or lipid in its walls, but it contained small amounts of the “hexagonal” layer. The holey layer, constituting the bulk of the wall, was similar in morphology and composition to that layer in the pigmented strain. Lysozyme did not remove the lipoprotein-polysaccharide component from the walls of the pigmented strains, and the hexagonally packed structure was not visibly affected, except for change in a minor structure. Most of the mucopeptide layer was solubilized by lysozyme, but a structureless bag-shaped residue was left. This residue contained phosphorus, carbohydrate, and limited amino acids, but it did not contain muramic acid, glucosamine, or ornithine. Aqueous phenol removed a lipoprotein component from strain R1, which contained limited fatty acids. It also removed meso- and ll-diaminopimelic acid.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLSOP J WORK E. Cell walls of Propionibacterium species: fractionation and composition. Biochem J. 1963 Jun;87:512–519. doi: 10.1042/bj0870512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- BLEIWEIS A. S., KRAUSE R. M. THE CELL WALLS OF GROUP D STREPTOCOCCI. I. THE IMMUNOCHEMISTRY OF THE TYPE 1 CARBOHYDRATE. J Exp Med. 1965 Aug 1;122:237–249. doi: 10.1084/jem.122.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- CHAPMAN J. A., MURRAY R. G., SALTON M. R. THE SURFACE ANATOMY OF LAMPROPEDIA HYALINA. Proc R Soc Lond B Biol Sci. 1963 Nov 19;158:498–513. doi: 10.1098/rspb.1963.0060. [DOI] [PubMed] [Google Scholar]
- DUC-NGUYEN H., WEED L. L. D-ORNITHINE AS A CONSTITUENT OF A BACTERIAL CELL WALL. J Biol Chem. 1964 Oct;239:3372–3376. [PubMed] [Google Scholar]
- GLAUERT A. M. The fine structure of bacteria. Br Med Bull. 1962 Sep;18:245–250. doi: 10.1093/oxfordjournals.bmb.a069988. [DOI] [PubMed] [Google Scholar]
- Glauert A. M. Moiré patterns in electron micrographs of a bacterial membrane. J Cell Sci. 1966 Dec;1(4):425–428. doi: 10.1242/jcs.1.4.425. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSELEY B. E., LASER H. REPAIR OF X-RAY IN MICROCOCCUS RADIODURANS. Proc R Soc Lond B Biol Sci. 1965 Apr 13;162:210–222. doi: 10.1098/rspb.1965.0035. [DOI] [PubMed] [Google Scholar]
- MOSELEY B. E., SCHEIN A. H. RADIATION RESISTANCE AND DEOXYRIBONUCLEIC ACID BASE COMPOSITION OF MICROCOCCUS RADIODURANS. Nature. 1964 Sep 19;203:1298–1299. doi: 10.1038/2031298a0. [DOI] [PubMed] [Google Scholar]
- Miller I., Plapp R., Kandler O. The amino acid sequence of the serine containing murein of Butybacterium Rettgeri. Biochem Biophys Res Commun. 1966 Nov 22;25(4):415–420. doi: 10.1016/0006-291x(66)90221-x. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R., CUMMINS C. S. CHEMICAL STRUCTURE OF BACTERIAL CELL WALLS. ORNITHINE AND 2,4-DIAMINOBUTYRIC ACID AS COMPONENTS OF THE CELL WALLS OF PLANT PATHOGENIC CORYNEBACTERIA. Nature. 1964 Mar 14;201:1105–1107. doi: 10.1038/2011105a0. [DOI] [PubMed] [Google Scholar]
- ROTH H., SEGAL S., BERTOLI D. THE QUANTITATIVE DETERMINATION OF GALACTOSE--AN ENZYMIC METHOD USING GALACTOSE OXIDASE, WITH APPLICATIONS TO BLOOD AND OTHER BIOLOGICAL FLUIDS. Anal Biochem. 1965 Jan;10:32–52. doi: 10.1016/0003-2697(65)90238-1. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., EHTISHAM-UD-DIN A. F. THE LOCALIZATION OF CYTOCHROMES AND CAROTENOIDS IN ISOLATED BACTERIAL MEMBRANES AND ENVELOPES. Aust J Exp Biol Med Sci. 1965 Jun;43:255–264. doi: 10.1038/icb.1965.24. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. A high speed shaker for the disruption of cells at low temperatures. Biochim Biophys Acta. 1957 Apr;24(1):203–204. doi: 10.1016/0006-3002(57)90168-3. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Horne R. W., Glauert A. M. The fine structure of Micrococcus radiodurans. Arch Mikrobiol. 1965 Jul 20;51(3):267–289. doi: 10.1007/BF00408143. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Lambert R., Saito Y. The composition of the cell wall of Lactobacillius bifidus var. pennsylvanicus. Arch Biochem Biophys. 1965 Oct;112(1):120–125. doi: 10.1016/0003-9861(65)90019-6. [DOI] [PubMed] [Google Scholar]
- WORK E. AMINO ACIDS OF WALLS OF MICROCOCCUS RADIODURANS. Nature. 1964 Mar 14;201:1107–1109. doi: 10.1038/2011107a0. [DOI] [PubMed] [Google Scholar]