Abstract
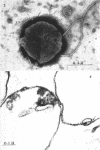
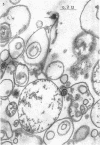
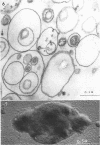
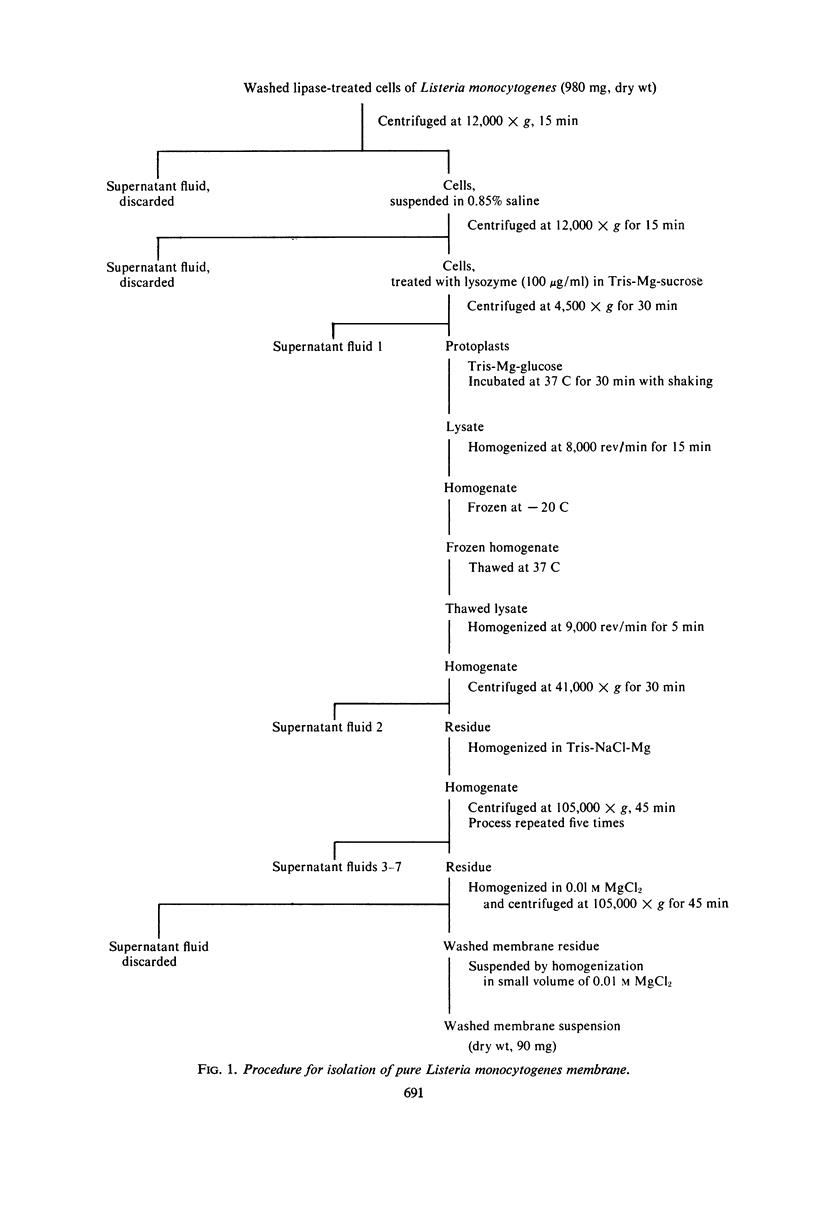
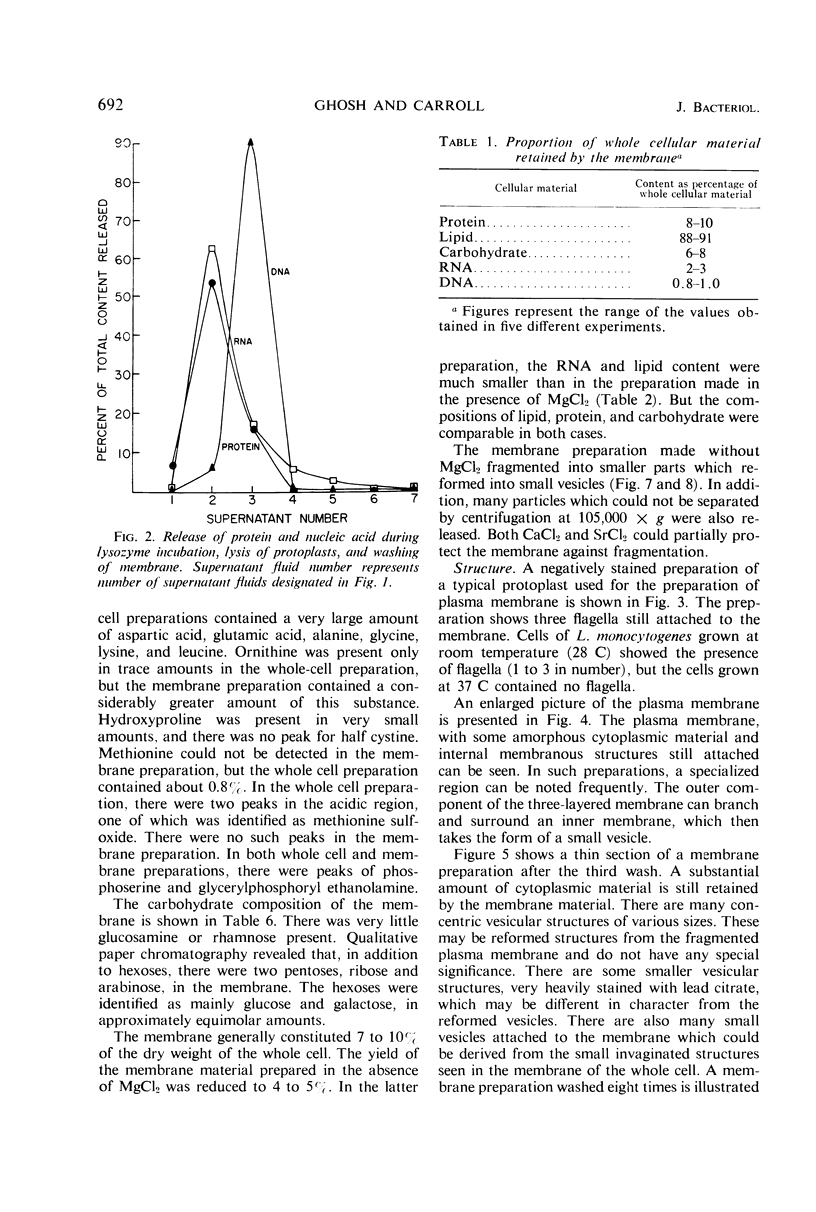
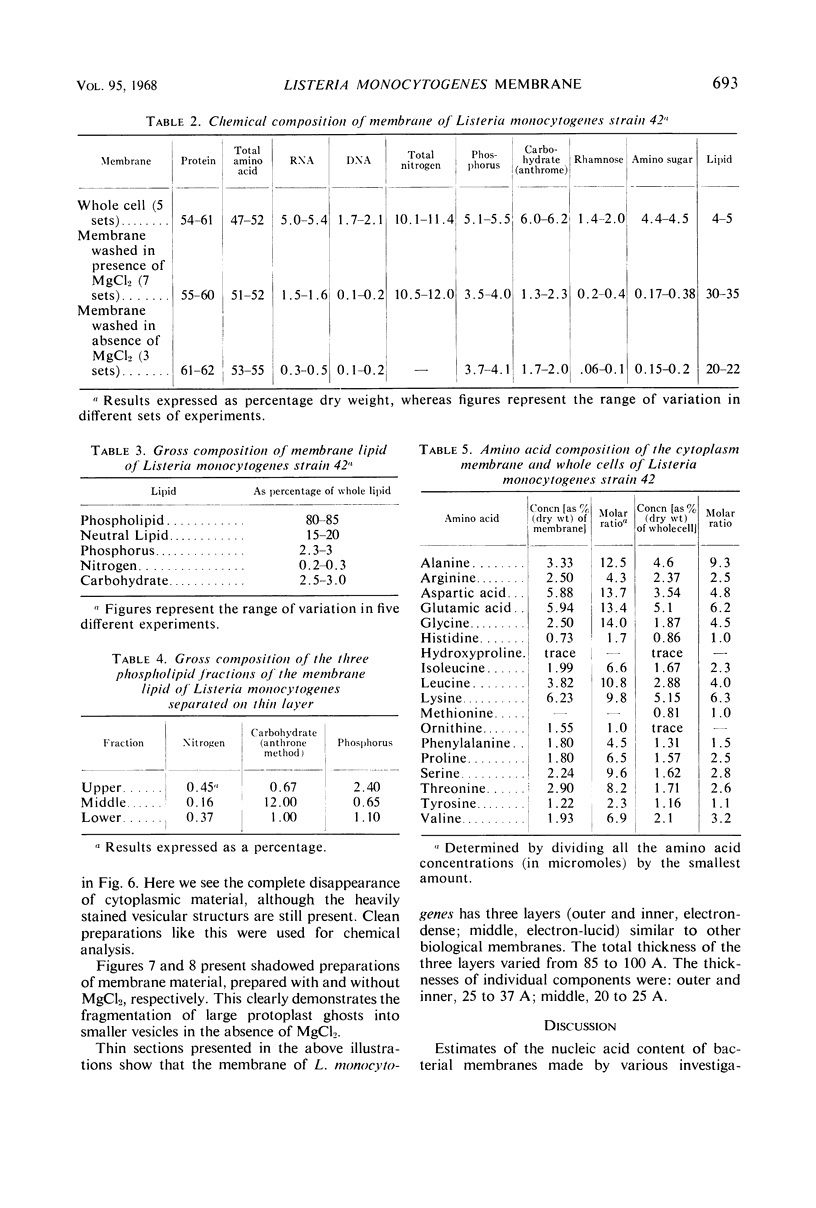
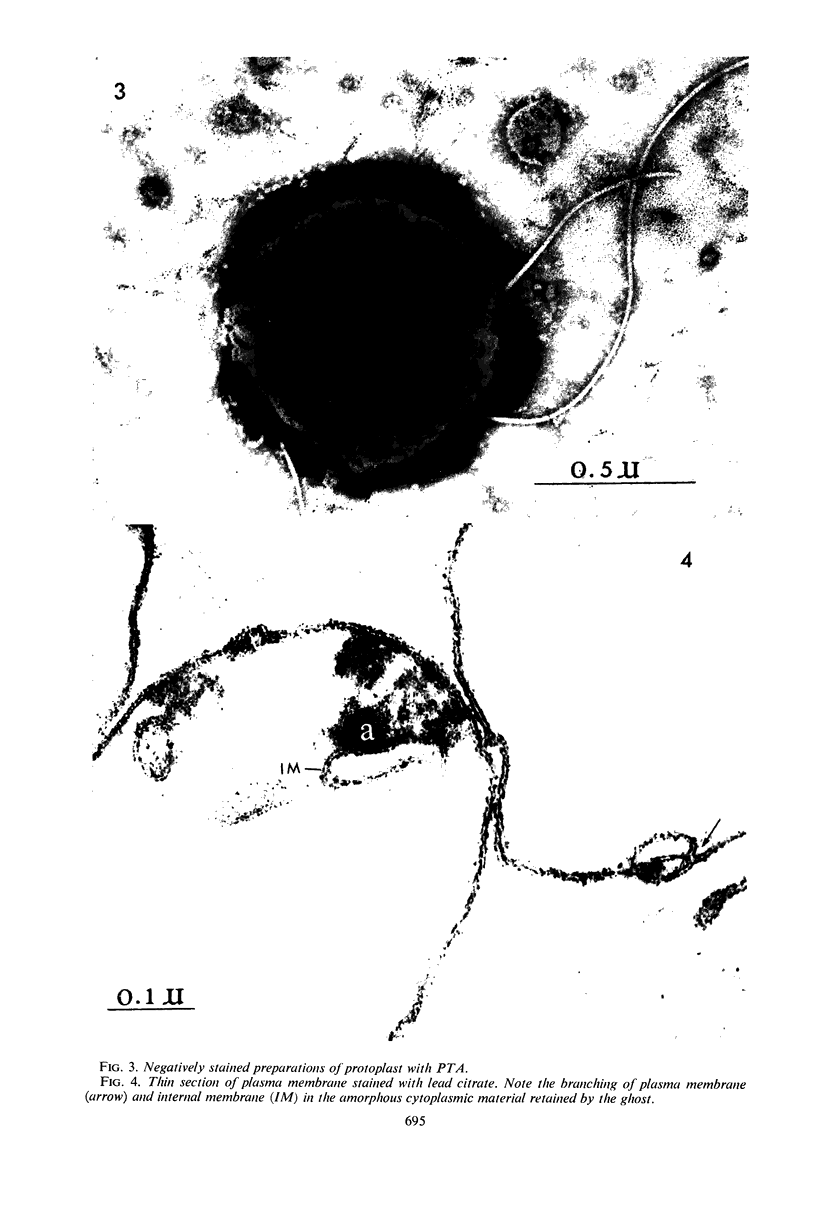
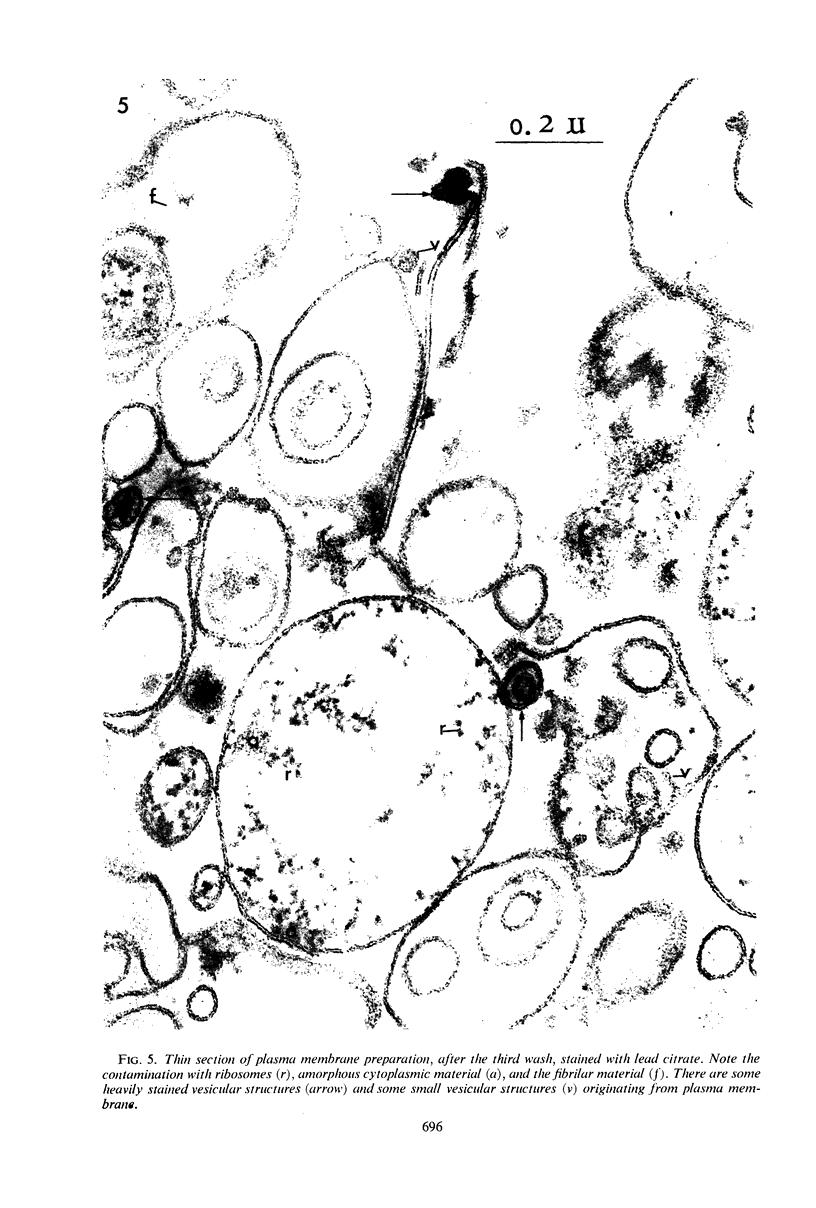
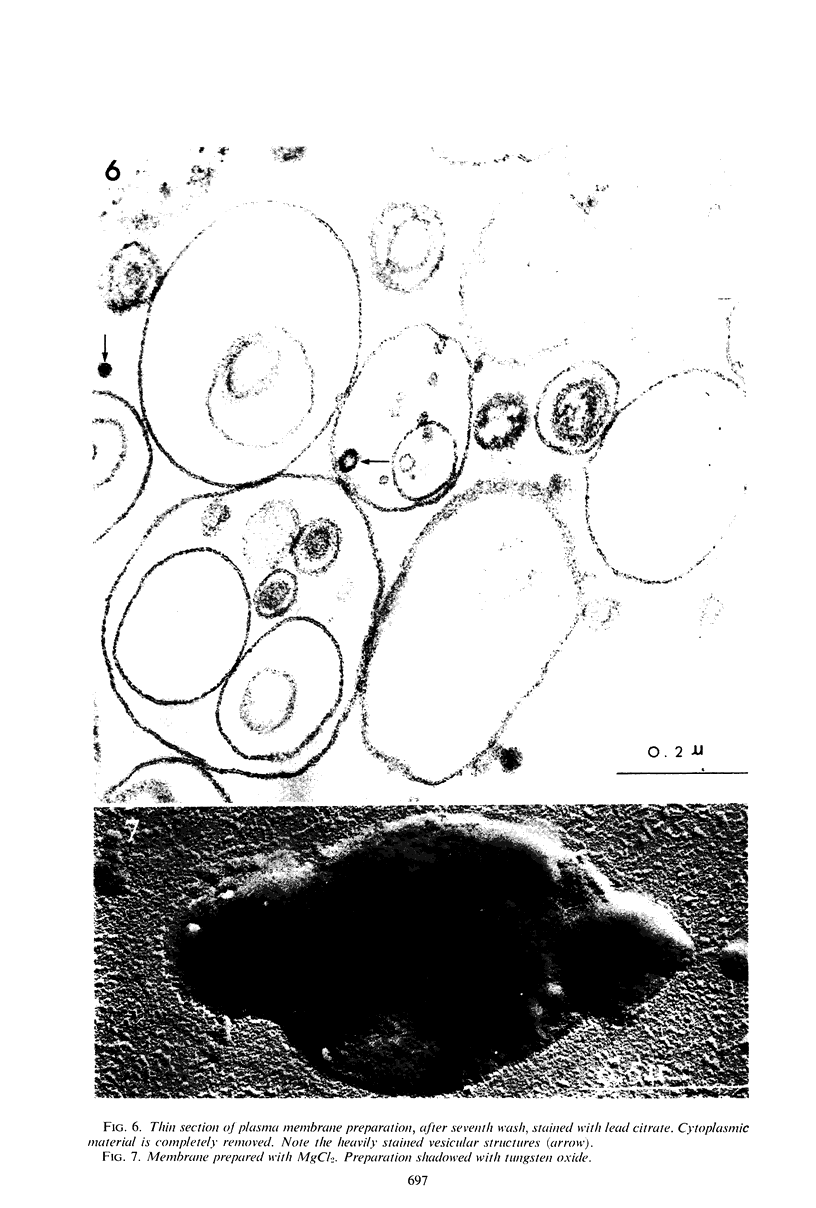
The plasma membrane of Listeria monocytogenes strain 42 was prepared by osmotic lysis of protoplasts with tris(hydroxymethyl)aminomethane (Tris) buffer, pH 8.2, containing MgCl2 and glucose, followed by washing with NaCl and MgCl2 in Tris buffer. Electron microscopy showed that the preparation was not contaminated with cytoplasmic material. The membrane preparation was composed of 55 to 60% protein, 1.5% ribonucleic acid, 0.1% deoxyribonucleic acid, 1.3 to 2.3% carbohydrate, 0.17 to 0.38% amino sugar, 0.2 to 0.4% rhamnose, 3.5 to 4.0% phosphorus, 10.5 to 12.0% nitrogen, and 30 to 35% lipid. Amino acid composition of the washed membrane showed some variation from that of the whole cells. Sulfur-containing amino acids were not present in the membrane hydrolysate. The membrane carbohydrate contained glucose, galactose, ribose, and arabinose. The membrane lipid was 80 to 85% phospholipid and 15 to 20% neutral lipid. The lipid contained 2.3 to 3.0% phosphorus, 2.5 to 3.0% carbohydrate, and a very small amount of nitrogen (0.2 to 0.3%). The phospholipid was of the phosphatidyl glycerol type. Electron micrographs of the washed membrane showed three layers. The outer and inner layers varied in thickness from 25 to 37 A and the middle layer from 20 to 25 A. The total thickness varied between 85 and 100 A. These preparations contained many vesicles which stained heavily with lead citrate. Some vesicles were also attached to the protoplast ghosts in the form of extrusions or intrusions, or both. Membrane preparations obtained by lysis of protoplasts in the absence of MgCl2 were fragmented and contained less lipid (20 to 22%) and ribonucleic acid (0.3 to 0.5%) than preparations prepared with MgCl2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Cohen M., Panos C. Membrane lipid composition of Streptococcus pyogenes and derived L form. Biochemistry. 1966 Jul;5(7):2385–2392. doi: 10.1021/bi00871a031. [DOI] [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIMER E. H. Studies on L forms and protoplasts of group A streptococci. II. Chemical and immunological properties of the cell membrane. J Exp Med. 1963 Mar 1;117:377–399. doi: 10.1084/jem.117.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., VENNES J. W. Immunologic comparison of isolated surface membranes of bacillus megaterium. Science. 1956 Sep 21;124(3221):535–536. doi: 10.1126/science.124.3221.535. [DOI] [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V., McQUILLEN K. The chemical composition of the protoplast membrane of Micrococcus lysodeikticus. Biochim Biophys Acta. 1958 Jul;29(1):21–29. doi: 10.1016/0006-3002(58)90141-0. [DOI] [PubMed] [Google Scholar]
- GODSON G. N., HUNTER G. D., BUTLER J. A. Cellular components of Bacillus megaterium and their role in protein biosynthesis. Biochem J. 1961 Oct;81:59–68. doi: 10.1042/bj0810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. The bacterial cytoplasmic membrane. J Gen Microbiol. 1962 Sep;29:39–46. doi: 10.1099/00221287-29-1-39. [DOI] [PubMed] [Google Scholar]
- Jermyn M. A., Isherwood F. A. Improved separation of sugars on the paper partition chromatogram. Biochem J. 1949;44(4):402–407. doi: 10.1042/bj0440402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUKOIANOVA M. A., GEL'MAN N. S., OPARIN A. I. [Oxidation of succinate in the structural elements of Micrococcus lysodeikticus in relation to desoxyribonucleic acid and magnesium ions]. Biokhimiia. 1963 Mar-Apr;28:334–339. [PubMed] [Google Scholar]
- Mizushima S., Ishida M., Kitahara K. Chemical composition of the protoplast membrane of Bacillus megaterium. J Biochem. 1966 Apr;59(4):374–381. doi: 10.1093/oxfordjournals.jbchem.a128312. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., BAKAY B., CONOVER M. J., TOENNIES G. Protoplast membrane of Streptococcus faecalis. J Bacteriol. 1963 Jan;85:168–176. doi: 10.1128/jb.85.1.168-176.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUART D. C., Jr Fine structure of the nucleoid and internal membrane systems of Streptomyces. J Bacteriol. 1959 Aug;78:272–281. doi: 10.1128/jb.78.2.272-281.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Netschey A. Physical chemistry of isolated bacterial membranes. Biochim Biophys Acta. 1965 Oct 18;107(3):539–545. doi: 10.1016/0304-4165(65)90198-4. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Intracytoplasmic membranes in Escherichia coli. J Bacteriol. 1966 Sep;92(3):780–783. doi: 10.1128/jb.92.3.780-783.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L. Biochemistry of the cell wall of Staphylococcus aureus. Ann N Y Acad Sci. 1965 Jul 23;128(1):59–61. doi: 10.1111/j.1749-6632.1965.tb11629.x. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. Characterization of the protoplasmic constituents of bacillus megaterium. J Bacteriol. 1953 Dec;66(6):696–702. doi: 10.1128/jb.66.6.696-702.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUDKIN M. D., DAVIS B. NATURE OF THE RNA ASSOCIATED WITH THE PROTOPLAST MEMBRANE OF BACILLUS MEGATERIUM. J Mol Biol. 1965 May;12:193–204. doi: 10.1016/s0022-2836(65)80293-5. [DOI] [PubMed] [Google Scholar]