Abstract
We show that CC chemokines induced a sustained increase in monocyte adhesion to intercellular adhesion molecule-1 that was mediated by Mac-1 (αMβ2) but not lymphocyte function–associated antigen-1 (LFA-1; αLβ2). In contrast, staining for an activation epitope revealed a rapid and transient up-regulation of LFA-1 activity by monocyte chemotactic protein-1 (MCP-1) in monocytes and Jurkat CCR2 chemokine receptor transfectants or by stromal-derived factor-1α in Jurkat cells. Differential kinetics for activation of Mac-1 (sustained) and LFA-1 (transient) avidity in response to stromal-derived factor-1α were confirmed by expression of αM or αL in αL-deficient Jurkat cells. Moreover, expression of chimeras containing αL and αM cytoplasmic domain exchanges indicated that α cytoplasmic tails conferred the specific mode of regulation. Coexpressing αM or chimeras in mutant Jurkat cells with a “gain of function” phenotype that results in constitutively active LFA-1 demonstrated that Mac-1 was not constitutively active, whereas constitutive activity was mediated via the αL cytoplasmic tail, implying the presence of distinct signaling pathways for LFA-1 and Mac-1. Transendothelial chemotaxis of monocytes in response to MCP-1 was dependent on LFA-1; however, Mac-1 was involved at MCP-1 concentrations stimulating its avidity, showing differential contributions of β2 integrins. Our data suggest that a specific regulation of β2 integrin avidity by chemokines may be important in leukocyte extravasation and may be triggered by distinct activation pathways transduced via the α subunit cytoplasmic domains.
INTRODUCTION
Integrins comprise a family of αβ heterodimeric transmembrane proteins that participate in cell adhesion processes (Springer, 1990; Hynes, 1992). The regulation of the β2 integrins lymphocyte function–associated antigen-1 (LFA-11; αLβ2; CD11a/CD18) and Mac-1 (αMβ2; CD11b/CD18), which are exclusively expressed on leukocytes, is important for inflammatory and immunological responses (Diamond and Springer, 1994). Cellular stimulation by CD3 cross-linking or phorbol ester can modulate the avidity of β2 integrins by affecting their surface distribution, e.g., via Ca2+-dependent release from cytoskeletal restraint mediated by calpain and subsequent lateral clustering, or by altering post-ligand–binding events, such as cell spreading (Dustin and Springer, 1989; van Kooyk et al., 1989; Kucik et al., 1996; Stewart et al., 1996; Lub et al., 1997a; Stewart et al., 1998). In contrast, divalent cations, such as Mg2+ or Mn2+, stimulatory mAbs, or L-selectin cross-linking can induce high-affinity ligand binding of integrins by imposing conformational changes that are reported by activation-specific mAbs (Dransfield et al., 1992; Diamond and Springer, 1994; Hwang et al., 1996; Stewart et al., 1996). Moreover, chemoattractants and chemokines can stimulate integrin adhesiveness via G-protein–coupled receptors, which can be mediated via the induction of conformationally active neoepitopes (Lo et al., 1989; Detmers et al., 1990; Diamond and Springer, 1993; Tanaka et al., 1993; Baggiolini et al., 1994; Weber et al., 1996b).
Recent evidence has emerged that the avidity of leukocyte integrins with various subunits, e.g., β1, β2, β3, and β7, can be activated with different and characteristic kinetics in response to stimulation with chemokines or formyl-methyl-leucine-phenylalanine (fMLP) stimulation (Carr et al., 1996; Weber et al., 1996b; Sadhu et al., 1998). For instance, CC chemokines can induce a sustained activation of Mac-1 but also a transient activation of α4β1 and a late increase in α5β1 adhesiveness, implying that integrins sharing the same β subunit can be differentially regulated (Weber et al., 1996a,b). Although a dynamic regulation of LFA-1 avidity appears to be required for leukocyte transendothelial chemotaxis, increased LFA-1 avidity in response to CC chemokines, e.g., monocyte chemotactic protein-1 (MCP-1), used has been undetectable in adhesion assays (Carr et al., 1996; Weber et al., 1997a). Recently, other chemokines, e.g., the CXC chemokines stromal-derived factor-1α (SDF-1α) and 10-kDa inflammatory protein (IP10), have been shown to induce a rapid and mostly transient adhesion of T cells in stasis and may mediate their arrest in shear flow on LFA-1 substrates or activated endothelium (Campbell et al., 1998; Piali et al., 1998).
Specific properties and interactions of the integrin α and β cytoplasmic domains with the cytoskeleton and specific regulatory proteins appear to be involved in the bidirectional (“inside-out” and “outside-in”) transmembrane signal transduction of integrin regulation (Yamada and Miyamoto, 1995; Dedhar and Hannigan, 1996). It has been shown that in transfectants, the cytoplasmic domains of β1, β2, and β7 can differentially regulate LFA-1 clustering and thus affect cell adhesion (Lub et al., 1997b). Regulatory proteins specifically associated with β1, β2, and β3 integrin cytoplasmic domains have been identified, providing further evidence for integrin-specific regulatory pathways (Shattil et al., 1995; Kolanus et al., 1996; Chang et al., 1997; Kashiwagi et al., 1997). It has also been shown that the α cytoplasmic domains may confer a functional specialization and affect integrin clustering (Chan et al., 1992; Kassner et al., 1995; Yauch et al., 1997). Together with findings on the sequential regulation of α4β1 and α5β1 (Weber et al., 1996a), this may imply that the α cytoplasmic domains may be critical for the specific activation and function of integrins stimulated by chemokines. The differential regulation of integrin avidity by chemoattractants or chemokines may also critically contribute to successful transmigration of leukocytes, which is primarily mediated by the β2 integrins LFA-1 and Mac-1 and by their ligand ICAM-1, intercellular adhesion molecule-1 (ICAM-1) (Smith et al., 1989; Kavanaugh et al., 1991; Meerschaert and Furie, 1995; Weber et al., 1996a).
Here, we studied the kinetics of the β2 integrin activation by chemokines in mononuclear cells. Although the activation of Mac-1 was sustained, the up-regulation of LFA-1 activity was extremely transient, as detected by an mAb reporting conformational changes. We show that the differential regulation of LFA-1 and Mac-1 by chemokines is mediated through the α subunit cytoplasmic domain and may be triggered by distinct signal transduction pathways.
MATERIALS AND METHODS
Reagents and mAbs
Human recombinant macrophage inflammatory protein-1α (MIP-1α), MCP-1, RANTES (regulated on activation, normal T cell expressed and secreted), and SDF-1α were purchased from Pepro Tech (Rocky Hill, NJ). 2′,7′-Bis-2-carboxyethyl-5-(6)-carboxyfluorescein-acethoxymethylester was purchased from Molecular Probes (Leiden, the Netherlands). All other reagents were from Sigma (Deisenhofen, Germany) unless otherwise stated. Soluble ICAM-1 purified from mutant Chinese hamster ovary Lec 3.2.8.1 cells that express high mannose carbohydrates by immunoaffinity chromatography with ICAM-1 mAb R6.5 coupled to Sepharose (Marlin et al., 1990) and the mAbs TS1/22 (αL), TS1/18 (β2) (Sanchez-Madrid et al., 1982), OKM-1 (αM), CBRM1/29 (αM) (Diamond and Springer, 1993), and CBR-IC2/2 (ICAM-2) (de Fougerolles et al., 1991) were purified with protein A and were kind gifts of Dr. T.A. Springer (The Center for Blood Research, Boston, MA). The mAb R6.5 (anti-ICAM-1) (Smith et al., 1989) was provided by Dr. R. Rothlein (Boehringer Ingelheim, Ridgefield, CT). The activating mAb LFA1/2 (anti-β2) was a gift from Dr. L. Petruzzelli (University of Michigan, Ann Arbor, MI) (Petruzzelli et al., 1995). The mAb 24 (anti-CD11α) was kindly provided by Dr. N. Hogg (Imperial Cancer Research Fund, London, UK) (Dransfield et al., 1992). CD32 mAb was from PharMingen (San Diego, CA).
Blood Cell Isolation
Leukocyte-rich plasma was prepared from citrate-anticoagulated blood by dextran sedimentation of erythrocytes. Peripheral blood mononuclear cells were separated from leukocyte-rich plasma by Ficoll-Hypaque density gradient centrifugation. Monocytes were isolated from lymphocytes by Nycomed (Oslo, Norway) 1.068 hyperosmotic gradient centrifugation of leukocyte-rich plasma, as described (Boyum, 1983; Weber et al., 1996a). Platelets were removed from monocytes by four washes at 300 × g. This protocol yielded a purity of ∼85% monocytes, as assessed by CD14 staining, and did not result in a substantial activation, because L-selectin was only moderately shed, and L-selectin functions in shear flow were fully maintained.
Construction and Transfection of Wild-Type and Chimeric α Subunit cDNA and Generation of Mutant Jurkat Cells
Jurkat T lymphoma cells and the αL-deficient Jurkat clone J-β2.7 were maintained as described (Weber et al., 1997b). Chimeric cDNAs containing the αM extracellular and transmembrane regions linked to the αL cytoplasmic domain (αME) or αL extracellular and transmembrane regions joined to the αM cytoplasmic domain (αLE) were constructed as follows. A DraI restriction site was introduced by site-directed and conservative mutagenesis (Kunkel, 1985) within the sequence encoding the GFFKR motif in αM and αL. These cDNAs were digested with DraI and HindIII or with DraI and XbaI, and the expression vector AprM8 was digested with HindIII and XbaI. The DraI–HindIII fragment encoding the cytoplasmic domain of one α subunit and the DraI–XbaI fragment encoding the extracellular and transmembrane regions of the other α subunit were inserted into the HindIII–XbaI fragment of AprM8 by three-way ligation. Restriction analysis with MscI and DNA sequencing confirmed correct ligation and orientation. Cells were cotransfected with the cDNA for αL, αM, αME, or αLE and selection vector pBSneo by electroporation. The generation of Jurkat J-β2.7 transfectants coexpressing CCR2 chemokine receptor has been described (Weber et al., 1997a). Transfected cells were selected with 0.75 mg/ml G418 (Life Technologies, Gaithersburg, MD), and α subunit surface expression was enriched by multiple rounds of immunopanning on plates coated with αL or αM mAb. The generation of a mutant Jurkat cell clone with constitutively active LFA-1 (J19) by radiation mutagenesis, immunopanning on immobilized ICAM-1 (Hollander et al., 1988), and limited dilution will be described in detail elsewhere (our unpublished data).
Cell Adhesion Assays
Cell adhesion to ICAM-1 or BSA adsorbed at 10 μg/ml and fibrinogen at 25 μg/ml was performed as described (Weber et al., 1996a,b). Proteins were coated onto 96-well microtiter plates (Linbro Titertek; JCN Pharmaceuticals, Eschwege, Germany), and nonspecific adhesion was blocked by the addition of 1% human serum albumin (HSA) treated at 56°C for 2 h. Cells were labeled with the fluorescent dye 2′,7′-bis-2-carboxyethyl-5-(6)-carboxyfluorescein-acethoxymethylester (1 μg/ml) and resuspended in HHMC (Hank’s balanced salt solution, 10 mM HEPES, pH 7.4, 1 mM Mg2+, 1 or 0.1 mM Ca2+) supplemented with 0.5% HSA. For mAb inhibition, cells were preincubated with saturating concentrations of mAb for 30 min on ice, and monocytes were incubated with 5% heat-inactivated human serum or CD32 mAb to block Fc receptors. Labeled cells (5 × 104 in 50 μl) were added to ligand-coated wells in the presence of assay medium (control) or stimuli at indicated concentrations and allowed to settle on ice. Plates were rapidly warmed and incubated for indicated periods at 37°C. Nonadherent cells were removed by a plate washer (monocytes) or by aspiration wash (Jurkat cells) as described (Weber et al., 1996a, 1997b). Fluorescence of input and adherent cells was quantified with a fluorescence plate reader (SLT; Tecan, Research Triangle Park, NC), and specific binding was expressed as percentage of input.
Flow Cytometry
Cells were reacted with αL, αM, and β2 mAbs or isotype control in HHMC and 0.5% HSA for 30 min on ice, stained with FITC-conjugated goat anti-mouse immunoglobulin G (IgG) mAb, and analyzed by flow cytometry in a fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA). For mAb 24 staining, monocytes, Jurkat cells, Jurkat transfectants, J19 cells, or J19 transfectants were reacted with mAb 24 or isotype control (5 μg/ml) in HHMC in the presence of 5 mM Mg2+ and 2 mM EGTA or in the presence of MCP-1 or SDF-1α at indicated concentrations and for indicated periods at 37°C and immersed in ice water, as previously reported (Dransfield et al., 1992; Stewart et al., 1996). Cells were stained with FITC or phycoerythrin-conjugated anti-mouse IgG mAb on ice and analyzed by flow cytometry with appropriate light scatter gates. mAb 24 expression was reported as mean fluorescence intensity (percentage of isotype control) as described (Hwang et al., 1996).
Transendothelial Chemotaxis Assay
Isolation and culture of human umbilical vein endothelial cells and transendothelial migration assays were performed as described (Carr et al., 1994; Weber et al., 1996a). Human umbilical vein endothelial cells were grown on collagen-coated, 6.5-mm-diameter Transwell inserts (Costar, Cambridge, MA; 8 μm pore size). For inhibition studies, cells were preincubated with mAbs for 30 min on ice. To prevent binding of blocking mAb to Fc receptors, monocytes were preincubated with 5% human serum or purified IgG. Chemokines in assay medium (RPMI-1640, medium 199, 0.5% HSA) were added to 24-well tissue culture plates. Transwells were inserted, and cells were added to the top chamber. A dilution of cells served as a measure of input. Monocytes were allowed to transmigrate for 1 h. Input and transmigrated cells were detached with 5 mM EDTA and counted in a fluorescence-activated cell sorter using appropriate light scatter gates for monocytes.
RESULTS
Differential Regulation of β2 Integrin by Chemokines in Monocytes
To investigate the regulation of β2 integrin avidity by CC chemokines, we studied the adhesion of monocytes to immobilized ICAM-1. MCP-1, RANTES, and MIP-1α induced a prolonged increase in the binding of monocytes to ICAM-1 that was evident at 15 min, peaked at 30 min, and sustained at later time points (Figure 1A). Dose-dependence assays demonstrated that the induction of monocyte binding was optimal at 100 ng/ml MCP-1, at 100 ng/ml RANTES, and at 10 ng/ml MIP-1α (Figure 1B and our unpublished results). Inhibition assays with mAbs showed that unstimulated binding of cells was mediated by LFA-1, whereas the binding of the chemokine-stimulated cells was inhibited by mAbs to αM and ICAM-1 but not αL at 30 min (Figure 1C), indicating that the increase in adhesion was mediated by Mac-1. Stimulation with the cellular agonist phorbol 12-myristate 13-acetate (PMA) or extracellular agonists, i.e. Mn2+ or activating CBR-LFA1/2 mAb, induced monocyte adhesion to ICAM-1 that was mediated by both LFA-1 and Mac-1, indicating that LFA-1 can be activated (Figure 1D). Thus, CC chemokines induced a sustained increase in Mac-1 but not LFA-1 avidity.
Figure 1.
Induction of Mac-1-dependent monocyte adhesion to ICAM-1 by CC chemokines. (A–C) Kinetics (A), dose dependence (B), and mAb inhibition (C) of chemokine-stimulated monocyte binding to ICAM-1. (D) Effect of PMA, CBR-LFA1/2 mAb, and Mn2+ on monocyte binding to ICAM-1. Monocytes were subjected to adhesion assays on ICAM-1 at 37°C in the presence of MIP-1α (10 ng/ml), RANTES (100 ng/ml), and MCP-1 (100 ng/ml) (A and C), MCP-1 at indicated concentrations (B), or PMA (10 ng/ml), activating β2 mAb CBR-LFA1/2 (1 μg/ml), or Mn2+ (1 mM) (D) for indicated periods (A) or for 30 min (B–D). Cells were preincubated with mAbs to αL (TS1/22), αM (CBRM1/29), or isotype control wells with ICAM-1 mAb R6.5 (B and C). Data are mean ± SD of three independent experiments performed in triplicate.
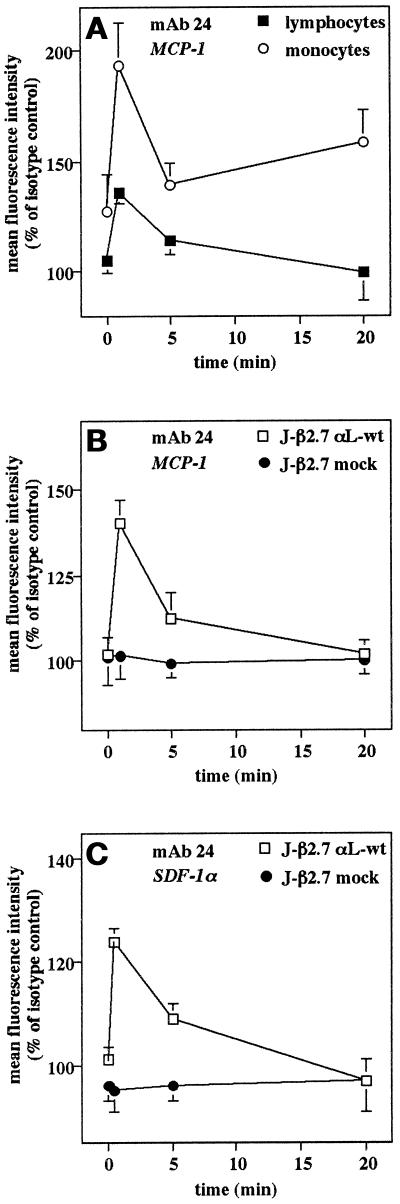
Dynamic regulation of LFA-1 avidity by chemokines is required for transendothelial chemotaxis; however, this regulation may be too transient or polarized to be detected in static adhesion assays (Weber et al., 1997a). To determine whether chemokines can induce a rapid and transient up-regulation in LFA-1 activity, we used the reporter mAb 24 (Dransfield et al., 1992), which recognizes a neoepitope on the active form of LFA-1, as has been described for lymphocyte stimulation by L-selectin cross-linking (Hwang et al., 1996). At the earliest time points (30 s), MCP-1 induced transient expression of the mAb 24 epitope on monocytes and less markedly on lymphocytes, which rapidly returned to lower or control levels at later time points (Figure 2A). The slightly increased expression at 20 min may be due to Mac-1 activation (Figure 2A). Similar experiments were performed with αL-deficient Jurkat J-β2.7 cells transfected without or with αL cDNA to restore LFA-1 expression and coexpressing MCP-1 receptor CCR2 (Weber et al., 1997a,b). Again, MCP-1 induced an early and transient up-regulation in mAb 24 expression on J-β2.7/αL but not J-β2.7/mock transfectants coexpressing CCR2 (Figure 2B). In addition, SDF-1α, a CXC chemokine shown to increase lymphocyte adhesion to ICAM-1 (Campbell et al., 1998), induced a transient induction of mAb 24 epitope in Jurkat J-β2.7/αL but not J-β2.7 mock transfectants, which was slightly more sustained than with MCP-1 (Figure 2C). Thus, SDF-1α and MCP-1 induced a very rapid and transient activation of LFA-1, whereas MCP-1 failed to induce LFA-1–mediated adhesion in a static adhesion assay. These data show that chemokines differentially regulate the avidity of the β2 integrins Mac-1 and LFA-1.
Figure 2.
Early, transient up-regulation of LFA-1 activity by MCP-1 and SDF-1α. Monocytes (A) and αL- or mock-transfected Jurkat J-β2.7 cells either coexpressing CCR2 (B) or not (C) were stimulated with MCP-1 (100 ng/ml) or SDF-1α (3 μg/ml) for indicated periods, in the presence of the CD11α activation epitope reporter mAb 24 or isotype control at 37°C, and then stained with FITC-conjugated mouse IgG mAb on ice and analyzed by flow cytometry with appropriate light scatter gates. Expression of the 24 epitope is reported as mean fluoresence intensity (percentage of isotype control). Data are mean ± SD of three separate experiments.
SDF-1α Specifically Regulates LFA-1 and Mac-1 Expressed in Lymphoid Cells
To further study the differential regulation of LFA-1 and Mac-1 by chemokines, Jurkat J-β2.7 cells were transfected with either αL or αM cDNA. Flow cytometric analysis revealed approximately equivalent surface expression of LFA-1 and Mac-1 on these transfectants (Figure 3, A and B). Static adhesion assays showed that stimulation with PMA induced an increase in the adhesion of Jurkat J-β2.7/αL or J-β2.7/αM transfectants to ICAM-1 (Figure 3, C and D). Inhibition with respective mAbs confirmed that the adhesion was specific for LFA-1 in αL transfectants and for Mac-1 in αM transfectants (Figure 3, C and D). The CXC chemokine SDF-1α was used for stimulation, because Jurkat cells express the SDF-1α receptor CXC receptor 4 (Hesselgesser et al., 1998), and SDF-1α can trigger lymphocyte adhesion to ICAM-1 under static and flow conditions (Campbell et al., 1998). Consistent with these data, SDF-1α induced a transient increase in adhesion of Jurkat J-β2.7/αL transfectants to ICAM-1 at 5 min, which was subsequently down-regulated at 15 min (Figure 3E). In a marked contrast, SDF-1α induced a prolonged increase in adhesion of Jurkat J-β2.7/αM transfectants to ICAM-1, which was evident at 5 min and sustained at 15 min (Figure 3F). These results parallel our findings in monocytes that chemokines induce a prolonged increase in the avidity of Mac-1, whereas they induce a rapid and transient activation of LFA-1.
Figure 3.
Differential regulation of LFA-1 and Mac-1 in lymphoid transfectants by SDF-1α. (A and B) Surface expression of LFA-1 and Mac-1 on Jurkat J-β2.7 transfectants. J-β2.7/αL and J-β2.7/αM transfectants were stained with mAbs to αL (TS1/22) or αM (CBRM1/29), respectively (solid line), and isotype control (dotted line). Shown is one representative experiment. (C–F) Adhesion of Jurkat J-β2.7 transfectants to ICAM-1. Cells were subjected to adhesion assays on ICAM-1 at 37°C with or without stimulation with PMA (100 ng/ml) for 30 min (C and D) or in the presence of SDF-1α (300 ng/ml) for the indicated periods (E and F). For mAb inhibition assays, cells were preincubated with saturating concentrations of mAbs for 30 min on ice. Data are mean ± SD of three independent experiments performed in triplicate. *, p < 0.05 versus unstimulated control.
Role of the α Subunit Cytoplasmic in β2 Integrin Regulation by Chemokines
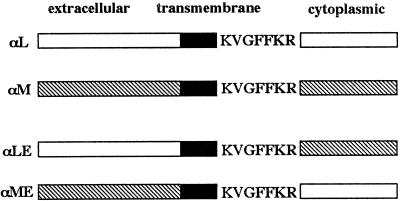
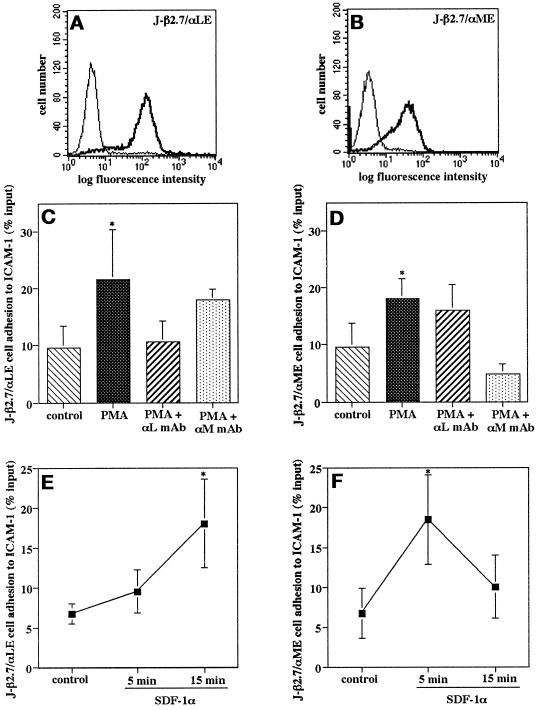
Our data show that integrins sharing the same β2 chain can be differentially regulated by chemokines in mononuclear cells, implying a regulatory role for the α subunit cytoplasmic domain. To test this hypothesis, we constructed chimeras consisting of the extracellular and transmembrane domains of αL joined to the intracellular domain of αM (termed αME) or vice versa (termed αLE) (Figure 4). We expressed these chimeras in the J-β2.7 cells and studied the regulation of avidity for ICAM-1 by SDF-1α. Flow cytometry confirmed approximately equivalent surface expression of the LFA-1 and Mac-1 extracellular domains (Figure 5, A and B). Adhesion assays to ICAM-1 revealed that the adhesion of both J-β2.7/αME and J-β2.7/αLE transfectants was increased after stimulation with PMA (Figure 5, C and D). This indicates that the cytoplasmic domains were functional in transducing an activation signal to the extracellular regions. A blocking mAb to LFA-1 but not to the Mac-1 extracellular domain inhibited adhesion of the J-β2.7/αLE cells, whereas only blocking mAb to Mac-1 inhibited adhesion of the J-β2.7α/ME cells (Figure 5, C and D). Upon stimulation of the J-β2.7/αLE cells with SDF-1α, adhesion to ICAM-1 was increased at 5 min and sustained at 15 min, similar to the prolonged adhesion of J-β2.7/αM transfectants (Figure 5E). In contrast, stimulation of J-β2.7/αME cells with SDF-1α resulted in a transient increase in adhesion to ICAM-1, as seen with J-β2.7/αL transfectants (Figure 5F). This clearly indicates that the αM cytoplasmic domain transduces a signal to the extracellular region, resulting in a sustained increase in adhesion, whereas the αL cytoplasmic domain triggers a transient activation. Thus, the different kinetics of integrin avidity regulation induced by chemokines may be mediated and determined by the α subunit cytoplasmic domains.
Figure 4.
Schematic diagram of αLE and αME chimeras. Chimeras of the α subunit were constructed as described in MATERIALS AND METHODS.
Figure 5.
Differential regulation of LFA-1 and Mac-1 avidity by SDF-1α is mediated by the α subunit cytoplasmic domain. (A and B) Surface expression of LFA-1 and Mac-1 extracellular regions on J-β2.7 cells transfected with chimeric α subunit cytoplasmic domain exchanges LE and ME. J-β2.7αLE and J-β2.7αME transfectants were stained with mAbs to αL (TS1/22) or αM (CBRM1/29), respectively (solid line), and isotype control (dotted line). Shown is one representative experiment. (C–F) Adhesion of J-β2.7 transfectants to ICAM-1. Cells were subjected to adhesion assays on ICAM-1 at 37°C with or without stimulation with PMA (100 ng/ml) for 30 min (C and D) or in the presence of SDF-1α (3 μg/ml) for the indicated periods (E and F). For mAb inhibition assays, cells were preincubated with saturating concentrations of mAbs for 30 min on ice. Data are mean ± SD of three independent experiments performed in triplicate. *, p < 0.05 versus unstimulated control.
Differential β2 Integrin Activation via the α Subunit Cytoplasmic Domains in a Mutant Jurkat Cell Line
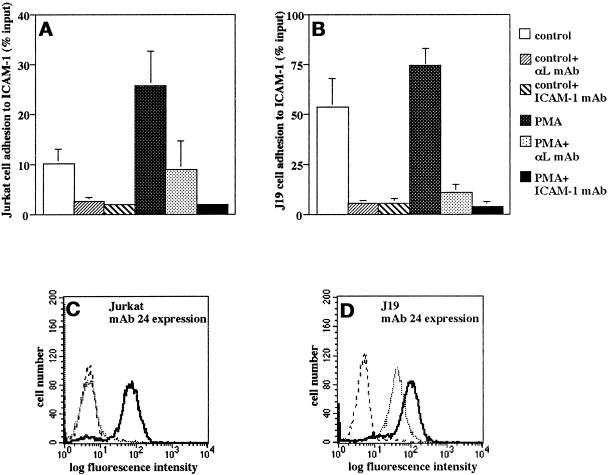
To further investigate the possibility that distinct signaling pathways account for the specific activation of the β2 integrins mediated by their α cytoplasmatic domain, as shown above, we used a mutant Jurkat cell clone (J19) with a “gain of function” phenotype, which expresses LFA-1 in constitutively active form. Flow cytometry, comparison of purified LFA-1 in adhesion assays, and DNA sequencing of αL and β2 cytoplasmic domain cDNA generated by reverse transcription PCR confirmed that the LFA-1 molecule itself was unaltered in J19 cells, indicating the presence of a specific signaling defect (our unpublished results). Adhesion assays and mAb inhibition revealed that, in contrast to wild-type cells, unstimulated J19 cells showed a constitutively high adhesion to ICAM-1, which was not significantly increased by PMA and was mediated by LFA-1 (Figure 6, A and B). This was paralleled by constitutive expression in J19 cells of the mAb 24 epitope, which is strongly induced by Mg2+ and EGTA in wild-type Jurkat cells and reflects an active form of LFA-1 (Figure 6, C and D).
Figure 6.
Differential β2 integrin activation via the α subunit cytoplasmic domains in the mutant Jurkat cell line J19. (A–D) Adhesion to ICAM-1 (A and B) and expression of the mAb 24 epitope (C and D) in J19 cells compared with wild-type Jurkat cells. (A and B) Cells were subjected to adhesion assays on ICAM-1 at 37°C with or without stimulation with PMA (100 ng/ml) for 30 min. For mAb inhibition assays, cells or wells were pretreated with saturating concentrations of mAbs for 30 min on ice. Data are mean ± SD of six independent experiments performed in triplicate. (C and D) Cells were reacted with the CD11α activation epitope reporter mAb 24 in the presence of 1 mM Ca2+ and 1 mM Mg2+ (dotted line) or 5 mM Mg2+ and 2 mM EGTA (bold line) and analyzed by flow cytometry. Staining with an isotype control mAb is shown (stippled line). Shown is a representative experiment.
Wild-type Jurkat and J19 cells were transfected with αM and αME cDNA, and equivalent levels of expression of the extracellular domain of Mac-1 were confirmed by flow cytometry (our unpublished results). Adhesion assays on the Mac-1 ligand fibrinogen revealed that both the unstimulated and PMA-stimulated adhesion of Jurkat/αM and J19/αM transfectants was comparable and was inhibited by a blocking Mac-1 mAb (Fig. 7A). Thus, the defect in the J19 mutants resulting in a constitutively active form of LFA-1 did not affect Mac-1 avidity or its cellular stimulation, suggesting the presence of distinct pathways of regulation for LFA-1 and Mac-1 function. In contrast, J19 cells expressing the αME chimera demonstrated constitutive binding to fibrinogen (Fig. 7B). In line with these results, the mAb 24 activation epitope was constitutively expressed in the J19/αM transfectants, whereas constitutive expression was slightly stronger in the J19/αME transfectants (Figure 7, C and D), possibly reflecting the additional presence of extracellular αM in an active conformation. In contrast, the mAb 24 epitope was not expressed in Jurkat/αM or Jurkat/αME transfectants (Figure 7, C and D). These experiments show that these distinct activation pathways are determined by the α subunit cytoplasmic domain.
Figure 7.
Constitutive LFA-1 activity in J19 cells is specific and mediated by the αL subunit cytoplasmic domain. (A–D) Adhesion to ICAM-1 (A and B) and expression of the mAb 24 epitope (C and D) in Jurkat or J19 cells transfected with αM (A and C) or the chimeric α subunit cytoplasmic domain exchange αME (B and D). (A and B) Cells were subjected to adhesion assays on the Mac-1 ligand fibrinogen at 37°C with or without stimulation with PMA (100 ng/ml) for 30 min. Data are mean ± SD of six independent experiments performed in triplicate. (C and D) Jurkat or J19 cells transfected with αM (C) or the chimeric αME (D) were reacted with CD11α activation epitope reporter mAb 24 (solid line, Jurkat; bold line, J19) or with an isotype control mAb (dotted line, Jurkat; stippled line, J19) in the presence of 1 mM Ca2+ and 1 mM Mg2+ and analyzed by flow cytometry. Shown is a representative experiment.
Role of β2 Integrin Avidity in Transendothelial Chemotaxis of Leukocytes
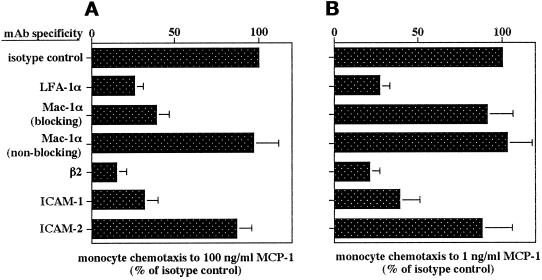
To investigate possible implications of the chemokine-induced regulation of LFA-1 and Mac-1, we studied transendothelial chemotaxis of monocytes in response to an MCP-1 gradient. Inhibition studies with mAbs confirmed that transendothelial chemotaxis was mediated by β2 integrins and ICAM-1 at all concentrations of MCP-1 studied. Transmigration of monocytes to MCP-1 (1 or 100 ng/ml) was inhibited by up to 70% with mAbs to αL, β2, or ICAM-1 (Fig. 8, A and B). In contrast, a mAb to αM inhibited transmigration induced by MCP-1 at concentrations that stimulated Mac-1 avidity (e.g., 100 ng/ml) but not by MCP-1 at 1 ng/ml, which did not up-regulate Mac-1 avidity (Figures 1B and 8, A and B). In contrast, a nonblocking mAb to αM or a blocking mAb to ICAM-2 had no effect (Fig. 8, A and B). These data indicate that although up-regulation of LFA-1 avidity by MCP-1 may not be observed in static adhesion assays, LFA-1 activity can nevertheless be transiently regulated and is undoubtedly crucial for transmigration in response to MCP-1. On the other hand, Mac-1 facilitated transmigration only in response to concentrations of MCP-1 which increased its avidity to ICAM-1.
Figure 8.
Role of β2 integrins in monocyte transendothelial chemotaxis. Inhibition by mAb of monocyte chemotaxis in response to MCP-1 at 100 (A) or 1 (B) ng/ml is shown. Monocytes were pretreated with mAb to β2 (TS1/18), αL (TS1/22), αM (CBRM-1/29, or nonblocking OKM-1) on ice, and endothelial monolayers were treated with mAb to ICAM-1 (R6.5), ICAM-2 (CBR-IC2/2), or isotype control for 20 min and washed. Spontaneous migration was 2.5 ± 0.6% of input, and migration in response to MCP-1 at 100 and 1 ng/ml (isotype control) was 45.4 ± 4.9 and 14.5 ± 2.4% of input, respectively. Data are mean ± SD of three independent experiments performed in duplicate.
DISCUSSION
We found that the CC chemokines MCP-1, MIP-1α, and RANTES induce a sustained increase in the avidity of Mac-1 but not LFA-1 for ICAM-1 in monocytes. This was consistent with previous findings that CC chemokines induced a prolonged activation of Mac-1 avidity in eosinophils (Weber et al., 1996b), but, however, failed to up-regulate LFA-1-mediated adhesion of T lymphocytes to ICAM-1 (Carr et al., 1996, Weber et al., 1997a), and that fMLP stimulated an increase in neutrophil binding to ICAM-1 that was dependent on Mac-1 and not LFA-1 (Smith et al., 1989). Kinetic studies using the reporter mAb 24 that recognizes an LFA-1 activation epitope (Dransfield et al., 1992) revealed that MCP-1 and the CXC chemokine SDF-1α induced a very rapid and transient up-regulation of LFA-1 activity in monocytes or lymphoid cells. These data show that CC chemokines differentially regulate the avidity of the β2 integrins Mac-1 and LFA-1 when expressed in the same cells and expand on previous studies, which demonstrated that chemokines can differentially regulate the avidity of integrins that share a common β subunit (Carr et al., 1996; Weber et al., 1996a; Sadhu et al., 1998).
A study in Jurkat cells that expressed mutant αL, rendering LFA-1 constitutively active or inactive, suggested that transendothelial chemotaxis induced by MCP-1 requires a dynamic regulation of LFA-1, which may be extremely transient, polarized to relevant areas (i.e. leading edge), or restricted to subsets of LFA-1 molecules and hence was undetectable in static adhesion assays (Weber et al., 1997a). Recently, other chemokines, e.g., the CXC chemokines SDF-1α and IP10, have been shown to trigger a rapid (at 1 min) and mostly transient increase in T cell adhesion and may mediate arrest under flow conditions on ICAM-1 or activated endothelium (Campbell et al., 1998; Piali et al., 1998). Here we show that SDF-1α induced a transient activation of LFA-1 and a sustained activation of Mac-1 in adhesion assays on ICAM-1 with transfectants selectively expressing either LFA-1 or Mac-1. Consistent with a previous study (Weber et al., 1997a), MCP-1 did not stimulate LFA-1 avidity in monocyte adhesion assays at time points as early as 5 min. However, detection of the mAb 24 activation epitope revealed that MCP-1 induced a very transient and slightly less sustained up-regulation of LFA-1 activity in monocytes or Jurkat transfectants coexpressing CCR2 than SDF-1α in Jurkat transfectants. This paralleled earlier findings that among CC chemokines tested, MCP-1 most rapidly activated α4β1 avidity (Weber et al., 1996a). We have found that MCP-1 is more crucial in mediating transmigration than arrest of monocytes in physiological shear flow (Weber et al., 1999). This may indicate that MCP-1 may be specialized in inducing mononuclear cell motility, whereas chemokines, such as IP10 and SDF-1α, may control lymphocyte arrest during inflammation or the localization during the surveillance and homeostasis of immune cells. As opposed to other chemokines, the rapidity of MCP-1 responses and its predominant role in monocyte migration may be due to differences between chemokine receptors in Gαi protein coupling and subsequent signaling (Amatruda et al., 1993). Regardless of the chemokine and the rapidity of the response, however, the mode of regulation is likely an intrinsic and specific characteristic of the integrin.
Chemokines have been found to sequentially induce an early, transient up-regulation of α4β1 avidity and a late, sustained activation of α5β1 in monocytes, showing that integrins sharing the same β subunit can be differentially regulated in one cell type (Weber et al., 1996a). This implied that differences in regulation were mediated via distinct α subunits. The transience in avidity regulation of α4β7 for vascular adhesion molecule-1 in lymphoid transfectants by fMLP or interleukin-8 further supports such a role for the α4 subunit (Sadhu et al., 1998). Our studies with αL/αM chimeras that consisted of αL and αM cytoplasmic tail exchanges showed that the α subunit cytoplasmic domains are responsible for conferring differential and specific modes of avidity regulation to β2 integrins. Different α subunit cytoplasmic tails can mediate specific β1 integrin-dependent cellular responses (Chan et al., 1992; Kassner et al., 1995), e.g., the α4 cytoplasmic domain promoted cell migration, whereas the α2 and α5 cytoplasmic domains facilitated collagen gel contraction and spreading. Our findings now demonstrate that the α subunit cytoplasmic domains may direct specific responses after chemokine stimulation.
The characterization of lymphoid cell mutants has proven a valuable genetic tool to study signal transduction pathways and has served to identify essential elements, e.g., the involvement of the lck tyrosine kinase in T cell receptor signaling (Straus and Weiss, 1992). In a similar approach, we used the mutant Jurkat cell clone J19 expressing LFA-1 in a constitutively high avidity state, as demonstrated by binding to ICAM-1 and mAb 24 activation epitope expression. This gain of function phenotype resulted from a signaling defect but not from changes in the integrin molecule. Expression of αM showed that this defect did not affect the activity of Mac-1, implying distinct pathways for the regulation of LFA-1 and Mac-1, whereas the high constitutive activity of the αME chimera indicated that such pathways may be specifically mediated via the α cytoplasmic domain. Ongoing studies to further characterize the nature of this signaling defect are under way. Other mutagenesis studies using Jurkat cells have revealed a defect downstream of protein kinase C affecting the avidity of both LFA-1 and α4β1 (Mobley et al., 1996) and have characterized αL- or β2-deficient cell lines (Weber et al., 1997b). Similar genetic analysis has provided new insights in structural and signaling defects of αIIbβ3 (Baker et al., 1997) and may be useful in further elucidating mechanisms of integrin regulation and adhesion.
Cellular inside-out signaling required for specific regulation of integrin affinity has been shown to be mediated via the integrin cytoplasmic domains (O’Toole et al., 1994). Notably, the α subunit cytoplasmic domains are well conserved among different species but, unlike the β subunit cytoplasmic domain, share little homology with each other (Hynes, 1992). Thus, the specificity of integrin regulation by the same agonist may be due to an involvement of regulatory proteins selective for the α cytoplasmic tail, e.g., a recently identified calcium-binding candidate regulatory protein that specifically interacts with the αIIb cytoplasmic tail (Naik et al., 1997). A differential regulation of β1, β2, β3, and β7 integrins by chemokines and chemoattractants has also been described (Carr et al., 1996; Weber et al., 1996b; Sadhu et al., 1998). Regulatory proteins specific for the β1, β2, and β3 cytoplasmic tails have been identified, which may be involved in these divergent pathways (Shattil et al., 1995; Kolanus et al., 1996; Chang et al., 1997). For example, overexpression of the β2-associated protein cytohesin-1 regulated LFA-1 but not α4β1 adhesiveness in Jurkat cells (Kolanus et al., 1996). Such regulatory proteins may also contribute to the specificity of integrin regulation by chemokines.
Transient up-regulation of LFA-1 activity was shown by induction of the mAb 24 epitope, which reports a conformational change indicative of increased affinity (Dransfield et al., 1992; Stewart et al., 1996). As seen with PMA, intracellular signals can result in increased LFA-1 affinity for ICAM-1 (Lollo et al., 1993). Deletion of the αL cytoplasmic tail after the GFFKR motif locked LFA-1 in a low-affinity state and prevented transendothelial chemotaxis induced by MCP-1, confirming that integrin cytoplasmic domains may regulate affinity via inside-out signals (O’Toole et al., 1994; Weber et al., 1997a). The inhibition of transendothelial chemotaxis by bivalent ICAM-1, which binds to high-affinity LFA-1, suggested that MCP-1 stimulation may involve an induction of LFA-1 affinity (Stewart et al., 1996; Weber et al., 1997a). Similarly, sustained Mac-1 activation by chemokines in granulocytes was associated with the induction of a conformationally altered neoepitope in a subpopulation of Mac-1, which correlates with increased affinity and mediates adhesion (Diamond and Springer, 1993; Weber et al., 1996b; Jones et al., 1998). Cross-linking of L-selectin, which is involved in leukocyte recruitment, also induced an increase in mAb 24 expression (Hwang et al., 1996), and platelet activation via G-protein-coupled receptors can cause conformational changes associated with increased affinity of αIIbβ3 (Sims et al., 1991). More recently, the myeloid S100 protein MRP-14 has been shown to increase Mac-1 affinity via a G-protein–coupled event in neutrophils (Newton and Hogg, 1998), which may sustain Mac-1 activation in myelomonocytic cells, but not when expressed in Jurkat cells. Together, these data suggest that affinity modulation is the primary mechanism promoting β2 integrin ligand binding in response to stimulation of G-protein–coupled receptors with chemokines. This is supported by findings that integrin affinity modulation is a predominant regulator of ligand binding and adhesion, although clustering may enhance responses or trigger outside-in signals (Lu and Springer, 1997; Hato et al., 1998).
The mechanisms of integrin regulation may also involve their cell surface distribution and the actin cytoskeleton. The release of LFA-1 from cytoskeletal restraints, e.g., by phorbol ester, cytochalasin D, or calpain protease in response to CD3 cross-linking, may allow lateral mobility and clustering on the cell surface, and with the induction of a high-affinity form may promote adhesion (Kucik et al., 1996; Lub et al., 1997a; Stewart et al., 1998). In contrast, cytochalasin D inhibited LFA-1 avidity in activated T cells or JY transfectants stimulated by interleukin-8 or fMLP, possibly because of a dual role of the actin cytoskeleton, which may also serve to maintain LFA-1 clustering (Lub et al., 1997a; Sadhu et al., 1998). We have found that LFA-1 on the surface of resting monocytes or Jurkat cells was clustered to some extent; however, this was not markedly modulated by chemokines not present in a gradient (our unpublished data). Cytochalasin D has been shown to affect Mac-1 activation by immune complexes but not by chemokines (Weber et al., 1996b; Jones et al., 1998), suggesting that involvement of the actin cytoskeleton depends on the stimulus and signal transduction pathways. Findings that deletion of the α4 cytoplasmic tail impairs lateral mobility and clustering of α4β1 integrin, thereby diminishing adhesion (Yauch et al., 1997), further imply that the α cytoplasmic tails may determine a differential regulation by the actin cytoskeleton. Integrin avidity may also be influenced by extracellular mechanisms, e.g., by urokinase receptor, which can physically associate with Mac-1 and increase its avidity (Xue et al., 1994; Simon et al., 1996). Such proteins may act to extracellularly stabilize an active conformation of Mac-1 but not LFA-1, thus leading to sustained versus transient regulation.
Monocytes use LFA-1 or Mac-1 for transendothelial migration in vitro (Meerschaert and Furie, 1995), but LFA-1 plays a more important role for monocyte migration into inflammatory sites induced by cytokines in vivo, because Mac-1 mAb was only inhibitory in combination with LFA-1 mAb (Issekutz, 1995). Our study shows that LFA-1 is involved in transendothelial chemotaxis of monocytes to all MCP-1 concentrations, underlining the importance of LFA-1 in transmigration. In contrast, Mac-1 contributed to leukocyte chemotaxis only to concentrations of MCP-1 that up-regulated Mac-1 avidity in a static adhesion assay. The sustained activation of Mac-1 avidity by chemokines may be relevant to monocyte arrest and may thus contribute to transmigration primarily via increased adhesion, as in comparison with PMA or Mn2+, its adhesive strength was relatively moderate to still allow optimal migration. A transient avidity regulation of α4β1 by chemokines has been shown to support the lateral monocyte migration to interendothelial junctions (Weber and Springer, 1998). A dynamic regulation of LFA-1 activity by chemokines would finally enable temporal coordination of traction and detachment to promote and complete transendothelial diapedesis (Weber et al., 1997a). Transient activation of LFA-1 was more rapid in response to MCP-1 than SDF-1α. This is consistent with a prominent role for MCP-1 in extravasation or trafficking of highly motile inflammatory cells, such as monocytes, whereas SDF-1α may be crucial for arrest, localization, or homeostasis of immune cells. Together, our data suggest that a coordinated regulation of integrins by chemokines and their specialization are crucially involved in the sequential process of successful leukocyte emigration.
ACKNOWLEDGMENTS
We thank Drs. N. Hogg, R. Rothlein, L. Petruzzelli, and T.A. Springer for kindly providing mAbs and reagents. K.S.C.W. was supported by the August-Lenz Stiftung. C.W. was supported by Deutsche Forschungsgemeinschaft grant We-1913/2.
Abbreviations used:
- fMLP
formyl-methyl-leucine-phenylalanine
- HSA
human serum albumin
- ICAM-1
intercellular adhesion molecule-1
- IgG
immunoglobulin G
- LFA-1
lymphocyte function-associated antigen-1
- MCP
monocyte chemotactic protein
- MIP
macrophage inflammatory protein
- PMA
phorbol 12-myristate 13-acetate
- RANTES
regulated on activation, normal T cell expressed and secreted
- SDF
stromal-derived factor
REFERENCES
- Amatruda TT, Gerard NP, Gerard C, Simon MI. Specific interactions of chemoattractant factor receptors with G-proteins. J Biol Chem. 1993;268:10139–10144. [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Baker EK, Tozer EC, Pfaff M, Shattil SJ, Loftus JC, Ginsberg MH. A genetic analysis of integrin function: Glanzmann thrombasthenia in vitro. Proc Natl Acad Sci USA. 1997;94:1973–1978. doi: 10.1073/pnas.94.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Isolation of human blood monocytes with Nycodenz, a new nonionic iodinated gradient medium. Scand J Immunol. 1983;17:429–436. doi: 10.1111/j.1365-3083.1983.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Hedrik J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Carr MW, Alon R, Springer TA. The C-C chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity. 1996;4:179–182. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein-1 is a major T lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BMC, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin α subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chang D, Wong C, Smith H, Liu J. ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J Cell Biol. 1997;138:1149–1157. doi: 10.1083/jcb.138.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signaling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR, Stacker SA, Schwarting R, Springer TA. Characterization of ICAM-2 and evidence for a third counterreceptor for LFA-1. J Exp Med. 1991;174:253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers PA, Lo SK, Olsen-Egbert E, Walz A, Baggiolini M, Cohn ZA. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Cabañas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Springer TA. T cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational changes in the adhesive and signaling functions of the integrin αIIbβ3. J Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity and HIV-1 infectivity. J Immunol. 1998;160:877–883. [PubMed] [Google Scholar]
- Hollander N, Selvaraj P, Springer TA. Biosynthesis and function of LFA-3 in human mutant cells deficient in phosphotidylinositol-anchored proteins. J Immunol. 1988;141:4283–4290. [PubMed] [Google Scholar]
- Hwang ST, Singer MS, Giblin PA, Yednock TA, Bacon KB, Simon SI, Rosen SD. GlyCAM-1, a physiologic ligand for L-selectin, activates β2 integrins on naive peripheral lymphocytes. J Exp Med. 1996;184:1343–1348. doi: 10.1084/jem.184.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Issekutz TB. In vivo blood monocyte migration to acute inflammatory reactions, IL-1α, TNF-α, IFN-γ, and C5a utilizes LFA-1, Mac-1, and VLA-4: the relative importance of each integrin. J Immunol. 1995;154:6533–6540. [PubMed] [Google Scholar]
- Jones SL, Knaus UG, Bokoch GM, Brown EJ. Two signaling mechanisms for activation of αMβ2 avidity in polymorphonuclear neutrophils. J Biol Chem. 1998;273:19556–19666. doi: 10.1074/jbc.273.17.10556. [DOI] [PubMed] [Google Scholar]
- Kashiwagi H, Schwartz MA, Eigenthaler M, Davis KA, Ginsberg MH, Shattil SJ. Affinity modulation of platelet integrin αIIbβ3 by β3-endonexin, a selective binding partner of the β3 integrin cytoplasmic tail. J Cell Biol. 1997;137:1433–1443. doi: 10.1083/jcb.137.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner PD, Alon R, Springer TA, Hemler ME. Specialized functional roles for the integrin α4 cytoplasmic domain. Mol Biol Cell. 1995;6:661–674. doi: 10.1091/mbc.6.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh AF, Lightfoot E, Lipsky PE, Oppenheimer-Marks N. Role of CD11/CD18 in adhesion and transendothelial migration of T cells: analysis utilizing CD18-deficient T cell clones. J Immunol. 1991;146:4149–4156. [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. αLβ2 integrin by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SK, Detmers PA, Levin SM, Wright SD. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989;169:1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollo BA, Chan KW, Hanson EM, Moy VT, Brian AA. Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J Biol Chem. 1993;268:21693–21700. [PubMed] [Google Scholar]
- Lu CF, Springer TA. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1 (LFA-1) J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- Lub M, van Kooyk Y, van Vliet SJ, Figdor CG. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol Biol Cell. 1997a;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lub M, van Vliet SJ, Oomen SPMA, Pieters RA, Robinson M, Figdor CG, van Kooyk Y. Cytoplasmic tails of β1, β2, and β7 integrins differentially regulate LFA-1 function in K562 cells. Mol Biol Cell. 1997b;8:719–728. doi: 10.1091/mbc.8.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin SD, Staunton DE, Springer TA, Stratowa C, Sommergruber W, Merluzzi V. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990;344:70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- Mobley JL, Ennis E, Shimizu Y. Isolation and characterization of cell lines with genetically distinct mutations downstream of protein kinase C that result in defective activation-dependent regulation of T cell integrin function. J Immunol. 1996;156:948–956. [PubMed] [Google Scholar]
- Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin αIIb cytoplasmic domain. J Biol Chem. 1997;272:4651–4654. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the β2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- O’Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L, Maduzia L, Springer TA. Activation of LFA-1 (CD11a/CD18) mimicked by an antibody directed against CD18. J Immunol. 1995;155:854–866. [PubMed] [Google Scholar]
- Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokines IP10 and Mig induce rapid and shear-resistant adhesion of activated T lymphocytes to endothelial integrin ligands via CXCR3. Eur J Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Staunton DE. Differential regulation of chemoattractant-stimulated β2, β3, and β7 integrin activity. J Immunol. 1998;160:5622–5628. [PubMed] [Google Scholar]
- Sanchez-Madrid F, Krensky AM, Ware CF, Robbins E, Strominger JL, Burakoff SJ, Springer TA. Three distinct antigens associated with human T lymphocyte-mediated cytolysis: LFA-1, LFA-2 and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, O’Toole T, Eigenthaler M, Thon V, Williams M, Babior BM, Ginsberg MH. β3-Endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin β2 subunit. J Cell Biol. 1995;131:807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DI, Rao NK, Xu H, Wie Y, Majdic O, Ronne E, Kobzik L, Chapman HA. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells Blood. 1996. 88, 3185–3194. [PubMed] [Google Scholar]
- Sims PJ, Ginsberg MH, Plow EF, Shattil SJ. Effect of platelet activation on the conformation of the plasma membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1991;266:7345–7352. [PubMed] [Google Scholar]
- Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–433. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stewart MP, Cabanas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- Stewart MP, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Adams DA, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, van de Wiel-van Kemande P, Weder P, Kuijpers TW, Figdor CF. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- Weber C, Alon R, Springer TA. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996a;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996b;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Lu C-F, Casasnovas J, Springer TA. Role of the αLβ2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J Immunol. 1997a;159:3968–3975. [PubMed] [Google Scholar]
- Weber C, Springer TA. VLA-4 avidity regulation by chemokines facilitates lateral migration of monocytes. J Immunol. 1998;161:6825–6834. [PubMed] [Google Scholar]
- Weber KSC, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential chemokine immobilization and hierarchical involvement of their receptors in monocyte arrest and transmigration on inflammatory endothelium. Eur J Immunol. 1999;29:700–712. doi: 10.1002/(SICI)1521-4141(199902)29:02<700::AID-IMMU700>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Weber KSC, York MJ, Springer TA, Klickstein LB. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines. J Immunol. 1997b;153:273–279. [PubMed] [Google Scholar]
- Xue W, Kindzelskii AL, Todd RF, Petty HR. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152:4630–4640. [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;5:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Felsenfeld DP, Kraeft S-K, Chen LB, Sheetz MP, Hemler ME. Mutational evidence for control of cell adhesion through integrin diffusion/clustering, independent of ligand binding. J Exp Med. 1997;186:1347–1355. doi: 10.1084/jem.186.8.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]