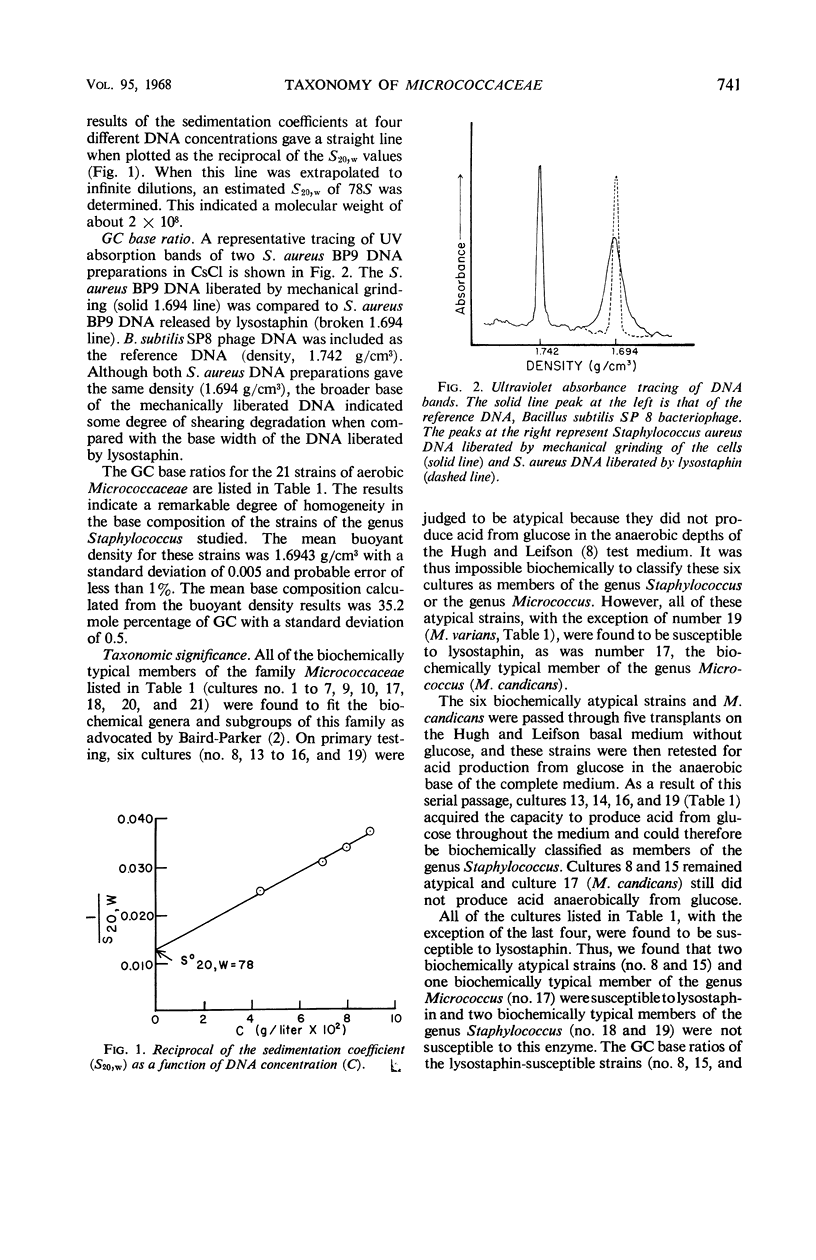
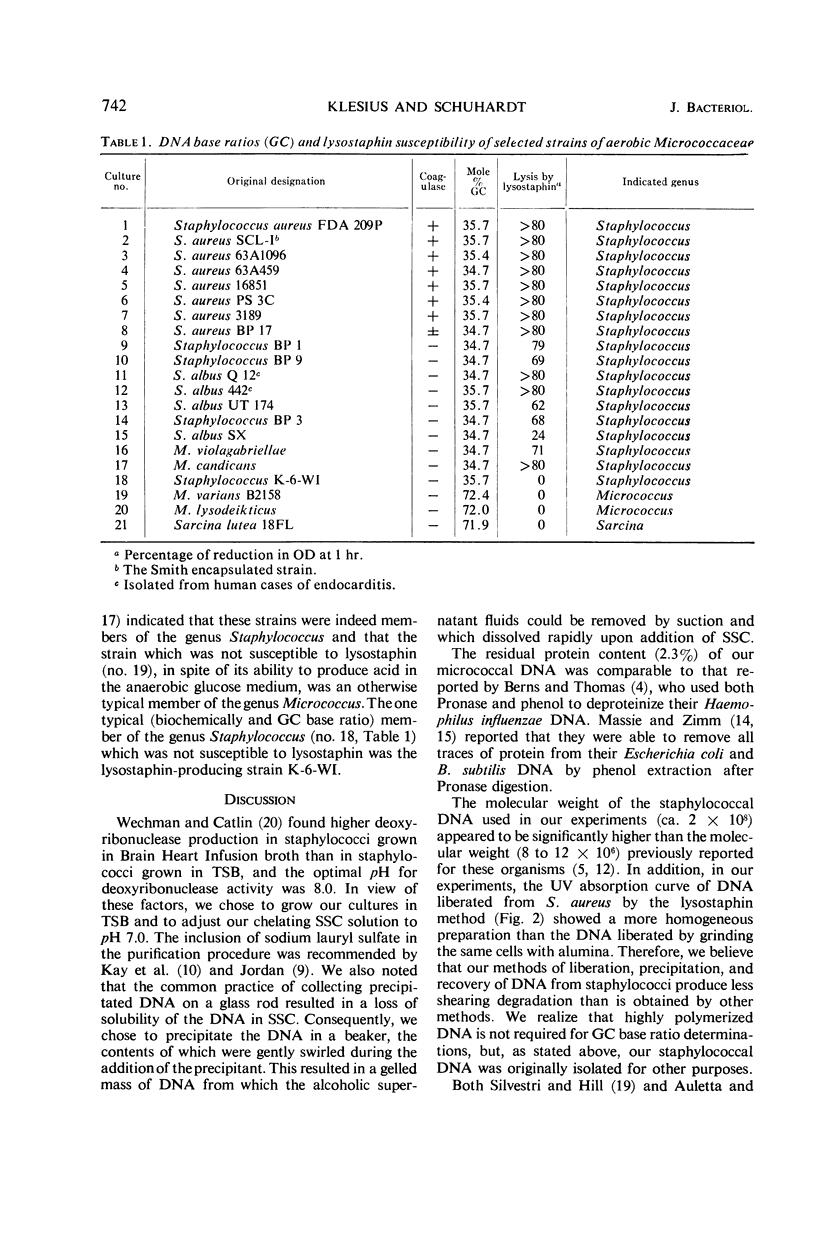
Abstract
By use of the staphylolytic enzyme lysostaphin, a method was devised for isolating and purifying highly polymerized deoxyribonucleic acid (DNA) from lysostaphinsusceptible Micrococcaceae. Staphylococcus aureus DNA isolated by this procedure gave an estimated molecular weight of ca. 2 × 108 and a residual protein content of 2.3%. The mole percentage of guanine + cytosine (GC) present in the DNA from 21 strains of aerobic Micrococceae was determined by buoyant density in cesium chloride. DNA from 12 biochemically typical members of the genus Staphylococcus gave a mean GC composition of 35.2 ± 0.5 mole per cent. Four biochemically atypical Staphylococcus strains and one biochemically typical strain of the genus Micrococcus (M. candicans) were found to be susceptible to lysostaphin and gave typical Staphylococcus spp. GC base ratios. One biochemically atypical member of the genus Micrococcus (M. varians) was not susceptible to lysostaphin and gave a typical Micrococcus spp. GC base ratio. Lysostaphin susceptibility is an easy test to perform, and the results of this test appear to correlate with GC base ratio studies of the genera of Micrococcaceae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auletta A. E., Kennedy E. R. Deoxyribonucleic acid base composition of some members of the Micrococcaceae. J Bacteriol. 1966 Jul;92(1):28–34. doi: 10.1002/path.1700920103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. A classification of micrococci and staphylococci based on physiological and biochemical tests. J Gen Microbiol. 1963 Mar;30:409–427. doi: 10.1099/00221287-30-3-409. [DOI] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Baird-Parker A. C. Staphylococci and their classification. Ann N Y Acad Sci. 1965 Jul 23;128(1):4–25. doi: 10.1111/j.1749-6632.1965.tb11626.x. [DOI] [PubMed] [Google Scholar]
- Blobel H. ISOLATION AND CHARACTERIZATION OF DEOXYRIBONUCLEIC ACID FROM A STRAIN OF STAPHYLOCOCCUS AUREUS. J Bacteriol. 1961 Sep;82(3):425–429. doi: 10.1128/jb.82.3.425-429.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty P., McGill B. B., Rice S. A. THE PROPERTIES OF SONIC FRAGMENTS OF DEOXYRIBOSE NUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 May;44(5):432–438. doi: 10.1073/pnas.44.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Zimm B. H. Molecular weight of the DNA in the chromosomes of E. coli and B. subtilis. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1636–1641. doi: 10.1073/pnas.54.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie H. R., Zimm B. H. THE USE OF HOT PHENOL IN PREPARING DNA. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1641–1643. doi: 10.1073/pnas.54.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. PURIFICATION AND PROPERTIES OF LYSOSTAPHIN--A LYTIC AGENT FOR STAPHYLOCOCCUS AUREUS. Biochim Biophys Acta. 1965 Feb 15;97:242–250. doi: 10.1016/0304-4165(65)90088-7. [DOI] [PubMed] [Google Scholar]
- Silvestri L. G., Hill L. R. Agreement Between Deoxyribonucleic Acid Base Composition and Taxometric Classification of Gram-Positive Cocci. J Bacteriol. 1965 Jul;90(1):136–140. doi: 10.1128/jb.90.1.136-140.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WECKMAN B. G., CATLIN B. W. Deoxyribonuclease activity of micrococci from clinical sources. J Bacteriol. 1957 Jun;73(6):747–753. doi: 10.1128/jb.73.6.747-753.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



