Abstract
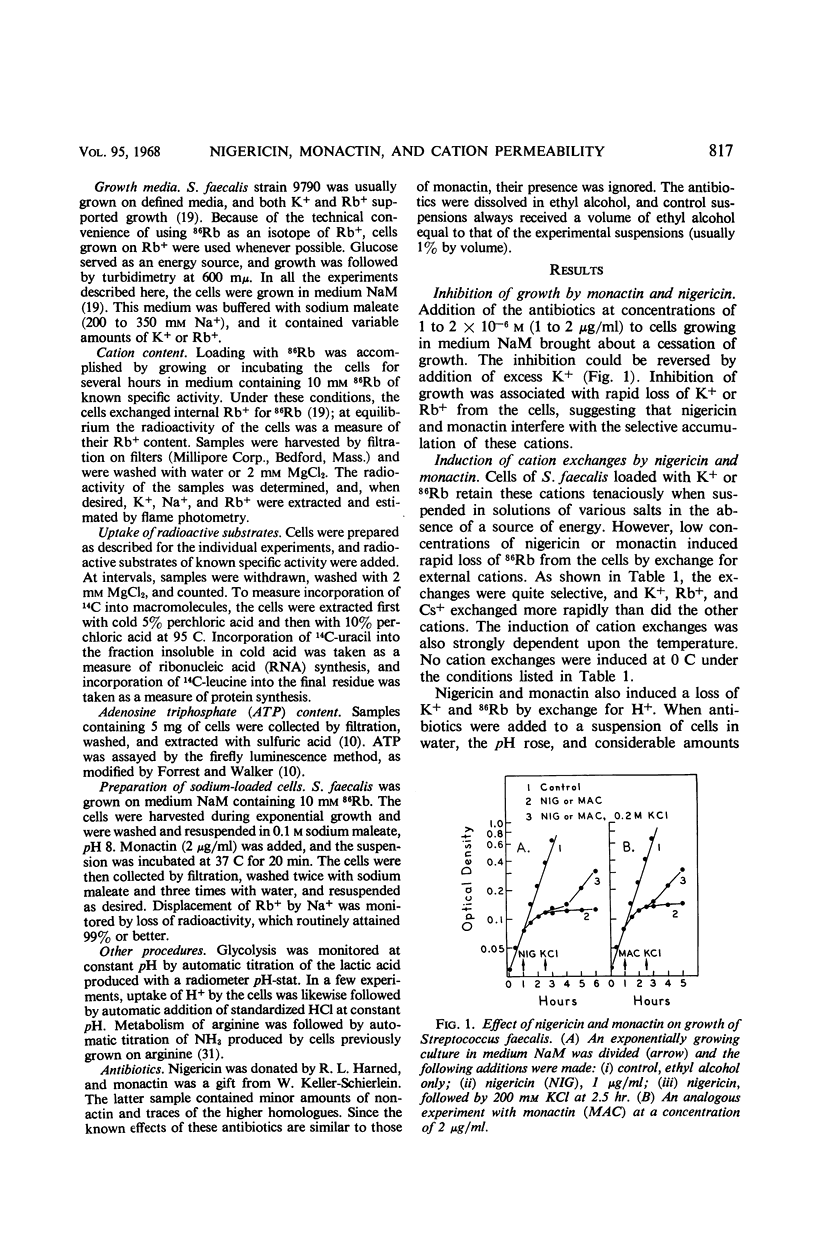
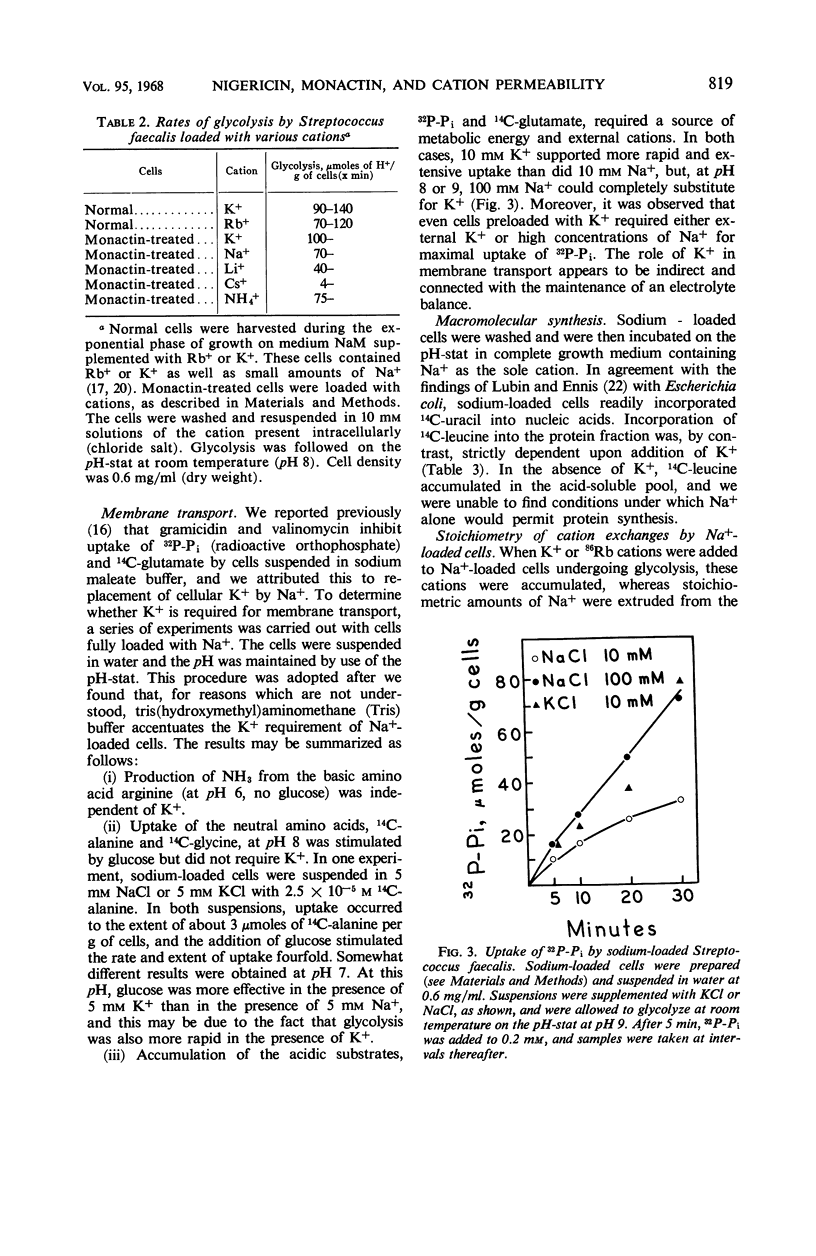
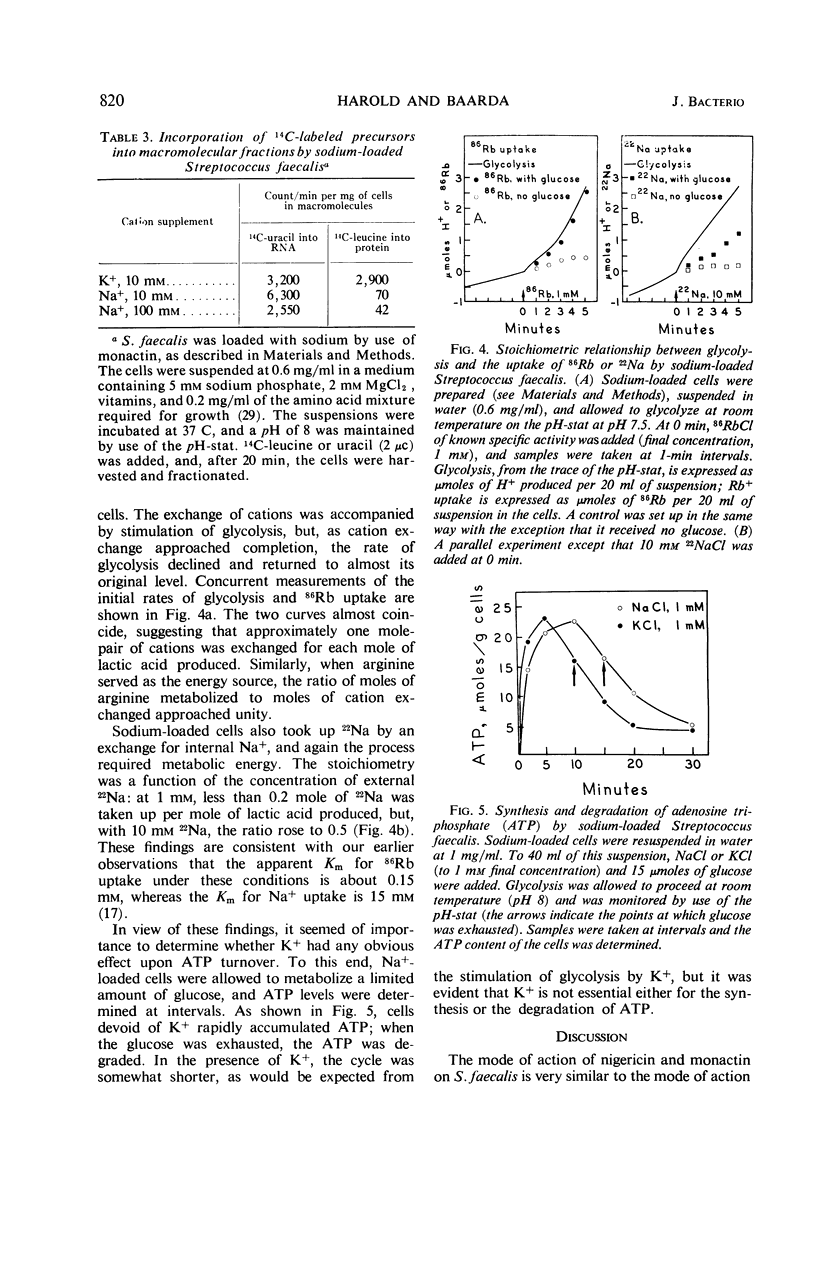
At a concentration of 10−6m, nigericin and monactin inhibited growth of Streptococcus faecalis, and the inhibition was reversed by addition of excess K+. In the presence of certain antibiotics, the cells exhibited increased permeability to certain cations; internal Rb+ was rapidly lost by exchange with external H+, K+ Rb+, and, more slowly, with Na+ and Li+. No effect was observed on the penetration of other small molecules. Cation exchanges induced by nigericin and monactin were metabolically passive and apparently did not involve the energy-dependent K+ pump. When the cells were washed, the cytoplasmic membrane recovered its original impermeability to cations. By use of monactin, we prepared cells whose K+ content had been completely replaced by other cations, and the metabolic characteristics of K+-depleted cells were studied. Cells containing only Na+ glycolyzed almost as well as did normal ones and, under proper conditions, could accumulate amino acids and orthophosphate. These cells also incorporated 14C-uracil into ribonucleic acid but incorporation of 14C-leucine into protein was strictly dependent upon the addition of K+. When K+ or Rb+ was added to sodium-loaded cells undergoing glycolysis, these ions were accumulated by stoichiometric exchange for Na+. From concurrent measurements of the rate of glycolysis, it was calculated that one mole-pair of cations was exchanged for each mole of adenosine triphosphate produced.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P., JOHNSON F. B. Adenosine triphosphatase in isolated bacterial cell membranes. J Biol Chem. 1960 Dec;235:3649–3662. [PubMed] [Google Scholar]
- ABRAMS A. Metabolically dependent penetration of oligosaccharides into bacterial cells and protoplasts. J Biol Chem. 1960 May;235:1281–1285. [PubMed] [Google Scholar]
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- Abrams A., Baron C. The isolation and subunit structure of streptococcal membrane adenosine triphosphatase. Biochemistry. 1967 Jan;6(1):225–229. doi: 10.1021/bi00853a035. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J., MOORE P. T. A sodium-yeast and some of its properties. Biochem J. 1954 Jul;57(3):523–528. doi: 10.1042/bj0570523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Gaffney H. M. The further preparation of inorganic cationic yeasts and some of their chief properties. Biochem J. 1966 Nov;101(2):385–391. doi: 10.1042/bj1010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-O S., Graven S. N., Lardy H. A. Potassium Ion-dependent hydrolysis of adenosine triphosphate induced by nigericin in mitochondria. J Biol Chem. 1967 Jun 25;242(12):2925–2932. [PubMed] [Google Scholar]
- FORREST W. W., WALKER D. J. SYNTHESIS OF RESERVE MATERIALS FOR ENDOGENOUS METABOLISM IN STREPTOCOCCUS FAECALIS. J Bacteriol. 1965 Jun;89:1448–1452. doi: 10.1128/jb.89.6.1448-1452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F. Assimilation of amino acids by Gram-positive bacteria and some actions of antibiotics thereon. Adv Protein Chem. 1953;8:285–391. doi: 10.1016/s0065-3233(08)60094-7. [DOI] [PubMed] [Google Scholar]
- Graven S. N., Estrada-O S., Lardy H. A. Alkali metal cation release and respiratory inhibition induced by nigericin in rat liver mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):654–658. doi: 10.1073/pnas.56.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven S. N., Lardy H. A., Estrada-O S. Antibiotics as tools for metabolic studies. 8. Effect of nonactin homologs on alkali metal cation transport and rate of respiration in mitochondria. Biochemistry. 1967 Feb;6(2):365–371. doi: 10.1021/bi00854a001. [DOI] [PubMed] [Google Scholar]
- Graven S. N., Lardy H. A., Johnson D., Rutter A. Antibiotics as tools for metabolic studies. V. Effect of nonactin, monactin, dinactin, and trinactin on oxidative phosphorylation and adenosine triphosphatase induction. Biochemistry. 1966 May;5(5):1729–1735. doi: 10.1021/bi00869a040. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of potassium transport by sodium in a mutant of Streptococcus faecalis. Biochemistry. 1967 Oct;6(10):3107–3110. doi: 10.1021/bi00862a018. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Cockrell R., Pressman B. C. Induced and spontaneous movements of potassium ions into mitochondria. Biochem J. 1966 Apr;99(1):200–213. doi: 10.1042/bj0990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M., ENNIS H. L. ON THE ROLE OF INTRACELLULAR POTASSIUM IN PROTEIN SYNTHESIS. Biochim Biophys Acta. 1964 Apr 27;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- MEYERS E., PANSY F. E., PERLMAN D., SMITH D. A., WEISENBORN F. L. THE IN VITRO ACTIVITY OF NONACTIN AND ITS HOMOLOGS: MONACTIN, DINACTIN AND TRINACTIN. J Antibiot (Tokyo) 1965 May;18:128–129. [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Induced active transport of ions in mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1076–1083. doi: 10.1073/pnas.53.5.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSTEIN A. Role of the cell membrane in the metabolism of inorganic electrolytes by microorganisms. Bacteriol Rev. 1959 Dec;23(4):175–201. doi: 10.1128/br.23.4.175-201.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit N., San Pietro A. K+ -dependent uncoupling of photophosphorylation by nigericin. Biochem Biophys Res Commun. 1967 Jul 21;28(2):277–283. doi: 10.1016/0006-291x(67)90441-x. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D. Quantitative amino acid assimilation in homofermentative metabolism. Arch Biochem Biophys. 1953 Aug;45(2):447–458. doi: 10.1016/s0003-9861(53)80021-4. [DOI] [PubMed] [Google Scholar]
- Weiden P. L., Epstein W., Schultz S. G. Cation transport in Escherichia coli. VII. Potassium requirement for phosphate uptake. J Gen Physiol. 1967 Jul;50(6):1641–1661. doi: 10.1085/jgp.50.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]


