Abstract
Src family protein-tyrosine kinases are implicated in signaling via glycosylphosphatidylinositol (GPI)-anchored receptors. Both kinds of molecules reside in opposite leaflets of the same sphingolipid-enriched microdomains in the lymphocyte plasma membrane without making direct contact. Under detergent-free conditions, we isolated a GPI-enriched plasma membrane fraction, also containing transmembrane proteins, selectively associated with sphingolipid microdomains. Nonionic detergents released the transmembrane proteins, yielding core sphingolipid microdomains, limited amounts of which could also be obtained by detergent-free subcellular fractionation. Protein-tyrosine kinase activity in membranes containing both GPI-anchored and transmembrane proteins was much lower than in core sphingolipid microdomains but was strongly reactivated by nonionic detergents. The inhibitory mechanism acting on Lck and Fyn kinases in these membranes was independent of the protein-tyrosine phosphatase CD45 and was characterized as a mixed, noncompetitive one. We propose that in lymphocyte plasma membranes, Lck and Fyn kinases exhibit optimal activity when juxtaposed to the GPI- and sphingolipid-enriched core microdomains but encounter inhibitory conditions in surrounding membrane areas that are rich in glycerophospholipids and contain additional transmembrane proteins.
INTRODUCTION
In contrast to transmembrane glycoproteins, surface molecules inserted into the plasma membrane via a glycosylphosphatidylinositol (GPI)1 membrane anchor are confined to the outer leaflet of the plasma membrane and do not directly communicate with the cell interior (Low, 1989). However, several GPI-anchored proteins have been shown to be potent signal transducers, because their cross-linking leads to increased protein-tyrosine phosphorylation, calcium fluxes, gene expression, and cell activation and/or proliferation (Robinson, 1991). GPI-anchored proteins such as neurotrophic factor receptors transduce signals by ligand-induced interactions with transmembrane receptor protein-tyrosine kinases (PTKs) (Massague, 1996), whereas other GPI-anchored molecules such as CD87 (uPAR), CD16B (FcγRIIIB), and CD14 (lipopolysaccharide receptor) interact with integrins (Petty and Todd, 1996) and appear to signal via integrin-dependent pathways. For most other GPI-anchored proteins, signaling is presumed to require association with sphingolipid microdomains (Romagnoli and Bron, 1997; Stulnig et al., 1997), and their coprecipitation with Src family PTKs has been documented in hematopoietic (Stefanova et al., 1991), epithelial (Shenoy-Scaria et al., 1992), and neuronal (Zisch et al., 1995; Kunz et al., 1996) cells. The molecular nature of this indirect association between GPI-anchored receptors and Src kinases, however, remains unresolved.
Sphingolipid microdomains are thought to consist of clusters of sphingolipids that achieve a liquid-ordered state in the presence of cholesterol (Ahmed et al., 1997; Schroeder et al., 1998) and resist solubilization by nonionic detergents. Such sphingolipid microdomains can be isolated as low-density, buoyant membrane complexes in equilibrium sedimentation gradients (Brown, 1992) or as membrane vesicles by gel filtration chromatography (Hoessli and Rungger-Brändle, 1985; Draberova and Draber, 1993; Cinek et al., 1995). In cells where caveolae are morphologically detectable, detergent-resistant membrane domains are also enriched in caveolin, the caveolar coat protein. However, recent morphological and biochemical investigations have provided evidence that sphingolipid microdomains enriched in GPI-anchored proteins and caveolin are not identical to caveolae (Schnitzer et al., 1995; Liu et al., 1997; Doyle et al., 1998). Other observations suggest that in cells expressing caveolin, sphingolipid microdomains are located close to caveolae, and some of their components may translocate to caveolae after interaction with physiological agonists (Sevinsky et al., 1996) or antibodies (Fujimoto, 1996). In cells lacking caveolin expression, sphingolipid microdomains have been difficult to demonstrate morphologically, but biochemical evidence suggests their importance in signaling through GPI-anchored surface molecules (van den Berg et al., 1995; Stulnig et al., 1997) as well as certain transmembrane receptors (Field et al., 1997; Deans et al., 1998). Moreover, in vitro reconstitution of detergent-resistant membranes has been achieved by mixing sphingolipids and cholesterol in suitable proportions (Ahmed et al., 1997; Schroeder et al., 1998), and single-particle–tracking studies on whole cells have documented transient confinement of GPI-anchored receptors and glycosphingolipids in the plane of the membrane (Sheets et al., 1997; Simson et al., 1998). Very recently, physical and biochemical studies have demonstrated the existence of submicron-sized GPI-domains in the plasma membrane (Friederichson and Kurzchalia, 1998; Varma and Mayor, 1998).
In this study, we have examined the catalytic activity of the Lck and Fyn kinases in different plasma membrane fractions from lymphoma cells. We show that Lck and Fyn kinases appear to be differentially regulated in different plasma membrane microdomains. This regulation is independent of the membrane-associated CD45 tyrosine phosphatase but dependent on the specific membrane microenvironment in which the kinases reside.
MATERIALS AND METHODS
Reagents and Antibodies
Triton X-100 (TX-100) was from Merck (Darmstadt, Germany); NP-40 was from Fluka (Buchs, Switzerland); octylglucoside (OTG) was from Alexis (San Diego, CA); and 3-([3-cholamidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate (CHAPS) was from Boehringer Mannheim (Mannheim, Germany). HRP-conjugated cholera toxin (CT) β subunit, enolase, and α-casein were purchased from Sigma (Buchs, Switzerland), and genistein was from Calbiochem (La Jolla, CA). The enhanced chemiluminescence (ECL) reagent was from Amersham (Little Chalfont, Buckinghamshire, United Kingdom).
Rat mAb against murine CD45 (mAb M1/9.3.4HL.2; ATCC TIB122), Thy-1.2 (mAb 30-H12; ATCC TIB107), and the heat-stable antigen (HSA) CD24 (M1/69.16.11.HL; ATCC TIB 125) were obtained from the American Type Culture Collection (Rockville, MD) and used as culture supernatants. Rat anti-murine CD26 hybridoma (H207.773) was a kind gift from Dr. H.-T. He (Center d’Immunologie, Institut National de la Santé et de la Recherche Médicale–Centre National de la Recherche Scientifique, Marseille, France). Rabbit polyclonal antibodies against Lck and Fyn and HRP-conjugated goat anti-mouse, goat anti-rat, and goat anti-rabbit immunoglobulin G were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Isolation of GPI-enriched Membranes by Equilibrium Density Gradient Centrifugation
The murine T lymphoma cell line P1798 obtained from Litton Bionetics (Bethesda, MD) was propagated in syngeneic BALB/c mice as ascites. Cells were washed in cold PBS twice and once in TKM buffer (50 mM Tris-HCl, pH 7.4, 25 mM KCl, 5 mM MgCl2, and 1 mM EGTA). The cell pellet was resuspended in TKM buffer containing 73% (wt/vol) sucrose and protease inhibitors leupeptin (1 μM), aprotinin (2 μg/ml), and Pefabloc SC (2 mM) (all from Boehringer Mannheim) at the ratio of 5 ml/109 cells. The cell suspension was homogenized using a motor-driven Potter-Elvehjem homogenizer (Kontes, Vineland, NJ) with a tight-fitting pestle. Aliquots of sucrose homogenate equivalent to 150 × 106 cells were incubated without or with various detergents at the indicated final concentrations for 20 min on ice; sucrose concentration was adjusted to 40% (wt/vol) in the final volume of 2.0 ml and subjected to equilibrium density gradient centrifugation as described earlier (Ilangumaran et al., 1996) and illustrated in Figure 1, A and B. Briefly, the homogenates were placed at the bottom of SW41 tubes (Beckman Instruments, Nyon, Switzerland), overlaid with 6.0 ml of 36% (wt/vol) sucrose followed by 3.0 ml of 5% (wt/vol) sucrose in TKM buffer, and centrifuged at 38,000 rpm (250,000 × g) for 16–20 h at 4°C. One-milliliter fractions were collected from the top, numbered 1–11, and stored at −20°C. Alternatively, the buoyant membranes forming a visible band at the 5–36% sucrose interface were collected, pooled, and stored at −20°C. The methodology and terminology used in this study are given in Figure 1, A and B. Alternatively, the same cell homogenate was centrifuged in a three-step sucrose gradient to obtain “light” and “heavy” membranes (Arni et al., 1996), as depicted in Figure 1C.
Figure 1.
Methodology and terminology used in this study. P1798 murine T lymphoma cells (109) were homogenized in TKM buffer containing 73% (wt/vol) sucrose. Aliquots of the cell homogenate (equivalent to 150 × 106 cells), treated with detergent or left untreated at 4°C for 20 min, were adjusted to 40% (wt/vol) sucrose and subjected to equilibrium density gradient centrifugation. One-milliliter fractions collected from the top and fractions 3–5 enriched for the GPI-anchored proteins were pooled, or the GPI-enriched floating membranes visible as a light-scattering band at the 5–36% sucrose interface were collected directly (∼3 ml) and stored at −20°C until use. (A) Untreated GPI-enriched membranes. (B) Detergent-treated GPI-enriched membranes. (C) Subcellular fractionation of the cell homogenate from 1–2 × 109 cells was carried out to obtain plasma membrane fractions with specific gravity of 1.034–1.09 g/ml (light membranes) and 1.09–1.136 g/ml (heavy membranes) as described previously (Hoessli and Rungger-Brändle, 1983; Arni et al., 1996). The most frequently used terms are boxed.
Detection of Cell Surface and Intracellular Molecules
Various cell surface and intracellular proteins in the density gradient fractions were evaluated by Western blotting. Twenty microliters of the gradient fractions were directly solubilized in 6× nonreducing SDS-PAGE sample buffer for the detection of cell surface antigens or in reducing sample buffer for intracellular kinases. Proteins separated using a minigel apparatus (Bio-Rad, Richmond, CA) were transferred to nitrocellulose (NC) filters (Schleicher & Schuell, Dassel, Germany). Alternatively, proteins were detected by dot immunoassay as described previously (Ilangumaran et al., 1996). Briefly, 10 μl of the gradient fractions were dotted onto NC filters in a 200-μl volume using a Bio-Rad dot blot apparatus. After blocking with Tween 20–Tris-buffered saline (TTBS; 10 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.1% Tween 20) containing 5% low-fat dry milk powder (TTBS-5% MP), the Western- and dot-blotted filters were incubated with antibodies in TTBS-1% MP for 60 min. After three washes in TTBS, the filters were incubated with appropriate HRP-conjugated second antibodies. After thorough washing, the filters were developed with the ECL reagent and exposed to Eastman Kodak (Rochester, NY) X-Omat S film. CT binding to membrane-associated ganglioside GM1 in the gradient fractions was evaluated by incubating the dot-blotted NC filter strips, after blocking in 5% MP-TTBS, with HRP-conjugated CT in 1% MP-TTBS at 1:20,000 dilution. After washing in TTBS the filters were developed with the ECL reagent. The luminograms were quantitated using a laser scanning densitometer (Molecular Dynamics, Sunnyvale, CA).
In Vitro Kinase Assays
To measure the total kinase activity, 0.5 ml of the pooled GPI-enriched membranes (fractions 3–5; corresponding to ∼25 × 106) was diluted at least fivefold with TKM buffer and centrifuged at 250,000 × g for 4–6 h. The sedimented membranes were suspended directly in 25 μl of kinase buffer (20 mM MOPS, pH 7.4, and 10 mM MgCl2). Kinase assay was set up in a final volume of 50 μl and initiated by the addition of 5 μCi of [γ-32P]ATP (Amersham, 5000 Ci/mmol) in 5 μl of kinase buffer. For cold kinase assays, unlabeled ATP was added to the final concentration of 50 μM. In some experiments 2.5 μg of acid-denatured enolase or α-casein were included in the same volume of reaction mixture. To measure the kinetics of enolase phosphorylation, varying amounts of enolase were added in the same reaction volume but containing [γ-32P]ATP of low specific activity (Amersham, 3 Ci/mmol). After incubation for 15 min at 25°C, the reaction was stopped by the addition of 6× sample buffer and boiling. Phosphorylated proteins separated in 5–20% gradient gels or 10% minigels were fixed in 10% trichloroacetic acid for 30 min and washed in 10% methanol:10% acetic acid solution for 6–12 h with several changes before drying and autoradiography. Phosphotyrosylated proteins were detected with 4G10 anti-phosphotyrosine mAb (Upstate Biotechnology, Lake Placid, NY) on Western blots.
For measuring kinase activities in membrane vesicles with Thy-1 or Lck exposed on the vesicle surface, 1 ml of the GPI-enriched membrane pool was diluted twofold with TKM buffer and precleared with 100 μl of Pansorbin (Calbiochem) for 30–60 min at 4°C with constant mixing. After the addition of anti-Thy-1 (100 μl of culture supernatant) or anti-Lck (10 μl) antibodies, the samples were incubated for 1 h at 4°C. After adding 25 μl of protein A/G-agarose beads preblocked with BSA (Santa Cruz Biotechnology), the samples were incubated for a further 4–16 h at 4°C with gentle mixing. The immune complexes were sedimented, washed three times in TKM buffer and once in kinase buffer, and subjected to kinase assay as described above. To test the effects of lipids, purified lipids were dried under N2 and added either as TX-100 mixed micelles, or in some cases as sonicated lipid vesicles in the absence of any detergent, to the membrane preparations and incubated for 15 min at 30°C before initiation of the kinase reaction.
Characterization of the In Vitro Phosphorylated Proteins
Immunoprecipitation.
After in vitro phosphorylation on TX-100-treated GPI-enriched membranes, SDS was added to the reaction mixture to a final concentration of 0.2% and incubated at room temperature for 15 min. After 10-fold dilution with TKM buffer containing 0.5% TX-100, the samples were precleared with Pansorbin for 30 min at 4°C. Anti-Lck or anti-Fyn antibodies were added, and the samples were incubated on ice for 30 min. The immune complexes were collected onto protein A/G-agarose beads and washed, and the phosphoproteins were eluted by boiling in sample buffer and analyzed by SDS-PAGE as described above.
Base Hydrolysis.
Phosphorylation on Tyr residues was distinguished from that on Ser and Thr residues by the sensitivity of the latter to base hydrolysis (Kamps and Sefton, 1989). In vitro–phosphorylated proteins were transferred to polyvinylidene difluoride (PVDF) membrane filters (Bio-Rad). After obtaining an autoradiogram, the blot was treated with 1 M KOH for 1.5 h at 55°C, rinsed with TTBS, and exposed to reveal alkali-resistant phosphorylations.
Phosphoamino acid Analysis.
After in vitro kinase assay on TX-100-treated GPI-enriched membranes, the proteins were transferred to a PVDF membrane and exposed to x-ray film, and the labeled phosphoprotein bands were cut out. The proteins were hydrolyzed in 5.7 N HCl for 2 h, and the hydrolysate was dried in a SpeedVac concentrator (Savant Instruments, Holbrook, NY), resuspended in 10 μl of water containing 1 mg/ml of standard phosphoamino acids (P-Ser, P-Thr, and P-Tyr), and separated by TLC (Munoz and Marshall, 1990). After three cycles of separation, the plate was dried and exposed to x-ray film. Positions of the labeled phosphoamino acids were determined with regard to the ninhydrin-stained standards.
Lipid Analysis
Whole cells, membrane vesicles, or TX-100–treated membranes were extracted in chloroform:methanol:pyridine:water (60:30:1:6 by vol) for 48 h at 50°C and analyzed by TLC as described earlier (Arni et al., 1996).
RESULTS
Isolation of GPI-enriched Plasma Membranes in the Absence and Presence of Detergents
GPI-enriched membrane vesicles from P1798 murine T lymphoma cells were isolated under detergent-free conditions by homogenization in dense sucrose solution followed by isopycnic density gradient centrifugation, as shown in Figure 1A. Five percent of the cellular proteins floated up to densities expected for plasma membranes, and the bulk of the remainder was recovered in the lower-most fraction 11 and pellet. The GPI-anchored proteins Thy-1 and HSA were enriched in buoyant membrane fractions 3–5 at the 36–5% sucrose interface (Table 1) and will be referred to as “untreated GPI-enriched membranes.”
Table 1.
Effects of various detergents on the floatability of cell surface and intracellular membrane-associated molecules
| Detergent | Thy-1
|
HSA
|
CD45
|
CD26
|
Lck
|
Fyn
|
GM1
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top | Bot | Top | Bot | Top | Bot | Top | Bot | Top | Bot | Top | Bot | Top | Bot | |
| None | 68 | 16 | 86 | 8 | 48 | 30 | 69 | 24 | 48 | 41 | 27 | 55 | 68 | 13 |
| TX-100, 1% | 70 | 12 | 87 | 6 | 23 | 40 | 51 | 25 | 41 | 34 | 35 | 43 | 62 | 12 |
| NP-40, 0.5% | 66 | 10 | 91 | 4 | 38 | 53 | 56 | 24 | 46 | 37 | 38 | 48 | 61 | 14 |
| CHAPS, 20 mM | 84 | 2 | 98 | 2 | 19 | 62 | 47 | 28 | 44 | 39 | 50 | 38 | 79 | 6 |
| OTG, 60 mM | 63 | 15 | 42 | 48 | 25 | 59 | 49 | 31 | 31 | 51 | 27 | 54 | 50 | 26 |
Aliquots of P1798 cell homogenate in 73% sucrose, equivalent to ∼150 × 106 cells, were treated with various detergents at the indicated concentrations or left untreated at 4°C for 20 min. After density gradient centrifugation as described in Figure 1, 1-ml fractions were collected, and 10 μl of each fraction from all the six gradients were analyzed by the ECL-based dot immunoassay for the indicated cell surface proteins, intracellular PTKs, or CT-binding ganglioside GM1, as described earlier (Ilangumaran et al., 1996). The luminograms were quantitated by laser scanning densitometry, and the amount in top (fractions 3–5) and bottom (Bot, fractions 9–11) fractions are represented as percentage of the sum of all fractions. The data shown are representative of two independent experiments.
We then examined how different detergents affect the recovery of membrane molecules in GPI-enriched membrane fractions by treating the cell homogenate before gradient centrifugation (Figure 1B). In addition to GPI-linked and transmembrane glycoproteins and intracellular kinases, we also followed the distribution of GM1 ganglioside in the density gradient fractions (Table 1). The proportion of buoyant Thy-1, HSA, CD26, and GM1 in fractions 3–5 (top) remained unchanged or increased after treatment with TX-100, NP-40, or CHAPS. On the other hand, OTG dissociated >50% of HSA and 30% of GM1 from the top fractions but did not affect the Thy-1 density distribution. Detergent treatment in general decreased the amounts of transmembrane type I CD45 recovered in the GPI-enriched buoyant fractions, whereas the recovery of type II transmembrane protein CD26, Lck, or Fyn was not significantly altered. A higher detergent-to-cell ratio dissociated more CD45, but not CD26 or the kinases, from the buoyant fractions (Ilangumaran et al., 1997). Only OTG moderately dissociated Lck. These results show that the association of GPI-linked proteins and membrane-associated intracellular PTKs with low-density membrane lipids are relatively resistant to most of the detergents used except OTG. The presence of GM1 ganglioside in the low-density fractions was also unaffected by detergents, except OTG, which has been reported to perturb interactions between sphingolipids (Melkonian et al., 1995).
Kinase Activities in GPI-enriched Membranes Are Markedly Enhanced after Detergent Treatment
The total protein profile of the pooled floating fractions showed marked quantitative and minor qualitative differences in various detergent-treated membrane samples (Figure 2A). However, Western blot detection showed comparable amounts of Thy-1, Lck, and Fyn in all detergent-treated membranes (Figure 2B), and >90% of these proteins were recovered in the 250,000 × g membrane pellet (our unpublished results). Therefore, the kinase activities were measured in GPI-enriched membranes sedimented from 0.5 ml of pooled fractions 3–5, corresponding to 2.5 × 107 cells.
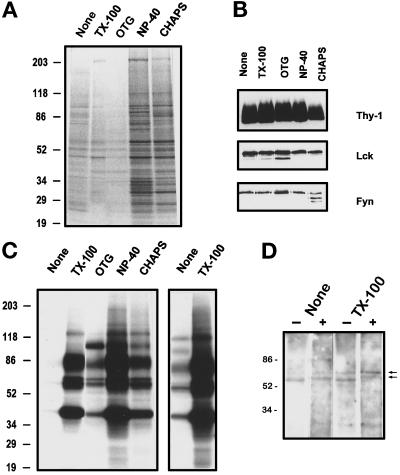
Figure 2.
Kinase activities in GPI-enriched membranes isolated using different detergents. Buoyant, GPI-enriched membranes (0.5 ml, equivalent to ∼25 × 106 cells) from the same cell homogenate, untreated (None) or after treatment with indicated detergents (1% TX-100, 60 mM OTG, 0.5% NP-40, or 20 mM CHAPS), were diluted in TKM buffer, and the GPI-enriched membranes sedimented at 250,000 × g for 4 h were suspended in kinase buffer. (A) Total protein profile after silver staining. (B) Western blot detection of Thy-1, Lck, and Fyn in 20 μl of the GPI-enriched membranes shown in A. (C) Phosphoprotein profile after in vitro phosphorylation in the presence of [γ-32P]ATP as described in MATERIALS AND METHODS. Phosphorylated proteins were separated in 5–20% SDS-PAGE gradient gels and detected by autoradiography after 4.5 h of exposure. For comparison a 20-h exposure of the first two lanes (none and TX-100) is shown in the right panel. Data representative of three independent experiments are shown. (D) Western blot detection of tyrosine-phosphorylated proteins with 4G10 anti-phosphotyrosine mAb after in vitro kinase assay on untreated and TX-100–treated GPI-enriched membranes in the absence (−) or presence (+) of 50 μM cold ATP. The hyperphosphorylated kinase bands are marked by arrowheads.
Total phosphotransferase assays on sedimented GPI-enriched membranes revealed strong phosphorylation of 40-, 58-, 59-, 60-, 80- to 85-, and 120-kDa proteins in TX-100–treated membranes (Figure 2C); longer exposure showed that most of these proteins were also phosphorylated in the untreated membranes, although much less efficiently (Figure 2C, right panel). NP-40– and CHAPS–treated membranes showed elevated kinase activities comparable to that of TX-100–treated ones (Figure 2C) but contained significantly more proteins (Figure 2A). OTG extraction dissociated most proteins from the buoyant membranes (Figure 2A) without loss of the kinases or their protein substrates (Figure 2C). Addition of detergents (at  of the concentration added to the cell homogenate before gradient centrifugation) directly to the untreated GPI-enriched membranes enhanced the kinase activities in a similar manner (see Figure 5 and our unpublished results). The anti-phosphotyrosine blot showed that hyperphosphorylated bands (Figure 2D, arrowheads) corresponding to Src family PTKs (see Figure 4A) appear after an in vitro phosphotransferase assay using cold ATP only in the TX-100–treated membranes but not in untreated membranes (Figure 2D).
of the concentration added to the cell homogenate before gradient centrifugation) directly to the untreated GPI-enriched membranes enhanced the kinase activities in a similar manner (see Figure 5 and our unpublished results). The anti-phosphotyrosine blot showed that hyperphosphorylated bands (Figure 2D, arrowheads) corresponding to Src family PTKs (see Figure 4A) appear after an in vitro phosphotransferase assay using cold ATP only in the TX-100–treated membranes but not in untreated membranes (Figure 2D).
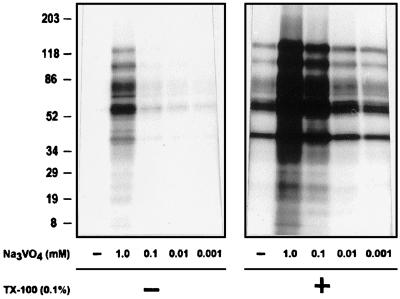
Figure 5.
Detergent-induced enhancement of kinase activities in GPI-enriched membranes is further augmented by vanadate. Kinase activities in untreated GPI-enriched membranes from 25 × 106 cells were measured by in vitro phosphorylation as described in Figure 2C in the presence of indicated concentrations of vanadate without or with 0.1% TX-100 in the reaction mixture.
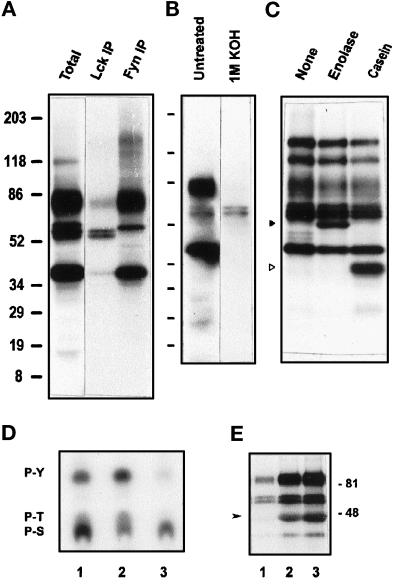
Figure 4.
GPI-enriched membranes harbor both Tyr and Ser/Thr kinase activities. (A) Immunoprecipitation of Lck and Fyn kinases. After the kinase assay, [32P]ATP on TX-100–isolated, GPI-enriched membranes (as in Figure 2C, lane 2), phosphorylated proteins were solubilized in SDS and immunoprecipitated with anti-Lck or anti-Fyn antibodies. The eluted phosphoproteins were separated by SDS-PAGE and detected by autoradiography after 4 d of exposure. (B) Base hydrolysis of the in vitro–phosphorylated proteins of GPI domains. After the kinase assay on TX-100–isolated, GPI-enriched membranes, the in vitro–phosphorylated proteins separated in 5–20% gels were transferred to a PVDF membrane and exposed to x-ray film for 1 d. Subsequently, the membrane was treated with 1 M KOH at 55°C for 1.5 h, rinsed, and exposed again for 15 d to reveal phosphorylations resistant to alkaline pH. (C) Phosphorylation of exogenous protein substrates by PTKs in GPI-enriched membranes. TX-100–treated GPI-enriched membranes (0.5 ml) were subjected to in vitro kinase assay as in Figure 2C, in the absence or presence of 5 μg of enolase or α-casein. Phosphoproteins separated in 5–20% gradient gels were revealed by autoradiography after overnight exposure. Phosphorylated enolase and α-casein are indicated by solid and open triangles, respectively. (D) Phosphoamino acid analysis of the major phosphoprotein bands. After in vitro kinase assay on TX-100–treated GPI-enriched membranes, the proteins were transferred onto a PVDF membrane and autoradiographed. Labeled bands corresponding to 80- to 85-kDa (lane 1), 56–59 Src kinase region (lane 2), and 40-kDa (lane 3) phosphoproteins were cut and acid hydrolyzed, and the phosphoamino acids were separated by TLC as described in MATERIALS AND METHODS. (E) Inhibition of phosphotransferase activities by genistein. TX-100–treated GPI-enriched membranes suspended in kinase buffer were incubated with 100 mM (lane 1) or 10 mM (lane 2) genistein or DMSO vehicle (lane 3) for 15 min at room temperature before the assay.
These results suggested that the observed differences in kinase activities between untreated and detergent-treated GPI-enriched membranes are likely to result from modifications of the membrane environment caused by detergents. A direct effect of the detergents on the catalytic activity on the kinases is unlikely because all detergents tested influenced the membrane-associated tyrosine kinases only when used at a concentration above their critical micelle concentration in an all-or-none manner (our unpublished results). In case of a direct effect of the detergent on kinase activity, a dose-dependent increase in enzymatic activity would be expected with increasing detergent concentration, which was not the case.
It could be envisaged that the elevated kinase activities in detergent-isolated, GPI-enriched membranes were due to facilitated access of the ATP substrate to the intravesicular kinases rather than an increase in phosphotransferase activity per se. The marked differences in the effects of various detergents on the membrane-associated kinase activities (Figure 2C) already argue against this possibility, because all detergents at the concentration used (above their critical micellar concentration values) will permeabilize the vesicles to ATP equally well. To settle this point, we carried out kinase assays on membrane vesicles recovered from solid-phase anti-Lck, corresponding to vesicles in the inside-out orientation (i.e., containing the kinases on their exposed leaflet) and compared their activity with that of right-side-out vesicles isolated on solid-phase anti-Thy-1 antibodies. The marked quantitative difference in phosphotransferase activity between the untreated and detergent-treated buoyant membranes was manifest in both inside-out (anti-Lck–selected) and right-side-out (anti-Thy-1–selected) vesicles. Much fewer inside-out vesicles were recovered with anti-Lck, suggesting that GPI-enriched membrane vesicles are predominantly in the right-side-out orientation and are best retained on solid-phase anti-Thy-1. However, the qualitative differences among the various detergent-treated membranes were negligible in either anti-Thy-1– or anti-Lck–selected vesicles, both of which showed a very similar pattern of phosphoproteins (40, 58–60, and 80–85 kDa; Figure 3). The proportionally stronger labeling of the 80- to 85-kDa band in anti-Lck–precipitated vesicles suggests an intracellular orientation for this phosphoprotein similarly to Lck. Controls with antibody-free or irrelevant antibody-bound beads did not show any significant phosphorylation (our unpublished results).
Figure 3.
Detergent-induced modulation of kinases activities in right-side-out and inside-out GPI-enriched membrane vesicles. One milliliter of the pooled GPI-enriched membrane fractions defined in Table 1 was immunoselected with anti-Thy-1 (A) or anti-Lck (B) antibody-coated protein A/G beads. The bound vesicles were washed in detergent-free buffer and subjected to in vitro kinase assay with [γ-32P]ATP as detailed in MATERIALS AND METHODS. Thy-1-associated kinase activities were revealed after 3 d of exposure to the film, whereas Lck-associated phosphorylations required 15 d of exposure.
Characterization of In Vitro–Phosphorylated Proteins in GPI-enriched Membranes
The 58- to 60-kDa phosphoproteins labeled in the TX-100–treated GPI-enriched membranes correspond to hyperphosphorylated species of the major Src family kinases Lck and Fyn present in murine T lymphoid cells (Figure 4A). Anti-Lck precipitated the 58- to 59-kDa doublet of hyperphosphorylated kinase, whereas anti-Fyn collected the 60-kDa protein in association with the 40- and 80- to 85-kDa phosphoproteins, an interaction that had apparently resisted the SDS solubilization after the kinase assay on GPI-enriched membranes. We next analyzed the sensitivity of the in vitro–phosphorylated proteins to base hydrolysis. After kinase assay on TX-100–isolated membranes, the phosphoproteins were transferred and autoradiographed before and after treatment with 1 M KOH. As shown in Figure 4B, the radiolabel incorporated into Src family PTKs was resistant, whereas that in the 40- and 80- to 85-kDa phosphoproteins was alkali sensitive. Longer exposures also revealed a weak alkali-resistant signal on the 80- to 85-kDa protein (our unpublished results). Phosphoamino acid analysis of the bands corresponding to 80–85, 58–60, and 40 kDa confirmed these results (Figure 4D). Interestingly, in addition to the 32P label incorporated in serines and threonines, the 80- to 85-kDa protein contained a significant amount of the 32P label in Tyr residues, which appeared to be alkali sensitive (Figure 4B). Significant amounts of phospho-Ser and -Thr were also present on bands corresponding to the Src kinase region. Together, these results show that the 58-, 59-, and 60-kDa kinase bands were mainly phosphorylated on Tyr residues and the 40-kDa phosphoprotein was predominantly phosphorylated on Ser and Thr residues. The 80- to 85-kDa band is a highly acidic phosphoprotein (pI 4–5) containing both phosphotyrosines and phosphoserines/threonines. It is sensitive to cleavage by cyanogen bromide and trypsin, and its interaction with Fyn was disrupted by reducing agents (our unpublished results); this phosphoprotein was found to be associated with GPI-linked surface receptors and glycolipids of different cell types by several groups (Stefanova et al., 1991; Arni et al., 1993; Garnett et al., 1993; Minoguchi et al., 1994; Marie-Cardine et al., 1997; Deans et al., 1998), but its identity remains elusive.
We also document the Tyr kinase activity in GPI-enriched membranes by direct phosphorylation of the exogenous Tyr kinase substrates enolase and α-casein (Figure 4C), and preincubation of the GPI-rich vesicles with genistein markedly reduced phosphorylation of Lck, Fyn, and the 80- to 85-kDa bands and completely abolished enolase phosphorylation at 100 μM (Figure 4E). Similar results have been obtained with herbimycin as an inhibitor. These results show that GPI-enriched membranes contain Tyr and Ser/Thr kinase activities that are both enhanced by nonionic detergents. The Ser/Thr kinase activity does not seem to include a conventional PKC, because it was not enhanced in the presence of Ca2+, phorbol myristate acetate, and phosphatidylserine (our unpublished results). The decrease in labeling of the 40-kDa phosphoprotein bands in response to genistein suggests that the Ser/Thr kinase activity may depend on Tyr phosphorylation.
Increase in PTK Activities in GPI-enriched Membranes by Detergent Does Not Involve Activation by the Protein-Tyrosine Phosphatase CD45
CD45 contains a protein-tyrosine phosphatase (PTPase) activity that dephosphorylates the C-terminal tyrosine residue of Lck (Tyr-505) and consequently activates Src kinases (Sieh et al., 1993). This raises a possibility that the added detergents could mediate at least part of their effects through activation of CD45 and probably other unidentified PTPases in GPI-enriched membranes. To test for a possible enhancing activity of the CD45 and/or other PTPases on Lck and Fyn kinase activities associated with GPI-enriched plasma membranes, we measured the kinase activities in the presence of sodium orthovanadate, a potent inhibitor of PTPases (Gordon, 1991). Addition of vanadate alone enhanced the kinase activities at 1 mM concentration (Figure 5, left panel), and this enhancement was further augmented by the presence of 0.1% TX-100, which alone enhanced the kinase activities (Figure 5, right panel). Enhancement of phosphorylation by either vanadate alone or TX-100 plus vanadate was observed on all protein substrates and showed a clear dependence on the concentration of vanadate, indicating that PTPases were active in both untreated and TX-100–treated GPI-enriched membranes. Had the CD45 PTPase activity been necessary to activate PTKs in GPI-enriched membranes treated with TX-100, inhibition of the PTPases with vanadate should have resulted in a decrease in PTK activity and not an increase as observed in Figure 5, right panel. These results strongly argue against a stimulatory role for CD45 on the TX-100–induced PTK activity in GPI-enriched membranes.
PTK Activity in GPI-enriched Membranes Is Regulated by a Mixed, Noncompetitive Type of Inhibition
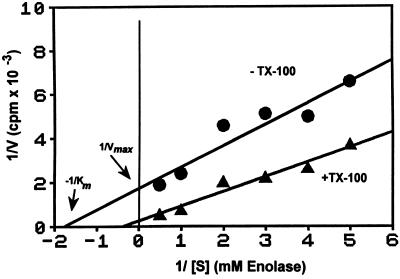
To characterize the regulatory mechanisms operating on Src family PTKs in GPI-enriched membranes, the PTK activity in untreated and TX-100–treated membranes was measured at varying enolase concentrations. The 32P radiolabel incorporated into enolase was measured, and the values were plotted against the reciprocal of substrate concentration in a Lineweaver–Burk plot (Figure 6). The difference in slopes shows that reaction velocity of PTKs is higher in TX-100–treated membranes than in untreated ones. Furthermore, PTKs in untreated and TX-100–treated GPI-enriched membranes apparently differ in their affinity toward the enolase substrate, as shown by differences in the intercepts (−1/Km). The observed pattern, with different 1/Vmax values and the slopes showing a tendency to converge, suggests that PTKs in untreated GPI-enriched membranes are regulated by a mixed, noncompetitive type of inhibition, a commonly encountered mode of down-regulating enzyme activity.
Figure 6.
Enzyme kinetics of PTK activity in GPI-enriched membranes isolated without or with TX-100. Untreated and TX-100–treated GPI-enriched membranes equivalent to 25 × 106 cells and originating from the same cell homogenate were sedimented and tested for in vitro kinase activity as in Figure 2C. The reaction mix contained low-specific-activity ATP (3 Ci/mmol) to a final concentration of 100 μM and varying amounts of the exogenous PTK substrate enolase. Phosphorylated proteins were separated by SDS-PAGE and autoradiographed. The enolase bands were excised, and the incorporated radiolabel was quantitated by liquid scintillation counting as a measure of the velocity of PTK activities. Western blotting of aliquots of untreated and TX-100–treated GPI-enriched membranes showed comparable amounts of Lck and Fyn kinases as in Figure 2B. The data from one of the three separate experiments, which gave similar results, are shown.
Membrane Lipids Modulate Phosphotransferase Activity of TX-100–treated GPI-enriched Membranes
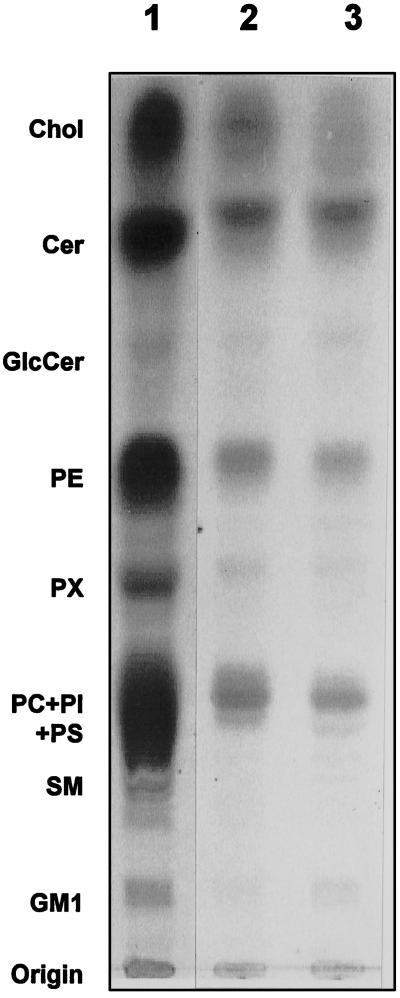
We observed that detergents added directly to the GPI-enriched membranes enhanced the kinase activities (Figure 5) only at concentrations above the critical micellar concentration values (our unpublished results), suggesting that bilayer lipids may act as regulators in this context. TLC analysis of the lipids (Figure 7) extracted from whole cells (lane 1) and untreated (lane 2) and TX-100–treated (lane 3) GPI-enriched membranes was qualitatively similar but showed a marked enrichment of ceramides in the TX-100–treated membranes. Although the molar ratios of ceramide to cholesterol were 0.73 and 0.64 for total cell extract and untreated GPI-enriched membranes, respectively, the TX-100–treated membranes had a ratio of 1.36. The latter membranes also contained proportionally more GlcCer, LacCer, and GM1 and less glycerophospholipids. To investigate whether any particular membrane lipid species would influence the activity of Src family PTKs in GPI-enriched membrane domains, several purified or synthetic membrane lipid species, including phosphatidylglycerol, phosphatidic acid, phosphatidylinositol, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, lysophosphatidylinositol, lysophosphatidylcholine, lysophosphatidylserine, lysophosphatidylethanolamine, ceramides from brain tissue, C-2 ceramide, C-8 ceramide, sphingomyelin, and GM1 ganglioside, were added to kinase reaction mixtures as mixed TX-100–lipid micelles or in some cases as sonicated lipid vesicles without any detergent. None of these lipids, over a wide concentration range, failed to inhibit enolase phosphorylation by PTKs in TX-100–treated GPI-enriched membranes or purified Lck (our unpublished results). Because phosphoinositides, particularly the phosphatidylinositol 3′-kinase (PI3K) product phosphotidylinositol 3,4-bisphosphate, have been shown to bind Src homology 2 containing proteins, including Lck (Rameh et al., 1995), we investigated whether any of the PI3K product could be inhibitory to Src kinases in GPI domains. Commercially available phosphatidylinositol 3-monophosphate, phosphatidylinositol 3,4-bisphosphate, phosphatidylinositol 4,5-bisphosphate, and inositol trisphosphate or PI3K products of brain-derived polyphosphoinositides did not modulate the PTKs in TX-100–treated GPI-enriched membranes to any significant extent (our unpublished results). However, only when the untreated membranes with their full complement of lipids were added to the TX-100–treated GPI-enriched membranes was the PTK activity markedly decreased. Reducing the input of untreated membranes paralleled the progressive restoration of the kinase activity (Figure 8).
Figure 7.
Lipid analysis of untreated and TX-100–treated GPI-enriched membranes. Untreated (lane 2) and TX-100–treated (lane 3), GPI-enriched membranes pelleted from one gradient (150 × 106 cells) were suspended in 200 μl of water and extracted in 4.8 ml of chloroform:methanol:pyridine:water (60:30:1:6 by vol) at 50°C for 48 h in sealed Pyrex tubes. For comparison total lipids were extracted from 200 × 106 cells (lane 1). The lipid extracts were desalted by reverse phase chromatography, applied to TLC plates (Silica Gel 60), and chromatographed in chloroform:methanol:0.22% aqueous CaCl2 (60:35:8 by vol). Lipids were detected by staining with a solution of phosphomolybdic acid (2.5%), cerium-IV-tetrahydrate (1%) containing 6% (vol/vol) concentrated sulfuric acid, followed by heating (Arni et al., 1996). Lipids were identified by comparing their migration coefficient values with those of the standards.
Figure 8.
Native GPI domains contain negative regulators of the associated kinases. Untreated and TX-100–treated GPI-enriched membranes equivalent to 25 × 106 cells and originating from the same cell homogenate were sedimented and tested for in vitro kinase activity as in Figure 2C. The effect of untreated GPI-enriched membranes was tested directly or at serial fourfold dilution on the kinase activity of the TX-100–treated GPI-enriched membranes by mixing both 10 min before the kinase reaction. All the assays were carried out in the same reaction volume containing 5 μCi of labeled ATP. Under the same assay conditions, the untreated GPI-enriched membranes did not contain demonstrable ATPase activity.
Modulation of PTK Activities by Detergents Reveals Heterogeneity in the Activation State of Lck and Fyn Kinases in Distinct Membrane Environments
We have previously reported that a three-step gradient in the absence of detergent separates the GPI-containing membranes into 1) a minor fraction of low density membranes (“light membranes”) highly enriched for GPI-anchored proteins and 2) a major fraction of high-density membranes (“heavy membranes”) containing both GPI-anchored proteins and transmembrane proteins (Arni et al., 1996; Figure 1C). The low-density light membranes probably represent the core of the sphingolipid and GPI-rich membrane domains, and the denser heavy membranes represent the core plus the surrounding regions where the GPI-anchored proteins undergo lateral interactions with transmembrane proteins (Ilangumaran et al., 1997; Ilangumaran and Hoessli, 1998). For equal amount of protein, the light membranes contain ∼20% of the amounts of Lck and Fyn kinases contained in heavy membranes (Arni et al., 1996). Yet, at comparable amounts of total protein, PTK activities in the light membranes were significantly higher than in heavy membranes (Arni et al., 1996; Figure 9). However, TX-100 resulted in a marked increase in phosphorylation of several protein substrates in heavy membranes, with only a marginal effect in light membranes (Figure 9). Treatment by other detergents (OTG and saponin) or pore-forming toxins (streptolysin-O [SLO] and α-toxin) caused a small but significant increase in phosphorylation in light membranes, similarly to TX-100. In contrast, phosphorylations in heavy membranes were markedly enhanced after TX-100, OTG, and saponin addition but not after addition of the pore-forming toxins SLO and α-toxin, suggesting that PTKs are selectively regulated by membrane constituents that are sensitive to the membrane disruption caused by nonionic detergents but not to pore-forming agents. Importantly, physical rearrangement of membranes (breakage and resealing of vesicles) by sonication had absolutely no effect on the phosphotransferase activity in either kind of membrane. Collectively, these results suggest that the activation state and regulation of PTKs within different local environments (microdomains) are sensitive to different membrane perturbants (detergents and pore-forming agents), emphasizing the importance of the membrane milieu on the activity of membrane-bound signaling molecules.
Figure 9.
Kinase actvities in heavy membranes are augmented by detergents but not by membrane pore-forming agents or sonication. Aliquots of light and heavy membranes containing 5 μg of total proteins were assayed for total phosphotransferase activity alone or in the presence of 0.1% TX-100, 6 mM OTG, 0.1% saponin (Sap), 100 U/ml SLO, or 10 U/ml α-toxin (a-Tox). Another set of aliquots was sonicated to disrupt the membrane vesicles before initiating the kinase reaction. Phosphorylated membrane proteins separated by SDS-PAGE were revealed by autoradiography after overnight exposure.
DISCUSSION
In lymphocytes, cross-linking the GPI-anchored surface receptors leads to rapid tyrosine phosphorylation of several intracellular protein substrates, including the nonreceptor PTKs Lck and Fyn (Hsi et al., 1989; Thompson et al., 1989), and cellular activation (Fischer et al., 1990; Korty et al., 1991; Shenoy-Scaria et al., 1992; Lund-Johansen et al., 1993; Morgan et al., 1993; Rowan et al., 1994; Stulnig et al., 1997). Signaling through GPI-anchored receptors is thought to require Src family PTKs, because several of them have been coprecipitated with GPI-anchored receptors (Stefanova and Horejsi, 1991; Stefanova et al., 1991; Cinek and Horejsi, 1992; Thomas and Samelson, 1992; Bohuslav et al., 1993; Garnett et al., 1993; Hatakeyama et al., 1994; Skubitz et al., 1995). The wealth of reports documenting these associations suggested the existence of transmembrane mechanism(s) for linking the GPI-anchored receptors to Src family PTKs, confined respectively to the outer and inner leaflets of the plasma membrane, but the precise nature of this interaction remains largely unresolved (Robinson, 1991; Brown, 1993). Although putative transmembrane connectors have been identified for Thy-1 (Lehuen et al., 1995) and CD55 (Kuraya and Fujita, 1998), the quest for a transmembrane linker to connect GPI-anchored receptors in general to intracellular PTKs has not been rewarding.
In recent years it has become apparent that GPI-anchored proteins are enriched in specialized regions of the plasma membrane (variously designated glycolipid-enriched membranes [Rodgers and Rose, 1996], detergent-resistant membranes [Brown and Rose, 1992], Triton X-100–insoluble floating fractions [Kurzchalia et al., 1995], sphingolipid “rafts” [Simons and Ikonen, 1997], and GPI domains [Arni et al., 1996]), wherein substantial amounts of membrane-associated Src family PTKs are also included that could account for the signaling capacity of GPI-anchored receptors. Mechanisms underlying the targeting of the doubly acylated PTKs to GPI-enriched membrane domains have been studied in detail (Shenoy-Scaria et al., 1993; van’t Hof and Resh, 1997). Whether these membrane domains also represent a unique site for Src family PTKs in terms of their regulation (Arni et al., 1996; Rodgers and Rose, 1996; Kabouridis et al., 1997) is only beginning to be addressed. In this paper, we show that Lck and Fyn kinases are differentially regulated in different plasma membrane environments. In membrane microdomains containing both GPI-anchored and transmembrane proteins, the associated PTKs are down-regulated by a mixed, noncompetitive mechanism of inhibition. This inhibition mechanism is abrogated by nonionic detergents, most likely upon disruption of the membrane structure, and can be restored by adding back the intact membranes.
The doubly acylated Lck interacts with the plasma membrane throughout the inner leaflet and does not require transmembrane CD4 receptor for membrane localization (Bijlmakers et al., 1997). The Fyn kinase was shown to rapidly associate with membranes after synthesis and then to more slowly localize to detergent-resistant membranes (van’t Hof and Resh, 1997). In resting cells, clustering of PTKs has not been demonstrated in any particular membrane region, but accumulation of PTKs underneath agonist-induced clusters of sphingolipid microdomains has been documented after antibody-mediated cross-linking of sphingolipid microdomain components (Zisch et al., 1995; Harder et al., 1998). The submembrane topology of Lck seems to be important in determining the signaling interactions of the molecule. For instance, Lck fused with a transmembrane protein can mediate the early steps of signaling via the T-cell receptor; however, the later steps in the T-cell receptor signaling pathway were only carried out by the doubly acylated Lck bound to the plasma membrane through hydrophobic and electrostatic interactions (Kabouridis et al., 1997). These findings indeed suggest that the hydrophobic and electrostatic interaction of Src family PTKs with the inner leaflet (Murray et al., 1998) could influence the conformation of the kinase molecule and thereby affect the modular interactions with substrates and the catalytic activity. In this study, we provide evidence for modulation of PTK activity by the local membrane environment of the kinase in the absence of agonist- or antibody-induced aggregation of sphingolipid microdomains. The membrane environment influences both the overall catalytic activity (increased phosphotransferase activity and enolase tyrosine phosphorylation) and autophosphorylation (appearance of hyperphosphorylated bands).
Sphingolipid microdomains may be viewed as sphingolipid- and cholesterol-rich core structures surrounded by a membrane area containing both GPI-anchored and transmembrane proteins. GPI-anchored proteins are probably tightly bound to the core sphingolipids, and both GPI-anchored proteins and glycosphingolipids can associate with transmembrane receptors at the periphery of the core sphingolipid microdomains through lateral, extracellular (integrins; Petty and Todd, 1996), and intramembraneous (CD44; Neame et al., 1995) interactions. Upon detergent extraction, the majority of the phospholipids are solubilized, disrupting these lateral interactions to a variable extent and leaving behind the core of the “detergent-insoluble” sphingolipid domains, still buoyant in density gradients (Ilangumaran et al., 1997; Ilangumaran and Hoessli, 1998). However, under these conditions, the amount of Lck and Fyn recovered in association with the sphingolipid domains was not significantly increased, suggesting that PTKs are not artifactually included in the detergent-resistant framework after extraction. Limited amounts of core sphingolipid domains could also be recovered under detergent-free conditions in a multistep sucrose gradient as light membranes, which are highly enriched for GPI-anchored proteins and deficient in transmembrane proteins. PTKs in the core sphingolipid microdomains (TX-100–treated GPI-enriched membranes and light membranes) were considerably more active than PTKs in sphingolipid microdomains associated with transmembrane proteins (untreated GPI-enriched membranes and heavy membranes). The down-regulated PTKs in the latter were efficiently reactivated by the TX-100, NP-40, CHAPS, and OTG detergents but not by the pore-forming α-toxin and SLO toxins, whereas the optimally active PTKs in the core sphingolipid microdomains (light membranes) were only minimally perturbed by the detergents or the pore-forming toxins. This enhancement of PTK activity probably results from modification of the PTK membrane environment by detergents and cannot be attributed to increased availability of ATP to the kinases, because neither sonication nor the pore-forming toxins resulted in the kind of enhancement observed after detergent treatment. Moreover, this enhancing effect of detergents is also observed in the minority of “inside-out” vesicles that were recovered on solid-phase anti-Lck, in which the PTKs are directly facing the extravesicular medium.
Adding back the untreated GPI-enriched membranes to the TX-100–treated ones restored the inhibition of PTK activity in the latter, probably after fusion of detergent-treated and untreated vesicles. This is, however, not a space-filling effect of the added lipids, because many different lipids (phosphoglycerides and ceramides), added as sonicated lipid vesicles or lipid–detergent mixed micelles to fully active kinases in detergent-treated membranes, failed to restore this inhibition. Despite the striking effect of nonionic detergents on membrane-bound PTK activity, the enhancement of kinase activity is attributable to the membrane perturbation induced by the detergent rather than a direct effect of the detergent on the kinase itself. Independently of detergents, membrane fractions of different densities and lipid–protein contents exhibit very distinct levels of PTK activity (light versus heavy membranes; Arni et al., 1996). This distinction is further exemplified by the different responses of light and heavy membrane PTKs to detergents and pore-forming agents. The membrane environment of PTKs therefore appears to be an important regulator of their catalytic activity.
Src family PTKs are regulated by an intramolecular interaction between the Src homology 2 domain and the C-terminal phosphotyrosine residue that locks the kinase in an inactive conformation (Cooper and Howell, 1993). Dephosphorylation of this regulatory tyrosine by PTPases such as CD45 relieves this repression (Mustelin et al., 1989). Enhancement of the PTK activities associated with GPI-enriched membranes by PTPase inhibitor sodium orthovanadate strongly suggests that Lck and Fyn PTKs residing in sphingolipid microdomains are neither in a “locked,” inactive conformation (Sieh et al., 1993) nor activated by the PTPase CD45 at the periphery of the microdomains, as proposed earlier (Rodgers and Rose, 1996). Actually, when the association of CD45 with detergent-resistant membranes was diminished by TX-100, NP-40, CHAPS, or OTG, we observed an increase, rather than a decrease, in PTK activity, strongly suggesting that removal of CD45 from membranes does not compromise PTK activity. Src family PTKs can also be activated by allosteric mechanisms that disrupt the intramolecular modular interactions (Moarefi et al., 1997; Williams et al., 1998), which “open” the kinase from the locked conformation without dephosphorylating the C-terminal Tyr-505 (Hardwick and Sefton, 1997). In addition, the locked conformation of the Src kinases is not static, because intermittent opening and closing mediated by allosteric mechanisms is believed to allow transient activation (“breathing”) of the kinase (Cooper and Howell, 1993). Recent evidence showing simultaneous phosphorylation of the regulatory and activation domain Tyr residues on the same Lck molecule support this notion (Hardwick and Sefton, 1997). In fact, PTKs in untreated GPIenriched membranes appear to be modulated by novel regulatory mechanisms. Despite the complexity of the experimental system involving at least two PTKs and several substrates, our kinetic data suggest that PTKs in untreated GPI-enriched membranes are down-regulated by a mixed, noncompetitive type of inhibition. In this situation, a negative regulator influences both Vmax and Km through binding to a site distinct from the active site and allosterically modifies the substrate binding. The kinetic difference in PTK activity observed between untreated and TX-100–treated GPI-enriched membranes is likely to be encountered when the enzyme:inhibitor dissociation constant (KI = [E][I]/[EI]) is greater than that of the enzyme-substrate:inhibitor (K′I = [ES][I]/[ESI]), i.e., when the affinity of the inhibitor for the enzyme alone is less than for the enzyme–substrate complex. This would suggest that in untreated GPI-enriched membranes the Lck and Fyn kinases are in the activated state forming complexes with their substrates, and that inhibition of their activity is brought about by regulatory mechanisms acting on protein domains outside the catalytic site of the enzyme. This regulatory input, which is perturbed by detergents, appears to be dependent on the specialized lipid environment of the GPI-rich membrane domains rather than any single membrane constituent.
Based on these results, we propose a model in which the PTKs associated with GPI-enriched core sphingolipid microdomains are catalytically active, but those interacting with the glycerophospholipid-rich surroundings are down-modulated by regulatory inputs probably acting on the modular, noncatalytic domains of the kinases. Within the core of sphingolipid microdomains the detergent-resistant membrane environment rich in glycosphingolipids and GPI-anchored receptors appears to promote both optimal conditions for high PTK activity and a close interaction of PTKs with other membrane protein substrates (i.e. with the 80-kDa phosphoprotein), whereas in membrane environments of a less restricted composition (i.e., the sphingolipid microdomains abutting the glycerophospholipid-rich membrane region), PTKs become liable to many more regulatory inputs. The removal of transmembrane proteins and glycerophospholipids by nonionic detergents apparently relieves PTKs from those regulatory inputs and restores a membrane environment that resembles that of core sphingolipid microdomains. Cross-linking of the surface GPI-linked receptors or gangliosides would also lead to coalescence of the sphingolipid domains and presumably reduces the quantum of external regulatory inputs, resulting in the reactivation of the sphingolipid domain–associated PTKs.
Transmembrane receptors that interact with sphingolipid microdomains may also acquire signaling competence from the microdomains, as it was shown for the FcεRI receptor (Field et al., 1995; Stauffer and Meyer, 1997), CD44 (Ilangumaran et al., 1998), and CD20 (Deans et al., 1998). Although the association of the GPI-anchored proteins with sphingolipid microdomains is thought to arise from the interaction of GPI anchor acyl chains with glycosphingolipids, the nature of interaction of the transmembrane proteins with sphingolipid domains is less clear. The CD44 transmembrane domain seems to confer the capacity to associate with detergent-resistant membrane domains, because swapping of the transmembrane domain abolished this capacity (Neame et al., 1995). For other transmembrane proteins, the mechanisms of association with sphingolipid domains have not yet been defined, but the possibility of extracellular interactions with gangliosides or GPIanchored receptors such as those reported for integrins (Petty and Todd, 1996) could be envisaged. Cross-linking these transmembrane receptors would then cross-link the sphingolipid microdomains and signal via the active Src family kinases present at the inner face of such remodeled domains. Such dependence of the T-cell receptor pathway on the integrity of sphingolipid microdomains was documented in several recent studies (Romagnoli and Bron, 1997; Montixi et al., 1998; Xavier et al., 1998).
Our results lend support to the view that PTKs associated with sphingolipid microdomains indeed represent a unique pool of signaling molecules that are controlled by the microdomain structure of the bilayer. Mechanisms underlying the higher activity of Src family PTKs within the core sphingolipid domains and the nature of the inhibition exerted on kinases outside microdomains require further definition. Likewise, how the microdomain-associated pool of kinases is linked to physiological means of cellular stimulation will have to be studied specifically in each of the different systems in which GPI-anchored receptors and transmembrane proteins interacting with the microdomains are implicated in cellular activation.
ACKNOWLEDGMENTS
We thank H. Chap (Institut National de la Santé et de la Recherche Médicale U 326, Toulouse, France) for experiments involving lipids and helpful discussions, K. Rose (Department de Biochimie Médicale, University of Geneva, Geneva, Switzerland) for help with enzyme kinetics, and M. Poincelet for excellent technical assistance. This work was supported by Swiss National Science Foundation grant 31-39-709-93, Swiss Cancer League grant 35-462-2-1997, and the Aargauische Krebsliga.
Abbreviations used:
- CHAPS
3-([3-cholamidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate
- CT
cholera toxin
- ECL
enhanced chemiluminescence
- GPI
glycosylphosphatidylinositol
- HSA
heat-stable antigen
- MP
milk powder
- NC
nitrocellulose
- OTG
octylglucoside
- PI3K
phosphatidylinositol 3′-kinase
- PTK
protein-tyrosine kinase
- PTPase
protein-tyrosine phosphatase
- PVDF
polyvinylidene difluoride
- SLO
streptolysin-O
- TTBS
Tween 20–Tris-buffered saline
- TX-100
Triton X-100
REFERENCES
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- Arni S, Ilangumaran S, van Echten-Deckert G, Sandhoff K, Poincelet M, Briol A, Rungger-Brändle E, Hoessli DC. Differential regulation of Src-family protein tyrosine kinases in GPI domains of T lymphocyte plasma membranes. Biochem Biophys Res Commun. 1996;225:801–807. doi: 10.1006/bbrc.1996.1254. [DOI] [PubMed] [Google Scholar]
- Arni S, Senaldi G, Poincelet M, Hoessli DC. Selective association of the p59fyn tyrosine kinase with murine T lymphoma membrane phosphoproteins. Oncogene. 1993;8:2485–2491. [PubMed] [Google Scholar]
- Bijlmakers MJJE, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–1040. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohuslav J, Cinek T, Horejsi V. Large, detergent-resistant complexes containing murine antigens Thy-1 and Ly-6 and protein tyrosine kinase p56lck. Eur J Immunol. 1993;23:825–831. doi: 10.1002/eji.1830230409. [DOI] [PubMed] [Google Scholar]
- Brown DA. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992;2:338–343. [PubMed] [Google Scholar]
- Brown DA. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993;5:349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cinek T, Hilgert I, Horejsi V. An alternative way of CD4 and CD8 association with protein kinases of the Src family. Immunogenetics. 1995;41:110–116. doi: 10.1007/BF00182321. [DOI] [PubMed] [Google Scholar]
- Cinek T, Horejsi V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992;149:2262–2270. [PubMed] [Google Scholar]
- Cooper JA, Howell BW. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Deans JP, Robbins SM, Polyak MJ, Savage JA. Rapid redistribution of CD20 to a low density detergent-insoluble membrane compartment. J Biol Chem. 1998;273:344–348. doi: 10.1074/jbc.273.1.344. [DOI] [PubMed] [Google Scholar]
- Doyle DD, Goings GE, Upshaw-Earley J, Page E, Ransch B, Palfrey HC. T-cadherin is a major glycophosphoinositol-anchored protein associated with noncaveolar detergent-insoluble domains of the cardiac sarcolemma. J Biol Chem. 1998;273:6937–6943. doi: 10.1074/jbc.273.12.6937. [DOI] [PubMed] [Google Scholar]
- Draberova L, Draber P. Thy-1 glycoprotein and Src-like protein-tyrosine kinase p53/p56lyn are associated in large detergent-resistant complexes in rat basophilic leukemia cells. Proc Natl Acad Sci USA. 1993;90:3611–3615. doi: 10.1073/pnas.90.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. Fc ε RI-mediated recruitment of p53/56(lyn) to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity Ig E receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Fischer GF, Majdic O, Gadd S, Knapp W. Signal transduction in lymphocytic and myeloid cells via CD24, a new member of phosphoinositol-anchored membrane molecules. J Immunol. 1990;144:638–641. [PubMed] [Google Scholar]
- Friederichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by cross-linking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. GPI-anchored proteins, glycosphingolipids, and sphingomyelin are sequestered to caveolae only after cross linking. J Histochem Cytochem. 1996;44:929–941. doi: 10.1177/44.8.8756764. [DOI] [PubMed] [Google Scholar]
- Garnett D, Barclay AN, Carmo AM, Beyers AD. The association of the protein tyrosine kinases p56lck and p60fyn with the glycosyl phosphatidylinositol-anchored proteins Thy-1 and CD48 in rat thymocytes is dependent on the state of cellular activation. Eur J Immunol. 1993;23:2540–2544. doi: 10.1002/eji.1830231024. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201B:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid-domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JS, Sefton BM. The activated form of the Lck tyrosine protein kinase in cells exposed to hydrogen peroxide is phosphorylated at both Tyr-394 and Tyr-505. J Biol Chem. 1997;272:25429–25432. doi: 10.1074/jbc.272.41.25429. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Kazuya I, Kazumasa O, Good RA, Onoé K. The murine c-fgr gene product associated with Ly6c and p70 integral membrane protein is expressed in cells of a monocyte/macrophage lineage. Proc Natl Acad Sci USA. 1994;91:3458–3462. doi: 10.1073/pnas.91.8.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli DC, Rungger-Brändle E. Isolation of plasma membrane domains from murine T lymphocytes. Proc Natl Acad Sci USA. 1983;80:439–443. doi: 10.1073/pnas.80.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli DC, Rungger-Brändle E. Association of specific cell-surface glycoproteins with a Triton X-100-resistant complex of plasma membrane proteins isolated from T-lymphoma cells (P1798) Exp Cell Res. 1985;156:239–250. doi: 10.1016/0014-4827(85)90278-2. [DOI] [PubMed] [Google Scholar]
- Hsi ED, Siegel JN, Minami Y, Luong ET, Klausner RD, Samelson LE. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989;264:10836–10842. [PubMed] [Google Scholar]
- Ilangumaran S, Arni S, Chicheportiche Y, Briol A, Hoessli DC. Evaluation by dot-immunoassay of the differential distribution of cell surface and intracellular proteins in glycosylphosphatidylinositol-rich plasma membrane domains. Anal Biochem. 1996;235:49–56. doi: 10.1006/abio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Briol A, Hoessli DC. Distinct interactions among GPI-anchored, transmembrane and membrane associated intracellular proteins, and sphingolipids in lymphocyte and endothelial cell plasma membranes. Biochim Biophys Acta. 1997;1328:227–236. doi: 10.1016/s0005-2736(97)00099-0. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Briol A, Hoessli DC. CD44 selectively associates with active Src-family kinases Lck and Fyn in glycosphingolipid-rich plasma membrane domains of human peripheral blood lymphocytes. Blood. 1998;91:3901–3908. [PubMed] [Google Scholar]
- Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signaling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps MP, Sefton BM. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989;176:22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Korty PE, Brando C, Shevach EM. CD59 functions as a signal-transducing molecule for human T cell activation. J Immunol. 1991;146:4092–4098. [PubMed] [Google Scholar]
- Kunz S, Ziegler U, Kunz B, Sonderegger P. Intracellular signaling is changed after clustering of the neural cell adhesion molecules axonin-1 and NgCAM during neurite fasciculation. J Cell Biol. 1996;135:253–267. doi: 10.1083/jcb.135.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraya M, Fujita T. Signal transduction via a protein associated with a glycosylphosphatidylinositol-anchored protein, decay-accelerating factor (DAF/CD55) Int Immunol. 1998;10:473–480. doi: 10.1093/intimm/10.4.473. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Hartmann E, Dupree P. Guilt by insolubility—does a protein’s detergent insolubility reflect caveolar location? Trends Cell Biol. 1995;5:187–189. doi: 10.1016/s0962-8924(00)88990-4. [DOI] [PubMed] [Google Scholar]
- Lehuen A, Beaudoin L, Bernard M, Kearney JF, Bach JF, Monteiro RC. T cell activation through Thy-1 is associated with the expression of a surface protein (p100) on a subset of CD4 cells. Int Immunol. 1995;7:607–616. doi: 10.1093/intimm/7.4.607. [DOI] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Low MG. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989;3:1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F, Olweus J, Symington FW, Arli A, Thompson JS, Vilella R, Skubitz K, Horejsi V. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur J Immunol. 1993;23:2782–2791. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- Marie-Cardine A, Bruyns E, Eckershorn C, Kirchgessner H, Meuer SC, Schraven B. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J Biol Chem. 1997;272:16077–16080. doi: 10.1074/jbc.272.26.16077. [DOI] [PubMed] [Google Scholar]
- Massague J. Neurotrophic factors—crossing receptor boundaries. Nature. 1996;382:29–30. doi: 10.1038/382029a0. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Chu T, Tortorella LB, Brown DA. Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry. 1995;34:16161–16170. doi: 10.1021/bi00049a031. [DOI] [PubMed] [Google Scholar]
- Minoguchi K, Swaim WD, Berenstein EH, Siraganian RP. Src family tyrosine kinase p53/56lyn, a serine kinase and Fc ε RI associate with α-galactosyl derivatives of ganglioside GD1b in rat basophilic leukemia RBL-2H3 cells. J Biol Chem. 1994;269:5249–5254. [PubMed] [Google Scholar]
- Moarefi I, Lafevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel M-A, Chauvin J-P, Pierres M, He H-T. Engagement of T cell receptor triggers irs recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP, Van den Berg CW, Davies EV, Hallett MB, Horejsi V. Cross-linking of CD59 and of other glycosyl phosphatidylinositol-anchored molecules on neutrophils triggers cell activation via tyrosine kinase. Eur J Immunol. 1993;23:2841–2850. doi: 10.1002/eji.1830231118. [DOI] [PubMed] [Google Scholar]
- Munoz G, Marshall SH. An alternate method for fast separation of phosphotyrosine. Anal Biochem. 1990;190:233–237. doi: 10.1016/0003-2697(90)90185-c. [DOI] [PubMed] [Google Scholar]
- Murray D, Hermida-Matsumoto L, Buser CA, Tsang J, Sigal CT, Ben-Tal N, Honig B, Resh MD, McLaughlin S. Electrostatics and the membrane association of Src: theory and experiment. Biochemistry. 1998;37:2145–2159. doi: 10.1021/bi972012b. [DOI] [PubMed] [Google Scholar]
- Mustelin T, Coggeshall KM, Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci USA. 1989;86:6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame SJ, Uff CR, Sheikh H, Wheatley SC, Isacke CM. CD44 exhibits a cell type dependent interaction with Triton X-100 insoluble lipid rich plasma membrane domains. J Cell Sci. 1995;108:3127–3135. doi: 10.1242/jcs.108.9.3127. [DOI] [PubMed] [Google Scholar]
- Petty HR, Todd RF. Integrins as promiscuous signal transduction devices. Immunol Today. 1996;17:209–212. doi: 10.1016/0167-5699(96)30013-3. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Chen C-S, Cantley LC. Phosphatidylinositol (3,45)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- Robinson PJ. Phosphatidylinositol membrane anchors and T-cell activation. Immunol Today. 1991;12:35–41. doi: 10.1016/0167-5699(91)90110-F. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56(lck) associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli P, Bron C. Phosphatidylinositol-based glycolipid-anchored proteins enhance proximal TCR signaling events. J Immunol. 1997;158:5757–5764. [PubMed] [Google Scholar]
- Rowan WC, Hale G, Tite JP, Brett SJ. Cross-linking of the CAMPATH-1 antigen (CD52) triggers activation of normal human T lymphocytes. Int Immunol. 1994;7:69–77. doi: 10.1093/intimm/7.1.69. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schroeder RJ, Ahmed SN, Zhu YZ, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- Sevinsky JR, Vijay Mohan Rao L, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets ED, Lee GM, Simson R, Jacobson K. Transient confinement of a glycosylphosphatidylinositol-anchored protein in the plasma membrane. Biochemistry. 1997;36:12449–12458. doi: 10.1021/bi9710939. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Gauen LKT, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor—interaction of glycosyl-phophatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993;12:315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Sphingolipid-cholesterol rafts in membrane trafficking and signaling. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simson R, Yang B, Moore SE, Doherty P, Walsh FS, Jacobson KA. Structural mosaicism on the submicron scale in the plasma membrane. Biophys J. 1998;74:297–308. doi: 10.1016/S0006-3495(98)77787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Ahmed K, Skubitz APN. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J Immunol. 1995;155:5382–5390. [PubMed] [Google Scholar]
- Stauffer TP, Meyer T. Compartmentalized IgE receptor-mediated signal transduction in living cells. J Cell Biol. 1997;139:1447–1454. doi: 10.1083/jcb.139.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Horejsi V. Association of the CD59 and CD55 cell surface glycoproteins with other membrane molecules. J Immunol. 1991;147:1587–1592. [PubMed] [Google Scholar]
- Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1018. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Stockinger H, Horejsi V, Waldhäusl W. Signal transduction via glycosyl phosphatidylinositol-anchored proteins in T cells is inhibited by lowering cellular cholesterol. J Biol Chem. 1997;272:19242–19247. doi: 10.1074/jbc.272.31.19242. [DOI] [PubMed] [Google Scholar]
- Thomas PM, Samelson LE. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992;267:12317–12322. [PubMed] [Google Scholar]
- Thompson LH, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5′-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989;143:1815–1821. [PubMed] [Google Scholar]
- van den Berg CW, Cinek T, Hallett MB, Horejsi V, Morgan BP. Exogenous glycosyl phosphatidyl-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca2+-signaling competent. J Cell Biol. 1995;131:669–677. doi: 10.1083/jcb.131.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59(fyn): selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Williams JC, Wierenga RK, Saraste M. Insights into Src kinase functions: structural comparisons. Trends Biochem Sci. 1998;23:179–184. doi: 10.1016/s0968-0004(98)01202-x. [DOI] [PubMed] [Google Scholar]
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Zisch AH, Dalessandri L, Amrein K, Ranscht B, Winterhalter KH, Vaughan L. The glypiated neuronal cell adhesion molecule contactin/F11 complexes with src-family protein tyrosine kinase Fyn. Mol Cell Neurosci. 1995;6:263–279. doi: 10.1006/mcne.1995.1021. [DOI] [PubMed] [Google Scholar]