Abstract
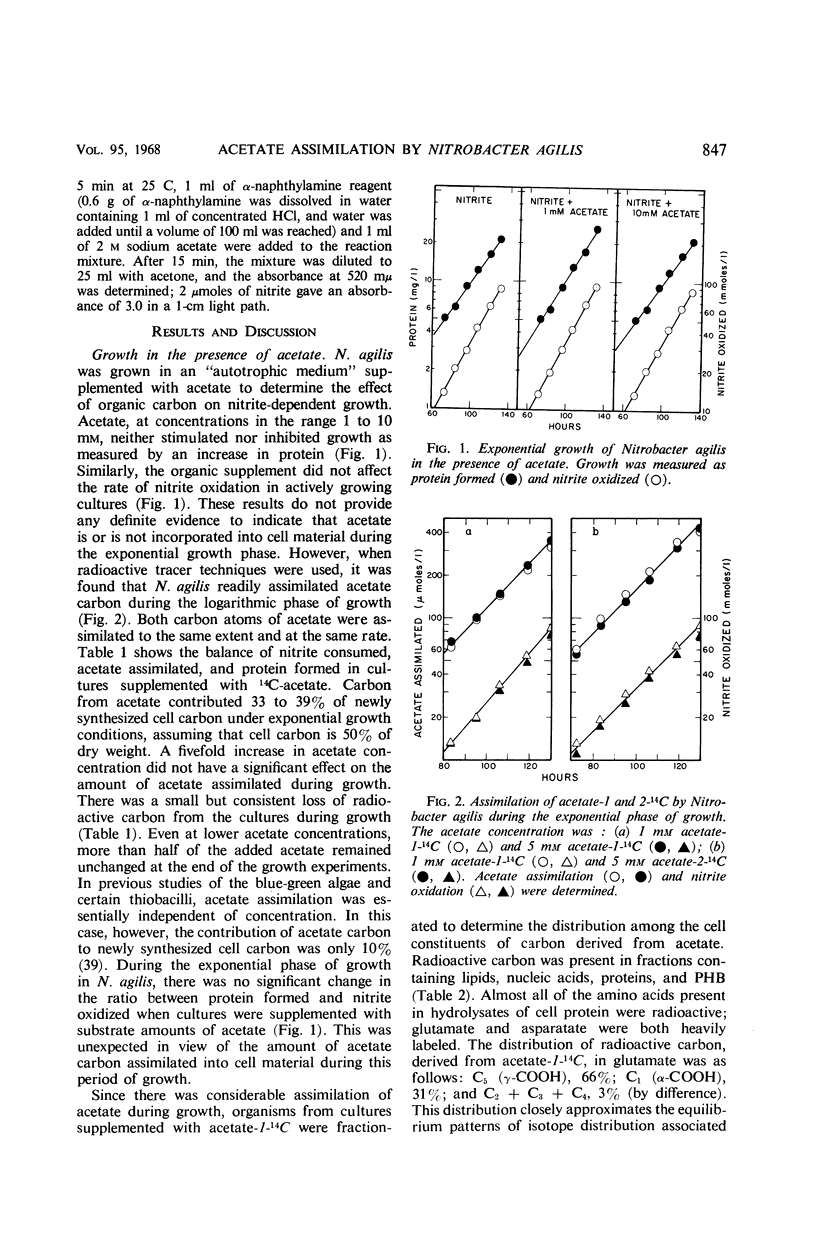
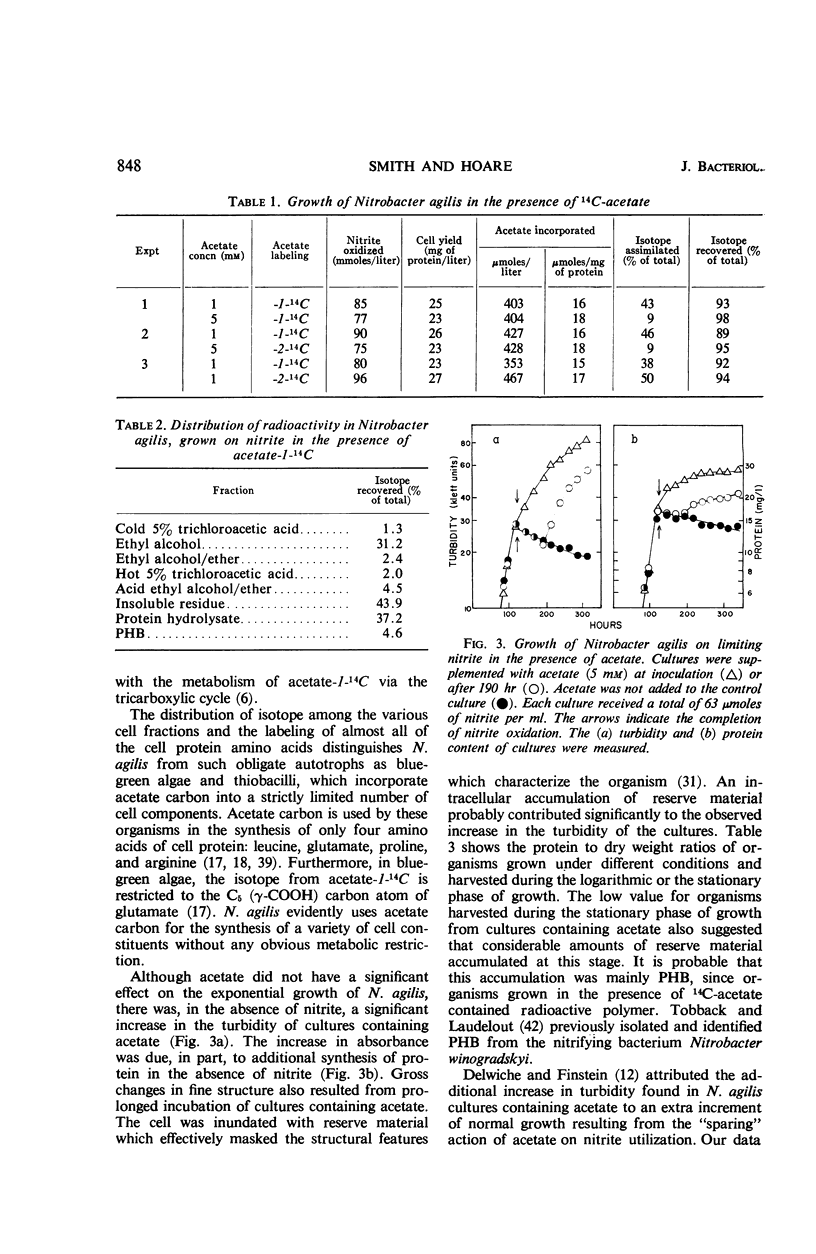
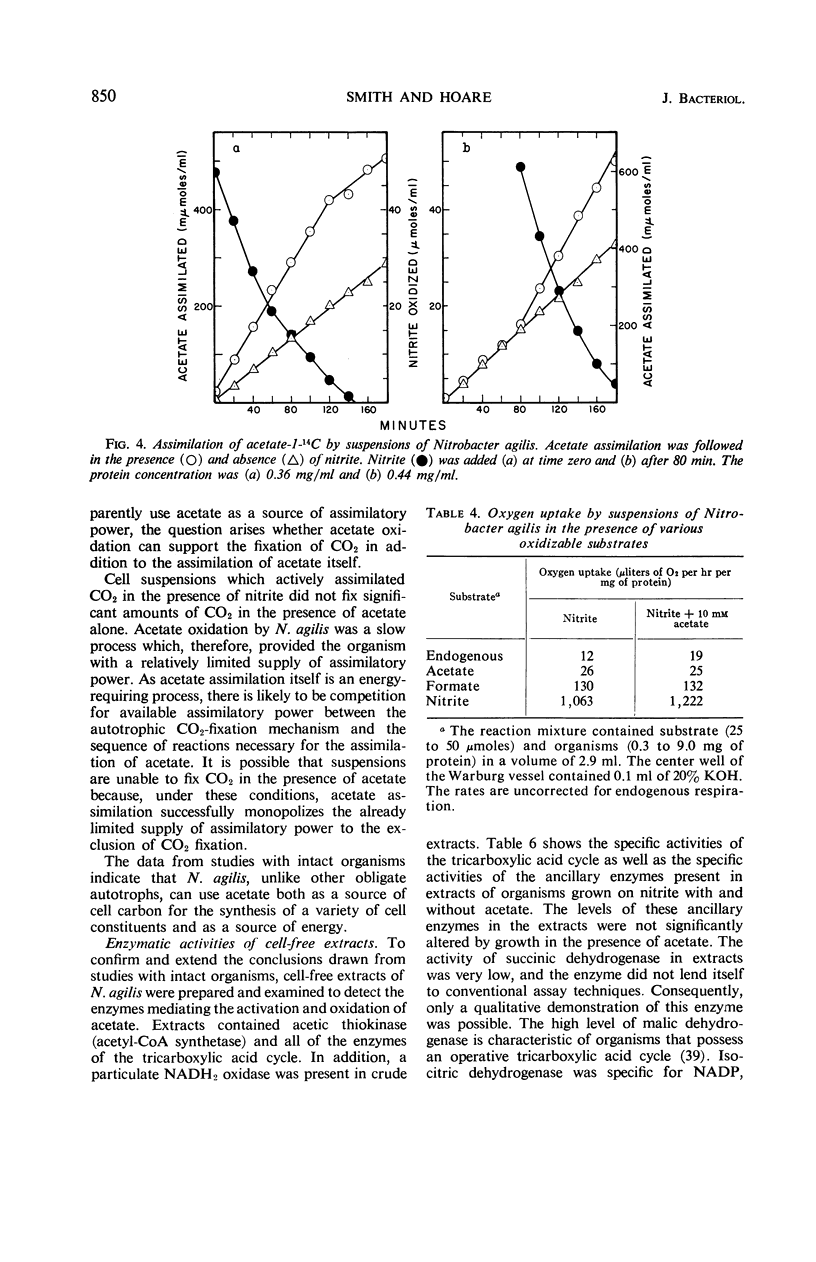
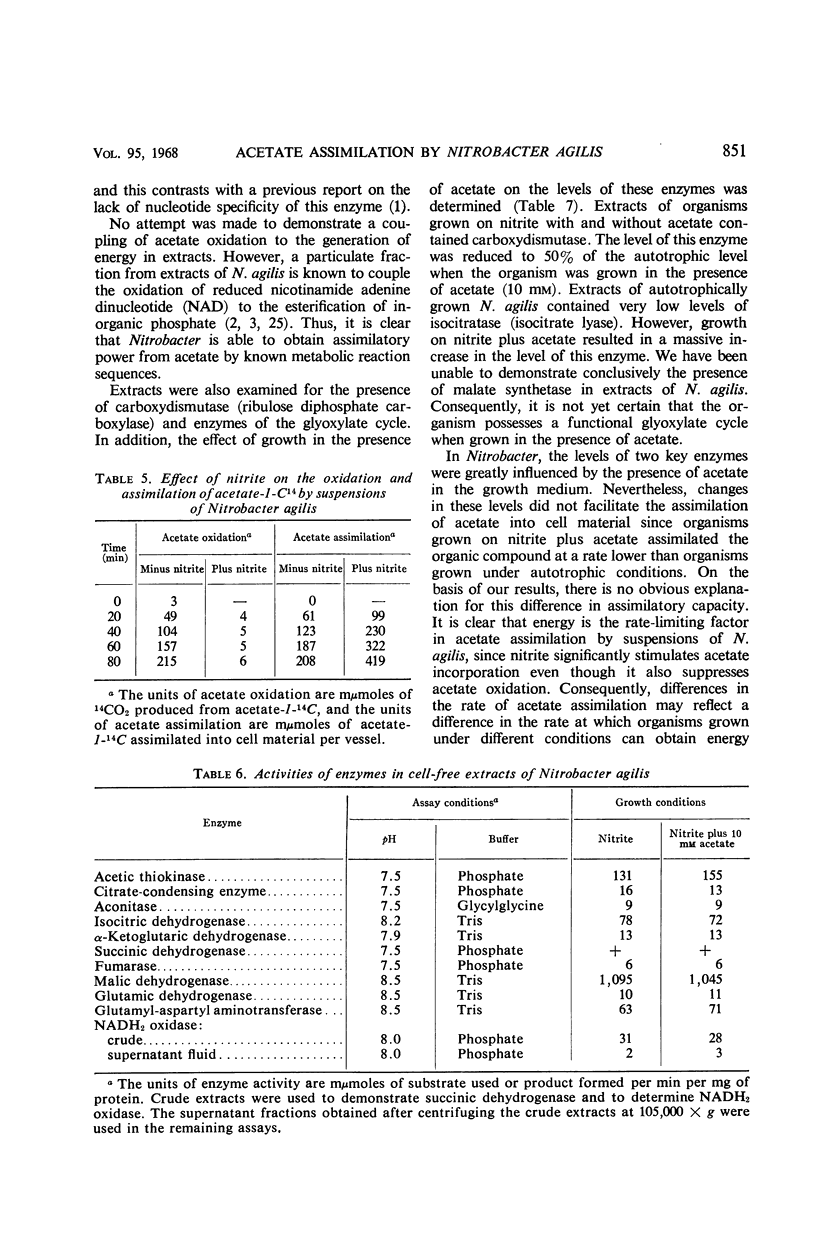
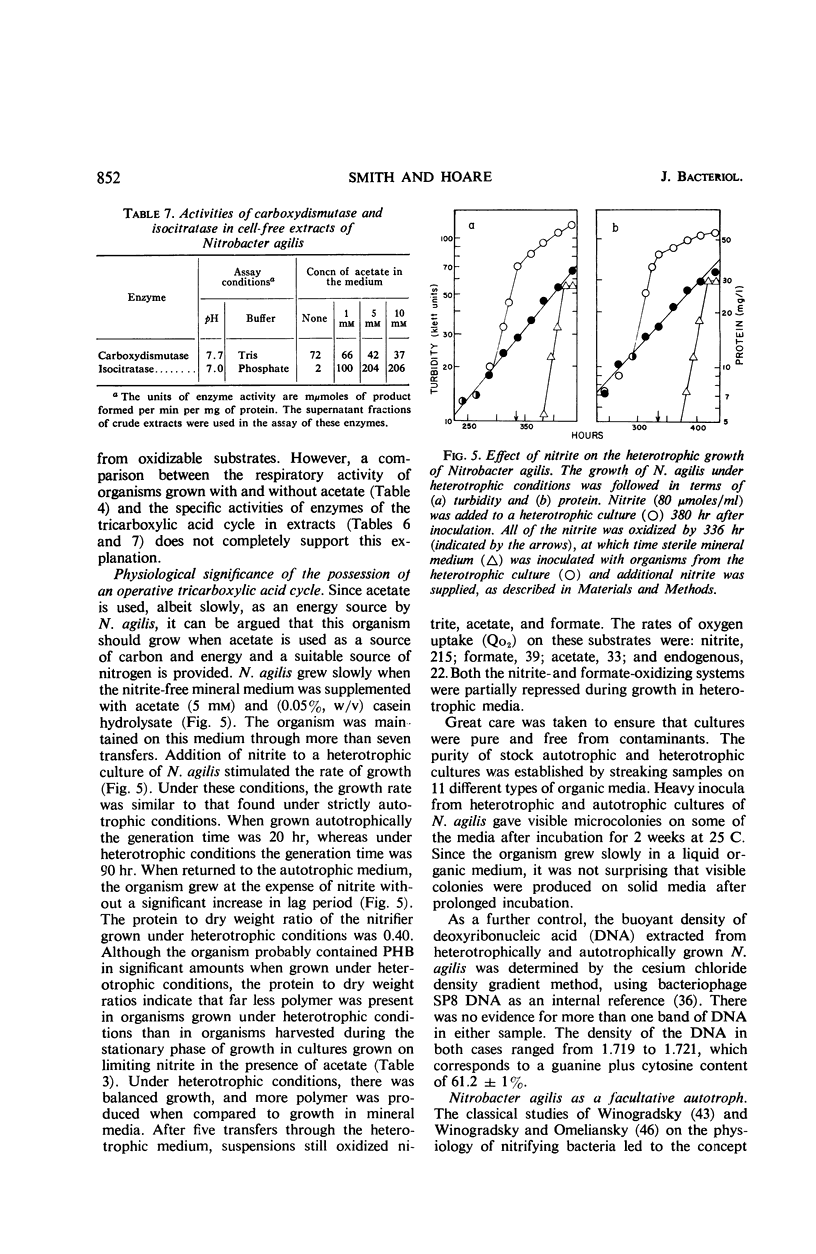
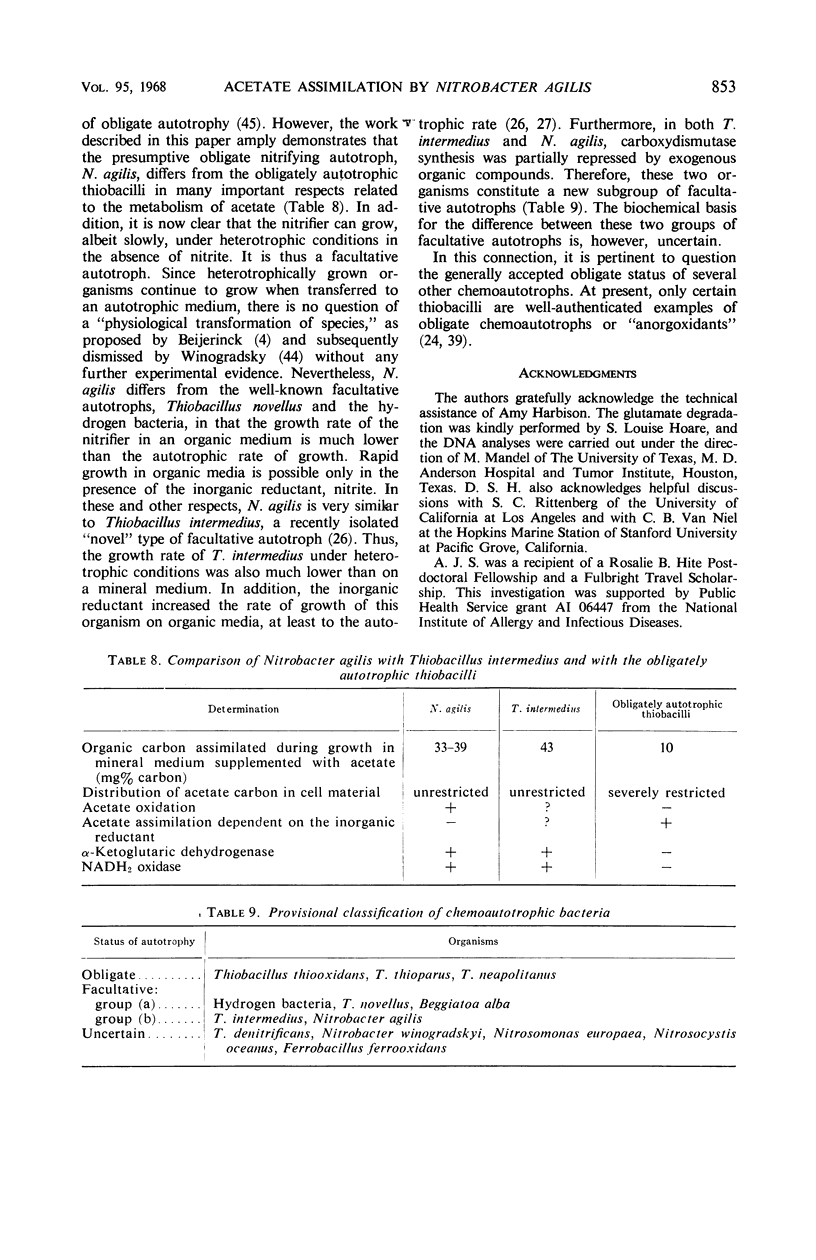
Acetate (1 to 10 mm) had no effect on the rate of nitrite oxidation or exponential growth by Nitrobacter agilis. However, acetate-1-14C and -2-14C were both assimilated by growing cultures, and acetate carbon contributed 33 to 39% of newly synthesized cell carbon. Carbon from acetate was incorporated into all of the major cell constituents, including most of the amino acids of cell protein and poly-β-hydroxybutyrate (PHB). Cultures grown in the presence of acetate showed a significant increase in turbidity, attributable in part to protein synthesis and the accumulation of PHB in the “post-exponential phase,” when the supply of nitrite was completely exhausted. Cell suspensons of N. agilis assimilated acetate in the absence of bicarbonate and even in the absence of nitrite. However, the addition of nitrite increased the rate of acetate assimilation by cell suspensions. The distribution of 14C-acetate incorporated by cell suspensions was qualitatively similar to that found with growing cultures. Cell suspensions of N. agilis slowly oxidized acetate to CO2. Addition of nitrite suppressed CO2 production from acetate but increased the assimilation of acetate carbon into cell material. N. agilis contained all the enzymes of the tricarboxylic acid cycle. Growth of N. agilis in the presence of acetate did not significantly affect the levels of the enzymes of the tricarboxylic acid cycle, but did result in a 100-fold increase in the specific activity of isocitratase. In contrast, carboxydismutase was partially repressed. N. agilis was grown heterotrophically through seven transfers on a medium containing acetate and casein hydrolysate. The addition of nitrite increased the rate of heterotrophic growth. Heterotrophically grown organisms still retained their ability to grow autotrophically with nitrite. However, these organisms oxidized nitrite at a slower rate. Organisms from autotrophic and heterotrophic cultures were analyzed to determine the mean guanine plus cytosine content of their deoxyribonucleic acid; in both cases this mean was 61.2 ± 1%. We concluded that N. agilis is not an obligate autotroph; it appears to be a facultative autotroph which resembles the novel facultative autotroph, Thiobacillus intermedius, very closely.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem M. I. Path of carbon and assimilatory power in chemosynthetic bacteria. I. Nitrobacter agilis. Biochim Biophys Acta. 1965 Aug 24;107(1):14–28. doi: 10.1016/0304-4165(65)90384-3. [DOI] [PubMed] [Google Scholar]
- BLACK A. L., KLEIBER M. The tricarboxylic acid cycle as a pathway for transfer of carbon from acetate to amino acids in the intact cow. Biochim Biophys Acta. 1957 Jan;23(1):59–69. doi: 10.1016/0006-3002(57)90285-8. [DOI] [PubMed] [Google Scholar]
- Butler R. G., Umbreit W. W. Absorption and utilization of organic matter by the strict autotroph, Thiobacillus thiooxidans, with special reference to aspartic acid. J Bacteriol. 1966 Feb;91(2):661–666. doi: 10.1128/jb.91.2.661-666.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Effect of mixed culture on Nitrosomonas europaea simulated by uptake and utilization of pyruvate. J Bacteriol. 1966 Jan;91(1):367–373. doi: 10.1128/jb.91.1.367-373.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Uptake and utilization of amino acids by resting cells of Nitrosomonas europaea. J Bacteriol. 1967 Apr;93(4):1309–1315. doi: 10.1128/jb.93.4.1309-1315.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Finstein M. S. Carbon and Energy Sources for the Nitrifying Autotroph Nitrobacter. J Bacteriol. 1965 Jul;90(1):102–107. doi: 10.1128/jb.90.1.102-107.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S. M., Burris R. H. Effect of exogenous substrates on the endogenous respiration of bacteria. J Bacteriol. 1967 Apr;93(4):1467–1470. doi: 10.1128/jb.93.4.1467-1470.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. S., Gibson J. Photoassimilation of acetate and the biosynthesis of amino acids by Chlorobium thiosulphatophilum. Biochem J. 1964 Jun;91(3):546–559. doi: 10.1042/bj0910546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. S., Moore R. B. Photoassimilation of organic compounds by autotrophic blue-green algae. Biochim Biophys Acta. 1965 Nov 29;109(2):622–625. doi: 10.1016/0926-6585(65)90192-5. [DOI] [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. E., Jr, Baldwin R. L. Enzymatic studies of pure cultures of rumen microorganisms. J Bacteriol. 1966 Nov;92(5):1321–1330. doi: 10.1128/jb.92.5.1321-1330.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIESOW L. ON THE ASSIMILATION OF ENERGY FROM INORGANIC SOURCES IN AUTOTROPHIC FORMS OF LIFE. Proc Natl Acad Sci U S A. 1964 Oct;52:980–988. doi: 10.1073/pnas.52.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Influence of amino acids and organic antimetabolites on growth and biosynthesis of the chemoautotroph Thiobacillus neapolitanus strain C. Arch Mikrobiol. 1967 Feb 20;56(2):91–105. doi: 10.1007/BF00408761. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Problems of the autotrophic micro-organisms. Sci Prog. 1967 Spring;55(217):35–51. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J., Rittenberg S. C. Effects of organic matter on the growth of Thiobacillus intermedius. J Bacteriol. 1966 Mar;91(3):1062–1069. doi: 10.1128/jb.91.3.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAVOLTA E., DELWICHE C. C., BURGE W. D. Formate odixation by cell-free preparations from Nitrobacter agilis. Biochim Biophys Acta. 1962 Feb 26;57:347–351. doi: 10.1016/0006-3002(62)91129-0. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SCHOEN G. UNTERSUCHUNGEN UEBER DEN NUTZEFFEKT VON NITROBACTER WINOGRADSKYI BUCH. Arch Mikrobiol. 1965 Jan 22;50:111–132. [PubMed] [Google Scholar]
- Sadler W. R., Stanier R. Y. THE FUNCTION OF ACETATE IN PHOTOSYNTHESIS BY GREEN BACTERIA. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1328–1334. doi: 10.1073/pnas.46.10.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBBACK P., LAUDELOUT H. POLY-BETA-HYDROXYBUTYRIC ACID IN NITROBACTER. Biochim Biophys Acta. 1965 Mar 8;97:589–590. doi: 10.1016/0304-4165(65)90173-x. [DOI] [PubMed] [Google Scholar]
- Van Gool A., Laudelout H. Formate utilization by Nitrobacter winogradskyi. Biochim Biophys Acta. 1966 Oct 31;127(2):295–301. doi: 10.1016/0304-4165(66)90384-9. [DOI] [PubMed] [Google Scholar]


