Abstract
We have proposed that reduced activity of inosine-5′-monophosphate dehydrogenase (IMPD; IMP:NAD oxidoreductase, EC 1.2.1.14), the rate-limiting enzyme for guanine nucleotide biosynthesis, in response to wild-type p53 expression, is essential for p53-dependent growth suppression. A gene transfer strategy was used to demonstrate that under physiological conditions constitutive IMPD expression prevents p53-dependent growth suppression. In these studies, expression of bax and waf1, genes implicated in p53-dependent growth suppression in response to DNA damage, remains elevated in response to p53. These findings indicate that under physiological conditions IMPD is a rate-determining factor for p53-dependent growth regulation. In addition, they suggest that the impd gene may be epistatic to bax and waf1 in growth suppression. Because of the role of IMPD in the production and balance of GTP and ATP, essential nucleotides for signal transduction, these results suggest that p53 controls cell division signals by regulating purine ribonucleotide metabolism.
INTRODUCTION
Beyond being the basic units of nucleic acids, nucleotides are ubiquitous molecular regulators. Nucleotides modulate diverse cellular processes by noncovalent allosteric regulation; covalent chemical activation via cleavage of high-energy phosphate bonds; covalent transfer of modulatory phosphate moieties; and providing the high energy output of phosphate bond cleavage for macromolecular conformational changes (Lehninger, 1975). Although many of the processes controlled by nucleotides are specific to their own biosynthesis and metabolism, they regulate a variety of other cellular processes as well (Lehninger, 1973; Criss and Pradhan, 1976; Pall, 1985).
Adenine and guanine ribonucleotides are the most frequently used nucleotides for molecular regulation, functioning to modulate important biochemical reactions in all aspects of cell function (Pall, 1985). Our interest in ATP and GTP as molecular regulators relates to their function as essential components of growth signal transduction pathways. Interestingly, in cellular signaling, ATP-dependent and GTP-dependent mechanisms appear to be restricted to one of two distinct phosphate cleavage reactions. ATP mechanisms employ covalent phosphoryltransfer (reviewed in Sibley et al., 1987; Aaronson, 1991; Cantley et al., 1991; Hunter, 1995), whereas GTP mechanisms use phosphate hydrolysis (reviewed in Reed, 1990; Simon et al., 1991; Hall, 1992; Chant and Stowers, 1995). Phosphoryltransfer modifies the catalytic or association properties of signaling molecules, whereas the energy of phosphate hydrolysis fuels structural rearrangements that lead to activation of GTP-bound proteins. Effectors of the balance of these distinct regulatory mechanisms may be important factors for integrated regulation of cellular growth signals.
Given the importance of ATP and GTP in signal transduction, their metabolism may be an important factor for cellular signaling (Pall and Robertson, 1988; Sherley, 1991). However, effects of nucleotide metabolism on cellular signaling have received relatively little attention (Franklin and Twose, 1977; Kharbanda et al., 1990; Rizzo et al., 1990). Certainly the concentration of GTP and ATP must be maintained above some critical level for normal cell signaling, and control of the GTP:ATP ratio may be an important mechanism for integrated signaling regulation. Thus, an important level of signaling control may be mechanisms that regulate adenine and guanine ribonucleotide concentrations.
Recently, we discovered an association between guanine ribonucleotide biosynthesis and cell growth regulation in response to expression of the wild-type p53 gene (Sherley, 1991; Sherley et al., 1995a; Liu et al., unpublished observations). The p53 gene is well known for its role in normal cell growth regulation in diverse mammalian tissues (reviewed in Levine and Momand, 1990; Donehower and Bradley, 1993; Gottlieb and Oren, 1996). Mutations that destroy wild-type p53 function are the most commonly observed genetic defect in diverse human tumors (Hollstein et al., 1991). Using cells that conditionally express physiological quantities of wild-type p53 protein, we showed that p53 expression causes growth suppression associated with a reduction in guanine ribonucleotide biosynthesis (Sherley, 1991). This biosynthetic defect was attributed to a p53-dependent reduction in the levels of inosine-5′-monophosphate dehydrogenase (IMPD; IMP:NAD oxidoreductase, EC1.2.1.14), the rate-limiting enzyme for guanine nucleotide biosynthesis (Sherley, 1991; Liu et al., unpublished data).
Although there are a number of hypotheses for the significance of p53 mutations in carcinogenesis, the normal cellular function of the protein remains an enigma (Vogelstein and Kinzler, 1992; Kinzler and Vogelstein, 1996; Ko and Prives, 1996). We have suggested that under physiological conditions p53 functions primarily to regulate IMPD activity by controlling the expression of IMPD mRNA (Sherley, 1991; Sherley, 1996; Liu et al., unpublished observations). As an essential, rate-limiting, branchpoint enzyme in the pathway for purine nucleotide biosynthesis, IMPD is a major regulator of the production and balance of GTP and ATP (Crabtree and Henderson, 1971; Lehninger, 1975). Reductions in its activity lead to decreased GTP concentration, a decreased GTP:ATP ratio, and a decreased GTP:GDP ratio (Lui et al. 1984; Jayaram et al., 1993). We have provided evidence that the first two alterations also occur in response to wild-type p53 expression and can account for p53-dependent growth suppression (Sherley, 1991; Sherley et al., 1995a). By analogy, p53 may regulate the cellular GTP:GDP ratio as well.
Previous studies indicate that beyond its role in the biosynthesis of nucleic acids, IMPD provides a regulatory function for cell growth (Cohen et al., 1981; Cohen and Sadée, 1983; Rizzo et al., 1990). IMPD expression is elevated in proliferative states like malignancy (Jackson et al., 1975; Jackson et al., 1976; Proffitt et al., 1983; Konno et al., 1991; Collart et al., 1992; Weber et al., 1992) and decreased during cell differentiation (Nagai et al., 1992). Consistent with the view that these associations reflect a regulatory function for IMPD in cell division control, inhibition of the enzyme with specific inhibitors causes growth arrest (Cohen et al., 1981; Cohen and Sadée, 1983; Lui, 1984; Lee et al., 1985; Turka et al., 1991) and differentiation (Kiguchi et al., 1990).
In previous studies, IMPD was predicted to play a unique role in the regulation of DNA replication by controlling guanine ribonucleotide pools (Cohen et al., 1981; Cohen and Sadée, 1983). We and others have shown that IMPD is distinct from other nucleic acid precursor synthesis enzymes with respect to its expression during the cell cycle (Szekeres et al., 1992) and its response to growth stimuli like serum (Stadler et al., 1994). Whereas nucleotide synthesis enzymes typically show greatly reduced activity in nonreplicative phases of the cell cycle and in response to growth factor withdrawal (Hochhauser et al., 1981), IMPD activity is maintained at near constant levels (Stadler et al., 1994; Liu et al., in preparation). We have proposed that this expression pattern reflects a special role for the enzyme in molecular processes that control the division potential of cells (Stadler et al., 1994; Liu et al., in preparation).
Previously, we have reported evidence that IMPD activity is a critical determinant of p53-dependent growth regulation. Specifically, we demonstrated that IMPD mRNA, protein, and activity are reduced in response to wild-type p53 protein expression (Sherley, 1991; Liu et al., unpublished data) and that nucleoside precursors that promote the formation of guanine ribonucleotides in the absence of IMPD function are able to prevent growth suppression by p53 (Sherley, 1991; Sherley et al., 1995a). In this report, we detail a direct test of this hypothesis by impd gene transfer. We demonstrate that transfection of a constitutively expressed IMPD cDNA abrogates p53-dependent growth suppression. The impd-transfected cells produced in this study are p53-resistant, exhibiting high rates of growth despite expressing growth-suppressive levels of wild-type p53 protein. This result solidifies our previous proposal that modest decreases in IMPD activity in response to p53 expression have profound consequences for cell growth (Sherley, 1991; Sherley et al., 1995a; Sherley, 1996; Liu et al., unpublished data).
These studies also shed light on the relationship between IMPD and other p53-responsive genes previously proposed as mediators of p53-dependent growth effects. In a previous study (Liu et al., unpublished data), we have shown that high level, p53-induced expression of bax and waf1, genes implicated as mediators of p53-dependent apoptosis and cell cycle arrest in response to DNA damage, respectively (El-Deiry et al., 1993; Miyashita et al., 1994; Selvakumaran et al., 1994; Deng et al., 1995), can occur in the absence of growth suppression. Similarly, the expression of these genes remains high in response to p53 expression in p53-resistant impd transfectants derived in the present study. Thus, the results presented herein not only confirm IMPD as a rate-determining mediator of p53-dependent growth regulation, but also indicate that if bax and waf1 function as p53 mediators in the absence of DNA damage, then IMPD activity prevents their growth suppression activity. Given the role of IMPD in adenine and guanine ribonucleotide metabolism, we postulate that p53-dependent growth regulation reflects the ability of p53 to control the level and/or balance of purine ribonucleotides engaged in integrated molecular regulation of cell growth signals.
MATERIALS AND METHODS
Materials
[α-32P]dCTP was purchased from ICN Biomedicals (Costa Mesa, CA). [125I]-rProtein A was supplied by New England Nuclear (Boston, MA). ZnCl2 and mycophenolic acid (MPA) were purchased from Sigma Chemical Company (St. Louis, MO).
Cell Culture
Zinc-dependent, p53-inducible Ind-4 and Ind-8 cells and control Con-2 and Con-3 cells were maintained as subconfluent monolayers in DMEM, supplemented with 10% dialyzed fetal bovine serum (JRH Biosciences, Lenexa, KS) and 5 μg/ml puromycin (Sigma), at 37°C in a humidified incubator with 5% CO2. The details of the derivation of these specialized lines are described elsewhere (Liu et al., unpublished observations). For routine maintenance, all cells were grown in 20 ml of culture medium per 75-cm2 culture flask area. Vector transfectant derivatives were maintained in the same manner, except culture medium contained 0.5 mg/ml G418-sulfate (Life Technologies, Gaithersburg, MD). Impd transfectant derivatives were maintained in the same manner as vector transfectants, except culture medium was supplemented with 45 μM ZnCl2. Before the initiation of experiments comparing zinc-free conditions to higher zinc levels, impd transfectants were cultured for 3 d in zinc-free medium.
In MPA resistance experiments, DMSO (Sigma) used as a carrier for the inhibitor was present in the culture medium at a final concentration of 0.017% (vol/vol). No changes were noted in the growth of control MPA-free cultures (as described in Figure 4) that were grown with the same concentration of DMSO.
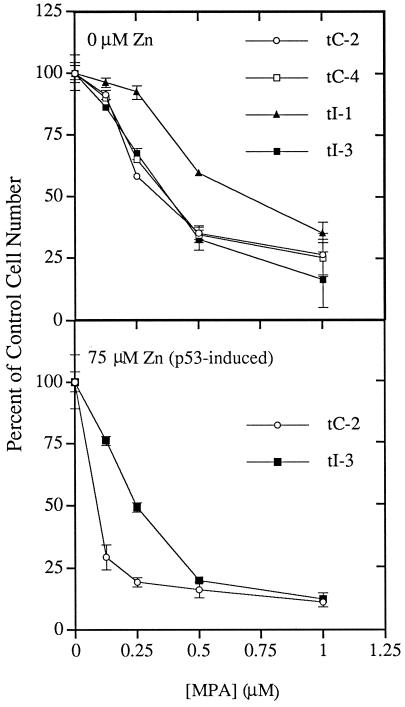
Figure 4.
p53-inducible, impd transfectants exhibit increased resistance to MPA, a potent inhibitor of IMPD. The indicated control, p53-inducible, vector transfectant (tC-2, tC-4) and impd transfectant (tI-1, tI-3) cells were grown under standard conditions in control medium (0 μM Zn; noninducing condition) or medium containing 75 μM Zn (p53-inducing condition), to which were added increasing amounts of the IMPD inhibitor MPA. The means of triplicate cell count data after 2 d of growth are plotted as a percentage of the mean value for growth in the absence of the drug. Error bars denote the SD of the data.
Derivation of Zinc-dependent, p53-inducible impd Transfectant Cells
Amplified plasmids for transfection were isolated by Qiagen column fractionation as specified by the supplier and further purified by CsCl equilibrium density gradient centrifugation. Five micrograms of either plasmid pCH2–5 (Collart and Huberman, 1988), which encodes the Chinese hamster IMPD cDNA under control of the simian virus-40 promoter in vector pcD-X (Okayama and Berg, 1983), or a derivative of pCH2–5 deleted for the entire IMPD cDNA sequence were cotransfected by the CaPO4 procedure as previously described (Sherley, 1991) into 1 × 106 Ind-8 cells with 2 μg of a plasmid conferring resistance to the antibiotic G418-sulfate (pSLneo; Sherley, 1991). The deletion derivative of pCH2–5 was prepared by digestion with BamHI followed by gel purification and circularization of the vector fragment.
At the time of selection in both 0.5 mg/ml G418-sulfate and 5 μg/ml puromycin, the transfected cells were replated at 1/10 density in parallel in selective medium or selective medium containing 40 μM ZnCl2 to induce p53-expression (e.g., see Figure 1). Impd transfectant cell lines were derived from ring-cloned resistant colonies that arose from pCH2–5 cotransfections under p53-inducing conditions (40 μM ZnCl2). These colonies were expanded in selective medium containing 40 μM ZnCl2, but thereafter maintained in selective medium containing 45 μM ZnCl2. Vector transfectants were derived with colonies from cotransfections in zinc-free medium with the derivative of pCH2–5 that contained a deletion of IMPD sequences. Multiple colonies of each type were expanded, cryo-preserved in liquid nitrogen at first passage, and tested for Mycoplasma. All cell clones tested negative. Thereafter, all experiments were performed with cells maintained in culture for less than 50 population doublings.
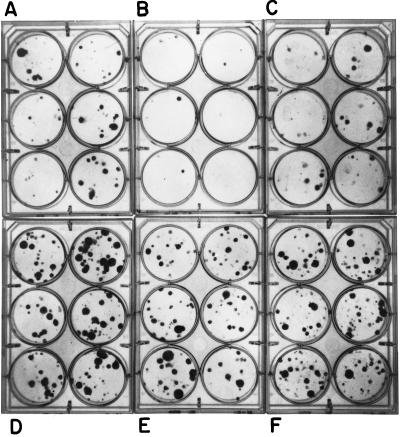
Figure 1.
IMPD gene transfer prevents p53-dependent growth suppression. Puromycin-resistant, zinc-dependent, p53-inducible cells (A–C, line Ind-4) and p53-null control cells (D-F, line Con-2) were cotransfected with 2 μg of a plasmid conferring resistance to neomycin and either 5 μg of a plasmid containing a hamster IMPD cDNA driven by the simian virus 40 early promoter (C and F) or 5 μg of a derivative plasmid deleted for the IMPD sequences (A, B, D, and E). Two days after transfection, the cells were trypsinized and replated at 1:10 their initial density into 6-well culture plates containing selective medium (i. e., growth medium containing both puromycin and neomycin; A and D) or selective medium containing 40 μM ZnCl2 to induce p53 expression (B, C, E, and F). Ten days later cell colonies were stained with crystal violet and photographed. Although not shown here, in the absence of ZnCl2, IMPD gene transfection did not significantly alter the frequency of resistant colony formation (see Figure 2). (A) p53-inducible line vector transfection, 0 Zn (low p53). (B) p53-inducible line vector transfection + Zn (p53 induced). (C) p53-inducible line IMPD transfection + Zn (p53 induced). (D) p53-null line vector transfection, 0 Zn (no p53). (E) p53-null line vector transfection + Zn (no p53). (F) p53-null line IMPD transfection + Zn (no p53).
Cell Growth Analyses
To initiate growth curve analyses, cells were grown over a 3-d period to about one-half confluency, trypsinized, and replated in zinc-free medium (i.e., DMEM supplemented with 10% dialyzed fetal bovine serum) at a cell:plating area:medium volume ratio of 1 × 105:25 cm2:5 ml. This ratio was held constant for all experiments, unless specified otherwise. Sixteen to 24 h later, the culture medium was replaced with the same volume of zinc-free medium or medium containing the specified concentration of ZnCl2. This time was designated as 0 h in the analyses. At subsequent times, cells were harvested by trypsinization from replicate flasks and counted with a Model ZM Coulter Counter. Doubling times were determined as previously described (Sherley et al., 1995a,b).
Northern Blot Analyses
The preparation of nucleic acid probes for the detection of iMPD, mdm2, bax, waf1, and L32 mRNAs in Northern blot analyses has been described elsewhere (Liu et al., unpublished observations). Northern blot analyses were performed using 5 μg of total cytoplasmic RNA essentially as described (Sambrook et al., 1989). Blots were exposed to preflashed radiographic film with a DuPont Cronex Lightning Plus enhancement screen at −80°C. For reprobing, blots were stripped by treatment with a Tris-buffered (50 mM, pH 8.0) 50% vol/vol solution of formamide at 72°C for 45 min.
Western Blot Analyses
Soluble Nonidet P-40 cell extracts were made as previously described (Stadler et al., 1994). Extract protein concentration was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). For analysis, 40 μg of extract protein for each examined sample were separated by SDS-PAGE in 10% polyacrylamide gels. Immunoblotting and IMPD protein detection were performed as previously described (Sherley and Kelly, 1988) by use of an affinity-purified IgG fraction of anti-hamster IMPD antiserum generously provided by Dr. F. Collart (Argonne National Laboratory, Argonne, IL; Collart and Huberman, 1988).
Densitometry
Autoradiograph band densities were quantified with an UltroScan XL scanning laser densitometer (Pharmacia LKB, Uppsala, Sweden). To ensure that all measurements were within a linear range, multiple exposures of varied time were compared for each gel, and only autoradiographic bands determined to be within the linear range of film response for a given exposure time were included in the analyses.
RESULTS
Gene Transfer Analysis of IMPD in p53-Dependent Growth Suppression
Recently, we have described murine fibroblast cell lines that conditionally express wild-type murine p53 at physiological levels in response to zinc (Ind-4, Ind-8; Liu et al., unpublished data). Upon p53 induction, these lines exhibit a reversible, partial cell cycle arrest in early S phase, without significant cell death or detectable apoptosis. In addition to the zinc-dependent p53-inducible lines, control lines for these studies were derived with zinc-inducible plasmid constructs deleted for p53 coding sequences (Con-2, Con-3). Using this series of cell lines, in addition to a temperature-dependent series of p53-inducible cells, we showed that IMPD activity, protein and mRNA are consistently reduced in response to p53 expression (Liu et al., unpublished data).
In earlier work, we performed experiments that suggested that reduced IMPD activity is the cause of p53-dependent growth suppression in p53-inducible cells (Sherley, 1991; Sherley et al., 1995a). Specifically, we showed that precursors that promote formation of guanine ribonucleotides in the absence of normal IMPD function prevent p53-dependent growth suppression. As a direct test of the hypothesis that reduced IMPD activity is required for p53-dependent growth suppression, we used gene transfer experiments to evaluate the ability of a constitutively expressed IMPD to prevent p53-dependent colony formation suppression in the zinc-dependent cell lines.
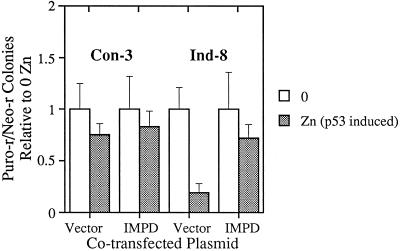
As shown in Figures 1 and 2, in colony formation assays p53-dependent growth suppression was prevented in two different zinc-dependent lines by transfection of a hamster IMPD cDNA expression plasmid. Quantification of four independent experiments performed in triplicate for the Ind-8 line indicated that IMPD transfection could effectively prevent p53-dependent colony formation suppression (Figure 2). Transfections with control cells demonstrated that the positive growth effect conferred by the hamster IMPD expression plasmid is only observed in cells undergoing p53-dependent growth suppression (Figure 1, compare E and F to B and C). Thus, the prevention of growth suppression by IMPD is not due to general growth stimulation; and in fact, transfection of higher amounts of the IMPD expression plasmid than used in these experiments resulted in general growth suppression (our unpublished observations).
Figure 2.
Quantitative analysis of IMPD gene transfer experiments. Puromycin-resistant, zinc-dependent, p53-inducible cells (Ind-8) and p53-null control cells (Con-3) were cotransfected with 2 μg of a plasmid conferring resistance to neomycin and either 5 μg of a plasmid containing a hamster IMPD cDNA driven by the simian virus-40 early promoter (IMPD) or 5 μg of a derivative plasmid deleted for the IMPD sequences (Vector). Two days after transfection, the cells were trypsinized and replated at 1:10 their initial density in selective medium (i.e., growth medium containing both puromycin and neomycin (0, open bars) or selective medium containing 40 μM ZnCl2 to induce p53 expression (Zn, closed bars). Ten days later, resistant cell colonies were stained with crystal violet and counted. For each pairwise comparison, the data were normalized to the number of colonies detected under noninducing conditions (i.e., 0 μM ZnCl2). The averaged colony formation data from four independent transfection experiments, each plated in triplicate, are graphed. Error bars denote the SD of the data.
To ensure that increased IMPD expression resulted in all IMPD transfections, we examined IMPD protein levels by immunoblot analysis of extracts from pooled transfected colonies. Compared with pooled vector transfectants (i.e., cells transfected with the expression plasmid deleted for IMPD coding sequences), pooled impd transfectants expressed 50% more IMPD protein on average (1.5 ± 0.16-fold increase; n = 4). This was true of transfected control cells (Con-2, Con-3; 1.5-fold and 1.5-fold, respectively) as well as of p53-inducible cells (Ind-4, Ind-8; 1.3-fold and 1.7-fold, respectively). This result confirmed that the failure of the IMPD plasmid to stimulate growth in control cells was not due to a smaller increase in IMPD protein, further highlighting the p53-specific nature of growth activation by IMPD.
Derivation and Characterization of Clonal p53-Resistant impd Transfectants
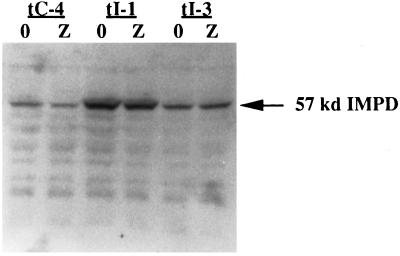
To investigate further properties of impd-transfected, p53-inducible cells, we expanded colonies derived from Ind-8 cells transfected with the hamster IMPD cDNA and selected under p53-inducing conditions (45 μM ZnCl2; Lines tI-1, tI-3, tI-5). To identify effects specific for impd transfection, we also derived control cell clones from Ind-8 cells transfected with vector DNA and selected in the absence of zinc (tC-2 and tC-4). The impd transfectants expressed a new 2.8-kb mRNA detected by Northern blot analysis with an IMPD-specific nucleic acid probe (see Figure 6, “trans”). The larger size of this mRNA is thought to arise from altered processing of the hamster IMPD message because of its construction with heterologous expression elements. Immunoblot analyses of protein extracts for impd transfectants indicate increased IMPD expression both in the presence and absence of zinc (Figure 3). In some impd transfectants the level of IMPD protein decreases upon p53 induction (Figure 3, tI-1) as in control vector transfectants (Figure 3, tC-4). However, for all examined impd transfectants, IMPD protein maintains a level greater than that of vector transfectants under noninducing conditions (tI-1, 270% more; tI-3, 20% more).
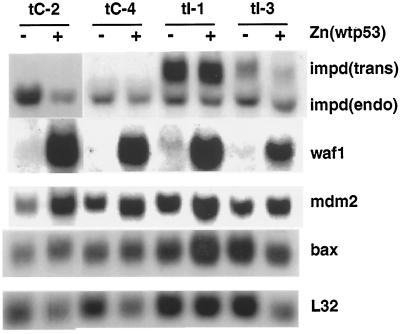
Figure 6.
p53-responsive gene expression is intact in p53-inducible impd transfectants. Five micrograms of total cytoplasmic RNA isolated from control, p53-inducible, vector transfectant (tC-2, tC-4), and impd transfectant (tI-1, tI-3) cells, grown for 2 d under standard conditions in Zn-free growth medium (−) or medium containing 75 μM ZnCl2 (+; wild-type p53-inducing condition), were examined by Northern blot analysis with sequential hybridization to specific DNA probes to detect the indicated mRNAs as described in MATERIALS AND METHODS. The faster mobility IMPD mRNA derives from the endogenous mouse impd gene (endo), and the slower mobility IMPD mRNA derives from the stably transfected hamster impd cDNA (trans).
Figure 3.
Increased IMPD protein levels in p53-inducible, impd transfectants. Immunoblot analysis of IMPD protein in soluble extracts prepared from p53-inducible, vector transfectant (tC-4), and impd transfectant (tI-1, tI-3) cells after 2 d of growth under either inducing (Z, 75 μM ZnCl2) or noninducing conditions (0, 0 μM ZnCl2). Forty micrograms of total protein from each extract sample was analyzed as described in MATERIALS AND METHODS with an anti-IMPD antiserum that recognizes both mouse and hamster IMPD proteins. Arrow, the migration position of mouse and hamster IMPD protein.
To evaluate whether increased IMPD protein in impd transfectants conferred increased IMPD activity, we examined their resistance to mycophenolic acid (MPA), a potent and specific inhibitor of the enzyme (Lee et al., 1985; Allison et al., 1993). This method of evaluation has the advantage over cell extract assays of providing an in vivo measure of IMPD function. Previous workers have shown that increased cellular IMPD activity confers resistance to growth arrest by MPA (Ullman, 1983; Collart and Huberman, 1987; Hodges et al., 1989; Lightfoot and Snyder, 1994). Consistent with their increased expression of IMPD protein, impd transfectants show greater resistance to MPA than control vector transfectants (Figure 4). Line tI-1, which exhibits the higher level of IMPD protein expression (compare Figure 3), exhibits greater resistance in the absence of p53 induction (Figure 4, 0 μM zinc). For line tI-3, which exhibits only a modest increase in IMPD protein, increased resistance is only manifest upon p53 induction (Figure 4, compare closed squares in 0 μM zinc and 75 μM zinc panels). This finding is entirely consistent with the fact that the greatest difference in IMPD expression noted between line tI-3 and control vector transfectants is under p53-inducing conditions when the control cells show increased sensitivity to MPA, a predicted consequence of p53-dependent IMPD repression (Figure 4, compare open circles in 0 μM zinc versus 75 μM zinc).
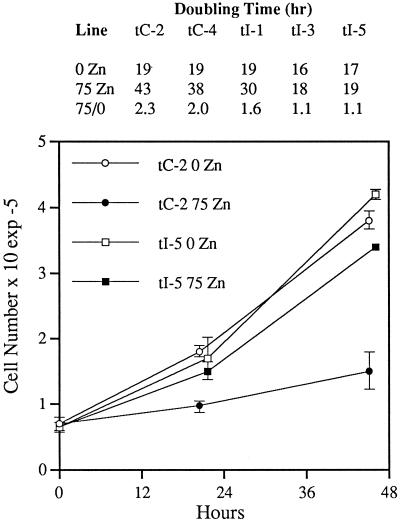
As a final evaluation of the effect of constitutive IMPD expression on p53-dependent growth suppression, we compared the growth response of clonally derived impd transfectants and control vector transfectants to p53 induction. As shown in Figure 5, whereas vector transfectant cells retain the property of p53-dependent growth suppression in the presence of zinc, all examined impd transfectant cell clones show a decreased response, with two clones (tI-3 and tI-5) exhibiting complete loss of zinc-dependent growth suppression. In contrast, under noninducing conditions, the doubling time of impd transfectants is not significantly different than that of control vector transfectants (see Figure 5, inset table), even though under this condition the total level of cellular IMPD in some impd transfectants is maximal owing to greater expression of endogenous IMPD in the absence of p53 induction (Figure 3, compare lane tI1–0 to tI1-zinc). Like the observation described earlier, that iMPD gene transfer did not enhance colony formation by p53-null control cells, this result further illustrates the point that the growth-enhancing effect of constitutive IMPD expression is restricted to cells undergoing p53-dependent growth suppression.
Figure 5.
p53-inducible, impd transfectants are resistant to p53-dependent growth suppression. The indicated control p53-inducible, vector transfectant (tC-2, tC-4) and impd transfectant (tI-1, tI-3, tI-5) cell lines were grown for 2 d under standard conditions in normal culture medium (0 Zn) or medium containing 75 μM ZnCl2 (75 Zn) to induce wild-type p53 expression. The graph shows representative growth data plotted as the mean of triplicate cell counts. Error bars denote the SD of the data. Growth data were fit to the ideal exponential growth equation to determine the doubling times shown in the inset table. 75/0, the ratio of the doubling time of p53-induced (75 Zn) cells to that of noninduced cells (0 Zn).
Expression of p53-Responsive Genes in p53-Resistant, impd Transfectants
Despite the loss of p53-dependent growth suppression, the impd transfectants still show zinc-dependent expression of p53 protein (our unpublished results) with wild-type gene activation function. The wild-type function of the induced p53 protein was confirmed by examination of the expression of mRNAs for three genes, waf-1, bax, and mdm-2, which are known to be induced by wild-type p53, but not by mutant forms of the protein (Barak et al., 1993; El-Deiry et al., 1993; Miyashita et al., 1994; Selvakumaran et al., 1994; Miyashita and Reed, 1995). All three mRNAs show similar level of zinc-dependent induction in impd transfectants as observed in the original untransfected p53-inducible cells (see Liu et al., unpublished data) and control vector transfected cells (Figure 6). The apparent reduction in bax mRNA expression for line tI-3 in Figure 6 is due to an underloaded gel lane, as indicated by the reduced signal for control L32 mRNA. After normalization to the reduced level of L32 mRNA, bax expression in line tI-3 is found to be induced 1.6-fold in response to zinc-induced p53 expression. The retention of the expression response of waf-1, bax, and mdm-2 confirms that conditional wild-type p53 function is intact in impd transfectants. Thus, this evaluation indicates that impd transfectants are “p53 resistant,” actively dividing despite expressing typically growth-suppressive levels of wild-type p53 protein.
DISCUSSION
Gene Transfer as a Tool to Investigate p53-Dependent Growth Mechanisms
There have been many previous reports of genes that are able to overcome p53-induced growth suppression effects in gene transfer experiments. These include genes for the simian virus-40 T-antigen (Michael-Michalovitz, 1991; Segawa et al., 1993; Quartin, et al., 1994), human papilloma virus E6 (Kessis et al., 1993; Spitkovsky et al., 1996) and E7 (Vousden et al., 1993), adenovirus E1A (Lin et al., 1995) and E1B (Shen and Shenk, 1994), mdm-2 (Finlay, 1993; Chen et al., 1994), activated H-ras (Lin et al., 1995), c-myc (Hermeking and Eick, 1994), bcl-2 (Chiou et al., 1994; Shen and Shenk, 1994; Guillouf et al., 1995; Wang et al., 1995), cdk4 and cdk6 (Latham et al., 1996), B-myb (Lin et al., 1994), MAP4 (Murphy et al., 1996). Some of these gene products (T-antigen, E6, E1B, and mdm-2) interfere with p53 function by direct interaction; and all affect cell growth by p53-independent pathways as well. This is an important distinction from our studies. The fact that previously studied p53-modulating genes also exhibit p53-independent growth effects confounds their assignments to p53-specific mechanisms of growth suppression. Finally, the issue of the physiological relevance of results obtained with supranormal expression of transfected genes is a concern for previous gene transfer experiments.
Our studies with IMPD avoid many of the problems encountered in previous gene transfer studies of p53 function. First, the fact that impd transfectants retain intact wild-type p53 transactivation activity, as measured by the induction of three different p53-responsive genes, suggests that IMPD does not interfere with p53 function directly. Second, the transfected impd minigene does not confer general growth stimulation. Although it enhances the growth of cells undergoing p53-dependent growth suppression, it has no detected growth effects on exponentially dividing control cells. This relationship strongly supports the interpretation that constitutive IMPD expression prevents p53-dependent growth suppression by intercession in p53-specific growth control mechanisms, and not by independent growth activation. Third, in fact, the amount of constitutive IMPD expression necessary for the production of p53-resistant cells approximates normal basal IMPD levels in noninduced cells (in Figure 3 compare tC-4, 0 to tI-3, zinc). This modest requirement underscores our original proposal that small changes in IMPD activity in response to physiological variation in wild-type p53 expression have profound effects on cell division (Sherley, 1991; Sherley et al., 1995a,b; Liu et al., unpublished observations).
p53, IMPD, and Purine Ribonucleotide Metabolism
Our studies (Sherley, 1991) are the first to suggest a relationship between p53-dependent growth suppression and nucleotide metabolism. Subsequently, other reports have appeared in which specific inhibitors of nucleotide metabolism were used to evaluate the function of the p53-dependent G1 checkpoint with respect to gene amplification or nucleotide pool perturbations. Two studies have shown that the p53-dependent checkpoint can function to prevent amplification of specific drug resistance genes (Livingstone et al., 1992; Yin et al., 1992). A third study found that whereas inhibitors that deplete deoxyribonucleotide pools cause an S-phase arrest independent of p53 status, inhibitors that deplete ribonucleotides cause a G1 arrest in cells with wild-type p53 function, but an S-phase arrest in p53 null cells (Linke et al., 1996). Conversely to our hypothesis, the authors of this study suggested that their results may indicate the ability of p53 to function as a sensor of ribonucleotide pool perturbations for the purpose of signaling a quiescent G1 arrest under conditions of poor nutrients. Because of the significant differences between the experimental strategies used, it is difficult to discern a relationship between our results of those of previous studies.
Beyond its role in controlling guanine nucleotide production, IMPD is an important enzyme for the synthesis of bioenergetic and regulatory adenine nucleotides, as GTP is required for de novo ATP biosynthesis (Lehninger, 1975). This relationship positions IMPD as one of two essential enzymatic regulators (succinyl-AMP synthetase being the other) of the GTP:ATP balance in cells. This balance may be a critical integration factor that links the regulation of different types of cell signals whose transduction is based on either GTP hydrolysis or ATP-dependent phosphotransfer. In support of this hypothesis, the GTP:ATP ratio across diverse cell types shows an extraordinarily small degree of variation (0.24 ± 0.11 for 14 independent determinations from 11 individual reports; Nelson et al., 1976; Jackson et al., 1977; Cohen et al., 1981; Cohen and Sadee, 1983; Gruber et al., 1985; Lee et al., 1985; Hodges et al., 1989; Glesne et al., 1991; Zhen et al., 1992; Balzarini et al., 1993; Jayaram et al., 1993).
In addition to its role in maintaining GTP levels and GTP:ATP balance, there is evidence that alterations in IMPD expression also affect the cellular GTP:GDP ratio (Lui, 1984; Jayaram et al., 1993). This guanine nucleotide ratio is believed to be a critical factor for proper functioning of a variety of signal transduction G proteins like the proto-oncogene ras. When GTP-bound, G proteins are competent for signal transduction. Hydrolysis of the γ phosphate of GTP renders them GDP bound and inactive for signaling. This signaling mechanism is thought to be insensitive to changes in overall cellular guanine ribonucleotide concentration, because the dissociation constants for GTP and GDP are many orders of magnitude less than estimates of cellular GTP and GDP concentration (Gibbs and Marshall, 1989; Bourne et al., 1990, 1991). In a number of studies, it has been shown that highly specific inhibitors of IMPD produce decreases in the GTP:GDP ratio that are sufficient to compromise the signaling activity of particular G proteins (Franklin and Twose, 1977; Kharbanda et al., 1990; Rizzo et al., 1990). In our studies, the reduction in guanine nucleotide synthesis observed in response to wild-type p53 expression is of similar magnitude (Sherley, 1991), and thus may produce similar signaling effects. Of course formally, we must consider that presently any of the guanine ribonucleotides downstream of the IMPD reaction [i.e., including xanthosine-5′-monophosphate (XMP), GMP, and cyclic GMP] is a viable candidate for the predicted critical nucleotide(s) whose concentration mediates p53-dependent growth suppression.
Relationship Between IMPD and Other p53-Responsive Genes
The finding that p53-resistant, impd transfectants retain p53-induced bax and waf1 expression is intriguing, as these two genes have been proposed as essential p53 targets that mediate p53-dependent growth suppression (El-Deiry et al., 1993; Miyashita et al., 1994; Selvakumaran et al., 1994; Deng et al., 1995). However, the studies leading to this conclusion were performed under conditions of DNA damage, growth factor withdrawal, or oncogene activation, which lead to apoptosis and cell death. In contrast, our studies are performed under normal cell culture conditions to minimize physiological perturbations (Sherley, 1991; Liu et al., unpublished observations). Given this difference, one possible explanation for the absence of growth suppression in response to waf1 and bax induction in impd transfectants is that the two proteins may not function to arrest cell growth in the absence of cellular damage. Consistent with this possibility, there is a striking absence of apoptosis in our studies (Sherley, 1991; Sherley et al., 1995a; Lui et al., unpublished data), and although p53-dependent cell cycle arrest is observed, it occurs in S phase (Sherley, 1991; Liu, et al., unpublished data) and not in G1 as described in studies of waf1 and p53-dependent growth suppression.
Studies with cells from mice with a germline disruption of the waf1 gene indicate that the growth suppression function of the waf1 protein is limited to conditions of DNA damage (Brugarolas et al., 1995; Deng et al., 1995). However, although waf1 overexpression has been shown independently to cause cell cycle arrest (El-Deiry et al., 1993; Chen et al., 1996), existing evidence does not support the hypothesis that it is rate-determining for p53-dependent growth suppression (Attardi et al., 1996). Studies of bax function in transgenic mice indicate that although it is a clear effector of cellular apoptosis (Oltvai et al., 1993; Knudson et al., 1995), it is not the rate-determining factor for p53-dependent apoptosis (Brady et al., 1996).
If bax and waf1 are active in growth suppression under the conditions of our studies, then these results demonstrate that their function requires a p53-dependent reduction in IMPD activity. A better understanding of the relationship between IMPD, bax, and waf1 will require more in-depth analyses of the expression (i.e., protein expression) and function of these proteins in p53-resistant impd transfectants. Although we are not aware of any reports of discordant expression of either bax or waf1 mRNA and protein, we have not yet ruled out the possibility that bax protein is not expressed in impd transfectants. In the case of waf1, in preliminary analyses, we have shown that both the expression of the protein and its presence in CDK-dependent kinase complexes are not significantly altered in impd transfectant cells (our unpublished results).
Our studies provide three examples of elevated bax and waf1 expression without detectable growth suppression in two different cell types (Liu et al., unpublished data; this report). However, in only one of these three examples is there direct experimental evidence that IMPD is responsible. Thus, at this stage of these studies some caution is warranted in the general conclusion that IMPD is epistatic to bax and waf1 under physiological conditions. Formally, it has not been established that the waf1 and bax proteins expressed in the fibroblasts used in this study are competent to induce cell cycle arrest and apoptosis, respectively. Of course, such reasoning leads to a conundrum, given the premise that p53-dependent growth suppression requires waf1 and/or bax induction. The question of the generality of this newly defined relationship among IMPD, waf1, and bax will be resolved when similar data are available from varied cell types.
Candidates for Sensors of p53/IMPD-Dependent Changes in Guanine Nucleotide Metabolism
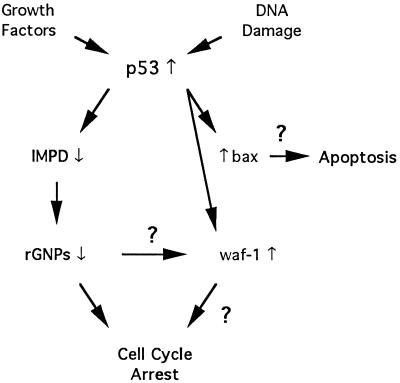
IMPD might overcome growth suppression effects of waf1 and bax by acting upstream, downstream, or independently of them in their respective genetic pathways for cell cycle arrest and apoptosis (see Figure 7). In any event, the finding that this enzyme mediates p53-dependent growth regulation predicts a regulatory role for guanine ribonucleotide metabolism in cell cycle control. Such regulation might occur by either of two mechanisms. First, our observations may reflect integrated regulation of internal cyclin-dependent kinase cell cycle programs and previously described GTP-dependent signal transduction pathways involved in the transduction of external growth stimuli. Second, they may indicate direct regulation by GTP-sensor proteins that monitor p53/IMPD-dependent changes in guanine ribonucleotide metabolism and respond by modulating internal signaling programs for cell division.
Figure 7.
Potential relationships between IMPD and putative mediators of p53-dependent growth suppression. The left branch of the flow diagram depicts IMPD in its role as a rate-determining mediator of p53-dependent growth suppression in the absence of cellular damage. Reduced IMPD activity in response to p53 expression is obligatory for p53-dependent growth suppression under normal growth conditions. This experimental response is proposed to reflect a physiological response to variation in growth factor levels or other tissue factors in vivo that serves to modulate the levels of a critical guanine ribonucleotide(s) (rGNPs) required for cell cycle progression. These studies call into question the presumed role of bax and waf1 as induced mediators of p53-dependent growth suppression. This apparent conflict may be resolved by a restriction of bax and waf1 function to states of DNA damage or by a dependence of their function on guanine ribonucleotides regulated by IMPD.
Integrated signaling regulation might occur either vertically in the same signaling pathway or horizontally between distinct signaling pathways. An example of vertical signal integration by the GTP:ATP ratio might occur in the path of external growth signals from ATP-dependent receptor tyrosine kinases; to GTP-dependent ras; to ATP-dependent raf and mitogen-activated protein kinases (Hunter, 1995). Horizontal GTP:ATP-dependent signal integration might occur in the recently described link between GTP-dependent ras signaling and cell cycle regulation control by the retinoblastoma gene product, which involves the ATP-dependent cyclin kinases (Peeper et al., 1997). In either scenario, p53-dependent IMPD regulation may limit the transmission of external signals by rapidly decreasing the activation state of G proteins like ras, due to an initial effect on the GTP:GDP ratio, and subsequently modulating signal propagation by ATP-dependent kinases like raf, mitogen-activated protein kinases, or cyclin kinases via secondary effects on ATP production. Such integrated regulation would quickly dampen incoming growth signals while providing a more gradual deceleration of overall signaling activity. This type of regulation would allow for measured, smooth transitions in signal reception while preserving the overall stability of intracellular signaling. Thus, p53 may function as a homeostatic factor for growth signal transduction via its regulation of IMPD and guanine ribonucleotide metabolism.
Another type of integrated signal regulation may occur at the level of individual signaling molecules, i.e., some signaling proteins can use either ATP or GTP as substrate. Because of differences in the chemical structure of the adenine and guanine base, important conformational differences may occur when a signaling protein binds one versus the other nucleotide. Since these structural differences could dictate the outcome of interactions with signaling partners, the GTP:ATP balance may regulate the quality and efficiency of signal transduction in certain pathways. Supporting the hypothesis that single-site, integrated signal regulation by the GTP:ATP ratio occurs, exceptions to the “ATP phosphotransfer-GTP hydrolysis rule” have been described. There are numerous examples of protein kinases that use GTP as substrate as well as ATP (Cochet et al., 1981; Pelech et al., 1987; Putnam-Evans et al., 1990; Stoehr and Smolen, 1990; Yamashita et al., 1992; Hide et al., 1994). Two ras-related GTPases have been described that bind and hydrolyze ATP (Miyazaki et al., 1988; Uritani and Miyazaki, 1988; Onozawa et al., 1995), and for one of them, ras1p of S. pombe, the possibility of ATP serving as an important effector has been discussed (Onozawa et al., 1995). It would be more surprising if signaling enzymes of this type did not exist, and in fact, more than anything else, the ATP phosphotransfer-GTP hydrolysis rule may actually reflect the fact that the nucleotide substrate specificity of many proteins described in cellular signaling has not been investigated.
The second mechanism suggested to account for growth regulation by p53 and IMPD, direct regulation of intracellular cell cycle programs by GTP-sensor proteins, has support from recent studies in this area. Three proteins were recently described with unique properties that make them excellent candidates for the predicted GTP sensors. The first candidates, Rho GTPases, have been implicated in modulating the activity of cyclin kinases that regulate cell cycle progression. In their active GTP-bound state, these proteins are reported to promote the degradation of the cyclin kinase inhibitor p27Kip1, a required event for G1-S transition (Hirai et al., 1997). There is evidence that the activity of the second candidate, the Ran GTPase, is required for the activation of Cdc2/cyclin B complexes for S phase-coupled mitotic progression of Xenopus egg extracts (Clarke et al., 1995). The third candidate GTP-sensor protein is the S. cerevisiae cell cycle control protein CDC6. CDC6 is a rate-determining factor for the initiation of DNA replication (Liang et al., 1995; Cocker et al., 1996) that exhibits ATP/GTPase activity (Zwerschke et al., 1994). Recently, it was found to interact with B-cyclin/Cdc28 complexes that regulate entry to mitosis. This interaction may indicate a mechanism to restrain mitosis until the completion of DNA replication (Elsasser et al., 1996). Evolutionarily conserved homologues of CDC6 have also been described in S. pombe, Xenopus laevis, and humans (Sanders et al., 1997). All three GTP-sensor candidates share the essential feature that a reduction in guanine ribonucleotide metabolism is predicted to lead to failed cell cycle progression because of a decrease in their GTP-dependent function. In particular, CDC6 is an especially attractive candidate, because its substrate specificity qualifies it as a target for single-site, integrated signal regulation by the GTP:ATP ratio as well.
IMPD, p53, and Cancer
Our studies implicate the impd gene as a p53 target whose repression is required for p53-dependent growth suppression. IMPD is an ideal choice to play a pivotal role in the function of a cancer gene like p53 that functions to regulate cell growth in diverse tissues. IMPD is an essential, ubiquitously expressed enzyme that is rate limiting for the biosynthesis of guanine ribonucleotides, which are universal regulators of diverse processes required for normal cell function and growth. Long before our notice of a functional relationship between p53 and IMPD, the enzyme was well known for its regulatory effects in cell growth, differentiation, and cancer.
IMPD has been a target for cancer chemotherapy for the past 30 years (Carter et al., 1969; Robins et al., 1982; Tricot et al., 1989; Zhen et al., 1992), and more recently it has also been targeted for antiviral (Streeter et al., 1973; Nelson et al., 1977; Colacino et al., 1993), antiparasitic, and immunosuppressive chemotherapy as well (Allison et al., 1993; Natsumeda and Carr, 1993). Because of dose-limiting toxicities, IMPD inhibitors have shown poor efficacy in cancer chemotherapy trials (Tricot et al., 1989; Natsumeda and Carr, 1993), and immunosuppression trials are ongoing (Allison et al., 1993). Our studies suggest that there is potential for significant clinical benefit from the continued elucidation of molecular aspects of IMPD function in the context of p53-dependent cell growth control. For example, knowledge of molecular differences between IMPD expression and function in the presence and absence of normal p53 function may lead to the design of compounds that selectively inhibit the enzyme in tumors containing p53 mutations, while sparing normal tissues with wild-type p53 function. Given the high frequency of p53 mutations in diverse human tumors (Hollstein et al., 1991), such compounds would have broad application in cancer treatment.
Our studies establish reduced IMPD activity as an essential requirement for p53-dependent cell cycle arrest in the absence of cellular damage. This initial definition does not preclude a role for the enzyme in p53-dependent growth regulation under nonphysiological conditions such as DNA damage, as its involvement in such processes has yet to be assessed. The hypothesis that p53 functions to regulate normal cell growth predates recent hypotheses that focus on p53 function in states of cellular damage (Finlay et al., 1989). These two schools of thought are not incompatible, and aspects of each may be relevant to normal tissue proliferation and the initiation and progression of cancer.
Although our goal has been to focus on molecular mechanisms involved in p53-dependent growth regulation in the absence of experimentally induced cellular damage, we cannot exclude the possibility that even under physiological conditions p53 requires a small degree of cellular damage to initiate cell cycle arrest (e.g., intrinsic DNA strand breakage and base mispairing that occur as a part of routine DNA metabolism). Experimental elevation of p53 may simply lower the cellular threshold for a cell cycle arrest response to such damage. On the other hand, there may be a distinct role for p53-dependent growth regulation in the absence of cellular damage as a cell growth homeostasis control to counter the effects of tissue factors that stimulate cell growth (see Figure 7). Evidence for such a role has been noted in skin wounding experiments in swine. At late times in the repair of skin incisions, when the normal epithelial architecture is nearly restored, high levels of p53 expression are observed in dividing basal cells (Antoniades et al., 1994). This finding is consistent with the idea of an induction of p53 to modulate the rapid division of these cells during the healing process.
Although the results presented herein are based in cell culture experiments, they have implications for the significance of p53 mutations in human cancers. They indicate that p53 mutations in human cancers reflect a significant requirement for increased guanine ribonucleotide biosynthesis for tumorigenesis. This requirement may be in effect even in tumors that exhibit wild-type p53 function. Such tumors are predicted to have other alterations that accomplish the same disruption as p53 mutation, i.e., increased guanine ribonucleotide levels with accompanying increases in GTP:GDP and GTP:ATP ratios. Given the high frequency of p53 mutations in diverse human cancer, such perturbation of purine ribonucleotide metabolism may be a universal requirement for tumorigenesis. We propose that such perturbations cause deregulation between specific points of cell signaling integration, resulting not only in aberrant responsiveness to external growth factors, but also a dysregulation of intracellular signals that automate cell division after activation by extracellular growth stimuli. We predict that cellular factors that control purine ribonucleotide-dependent signaling integration in response to effects of p53-dependent IMPD regulation will be an important new class of molecules to target for the treatment of cancer and other diseases of cellular proliferation.
ACKNOWLEDGMENTS
We thank Drs. J. Burch, R. Strich, J. Chernoff, and G. D. Markham for reviewing the manuscript and providing helpful suggestions for its completion. This work was supported by research grants from the National Institutes of Health National Cancer Institute (CA-58619 and CA-06927); the Pew Scholars Program in Biomedical Sciences; U. S. Healthcare, Inc.; and an appropriation from the Commonwealth of Pennsylvania.
REFERENCES
- Aaronson SA. Growth factors and cancer. Science. 1991;254:1146–1152. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Allison AC, Kowalski WJ, Muller CD, Eugui EM. Mechanisms of action of mycophenolic acid. Ann NY Acad Sci. 1993;696:63–87. doi: 10.1111/j.1749-6632.1993.tb17143.x. [DOI] [PubMed] [Google Scholar]
- Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. p53 expression during normal tissue regeneration in response to acute cutaneous injury in swine. J Clin Invest. 1994;93:2206–2214. doi: 10.1172/JCI117217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi LD, Lowe SW, Brugarolas J, Jacks T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 1996;15:3693–3701. [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Karlsson A, Wang L, Bohman C, Horská K, Votruba I, Fridland A, Aerschot AV, Herdewijn P, DeClercq ED. Eicar (5-ethynyl-1-β-D-ribofuranosylimidazaole-4-carboxamide): A novel potent inhibitor of inosinate dehydrogenase activity and guanylate biosynthesis. J Biol Chem. 1993;268:24591–24598. [PubMed] [Google Scholar]
- Barak Y, Juven T, Haffner R, Oren M. Mdm2 expression is induced by wild-type p53 activity. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: Conserved switch for diverse cell functions. Nature. 1990;348:125–131. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brady HJ, Salomons GS, Bobeldijk RC, Berns AJ M. T cells from baxα transgenic mice show accelerated apoptosis in response to stimuli but do not show restored DNA damage-induced cell death in the absence of p53. EMBO J. 1996;15:1221–1230. [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carter SB, Franklin TJ, Jones DF, Leonard BJ, Mills SD, Turner RW, Turner WB. Mycophenolic acid: an anti-cancer compound with unusual properties. Nature. 1969;223:848–850. doi: 10.1038/223848a0. [DOI] [PubMed] [Google Scholar]
- Chant J, Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995;81:1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Oliner JD, Zhan Q, Fornace AJ, Jr, Vogelstein B, Kastan MB. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA. 1994;91:2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- Chiou S-K, Rao L, White E. Bcl-2 blocks p53-dependent apoptosis. Mol Cell Biol. 1994;14:2556–2563. doi: 10.1128/mcb.14.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Klebe C, Wittinghofer A, Karsenti E. Regulation of Cdc2/cyclin B activation by Ran, a Ras-related GTPase. J Cell Sci, 1995;108:1217–1225. doi: 10.1242/jcs.108.3.1217. [DOI] [PubMed] [Google Scholar]
- Cochet C, Job D, Pirollet F, Chambaz EM. Cyclic nucleotide independent casein kinase (G type) in bovine adrenal cortex. Purification and properties of two molecular forms. Biochim Biophys Acta. 1981;658:191–201. doi: 10.1016/0005-2744(81)90289-8. [DOI] [PubMed] [Google Scholar]
- Cocker JH, Platti S, Santocanale C, Nasmyth K, Diffley JF X. An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature. 1996;379:180–182. doi: 10.1038/379180a0. [DOI] [PubMed] [Google Scholar]
- Cohen MB, Sadée W. Contributions of the depletions of guanine and adenine nucleotides to the toxicity of purine starvation in the mouse T lymphoma cell line. Cancer Res. 1983;43:1587–1591. [PubMed] [Google Scholar]
- Cohen MB, Maybaum J, Sadée W. Guanine nucleotide depletion and toxicity in mouse T lymphoma (S-49) cells. J Biol Chem. 1981;256:8713–8717. [PubMed] [Google Scholar]
- Colacino JM, Birch GM, Tang JC. Cellular metabolism and anti-influenza activity of 1,3,4-thiadiazol-2-yl-cyanamide ( LY217896) Antiviral Chem Chemother. 1993;4:271–280. [Google Scholar]
- Collart FR, Huberman E. Amplification of the IMP dehydrogenase gene in Chinese hamster cells resistant to mycophenolic acid. Mol Cell Biol. 1987;7:3328–3331. doi: 10.1128/mcb.7.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart FR, Huberman E. Cloning and sequence analysis of the human and Chinese hamster inosine-5′-monophosphate dehydrogenase cDNAs. J Biol Chem. 1988;263:15769–15772. [PubMed] [Google Scholar]
- Collart FR, Chubb CB, Mirkin BL, Huberman E. Increased inosine-5′-phosphate dehydrogenase gene expression in solid tumor tissues and tumor cell lines. Cancer Res. 1992;52:5826–5828. doi: 10.2172/10148922. [DOI] [PubMed] [Google Scholar]
- Crabtree GW, Henderson JF. Rate-limiting steps in the interconversion of purine ribonucleotides in Ehrlich ascites tumor cells in vitro. Cancer Res. 1971;31:985–991. [PubMed] [Google Scholar]
- Criss WE, Pradhan TK. Regulation of tumor cell metabolism by the adenylate and guanylate energy charges. In: Criss WE, Ono T, editors. Control Mechanisms in Cancer. J.R. Sabine, New York: Raven Press; 1976. pp. 401–410. [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Bradley A. The tumor suppressor p53. Biochim Biophys Acta. 1993;1155:181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Elasser S, Lou F, Wang B, Campbell JL, Jong A. Interaction between yeast Cdc6 protein and B-type cyclin/Cdc28 kinases. Mol Biol Cell. 1996;7:1723–1735. doi: 10.1091/mbc.7.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay CA. The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol Cell Biol. 1993;13:301–306. doi: 10.1128/mcb.13.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Franklin TJ, Twose PA. Reduction in β-adrenergic response of cultured glioma cells following depletion of intracellular GTP. Eur J Biochem. 1977;77:113–117. doi: 10.1111/j.1432-1033.1977.tb11648.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JB, Marshall MS. The ras oncogene—an important regulatory element in lower eucaryotic organisms. Microbiol Rev. 1989;53:171–185. doi: 10.1128/mr.53.2.171-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glesne DA, Collart FR, Huberman E. Regulation of IMP dehydrogenase gene expression by its end products, guanine nucleotides. Mol Cell Biol. 1991;11:5417–5425. doi: 10.1128/mcb.11.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb TM, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Jansen I, Willis RC, Seegmiller JE. Alterations of inosinate branchpoint enzymes in cultured human lymphoblasts. Biochim Biophys Acta. 1985;846:135–144. doi: 10.1016/0167-4889(85)90119-3. [DOI] [PubMed] [Google Scholar]
- Guillouf C, Grana X, Selvakumaran M, De Luca A, Giordano A, Hoffman B, Liebermann DA. Dissection of the genetic programs of p53-mediated G1 growth arrest and apoptosis: blocking p53-induced apoptosis unmasks G1 arrest. Blood. 1995;85:2691–2698. [PubMed] [Google Scholar]
- Hall A. Signal transduction through small GTPases—a tale of two GAPs. Cell. 1992;69:389–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Eick D. Mediation of c-myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Hide G, Graham T, Buchanan N, Tait A, Keith K. Trypanosoma brucei: characterization of protein kinases that are capable of autophosphorylation in vitro. Parasitology. 1994;108:161–166. doi: 10.1017/s0031182000068256. [DOI] [PubMed] [Google Scholar]
- Hirai A, Nakamura S, Noguchi, et al. Geranylgeranylated rho small GTPase(s) are essential for the degradation of p27Kip1 and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. J Biol Chem. 1997;272:13–16. [PubMed] [Google Scholar]
- Hochhauser SJ, Stein JL, Stein GS. Gene expression and cell cycle regulation. Int Rev Cytol. 1981;71:95–243. doi: 10.1016/s0074-7696(08)61183-3. [DOI] [PubMed] [Google Scholar]
- Hodges SD, Fung E, McKay DJ, Renaux BS, Synder FF. Increased activity, amount, and altered kinetic properties of IMP dehydrogenase from mycophenolic acid-resistant neuroblastoma cells. J Biol Chem. 1989;264:18137–18141. [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Jackson RC, Morris HP, Weber G. Partial purification, properties and regulation of inosine-5′-phosphate dehydrogenase in normal and malignant rat tissues. Biochem J. 1977;166:1–10. doi: 10.1042/bj1660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RC, Weber G, Morris HP. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature. 1975;256:331–333. doi: 10.1038/256331a0. [DOI] [PubMed] [Google Scholar]
- Jayaram HN, Zhen W, Gharehbaghi K. Biochemical consequences of resistance to tiazofurin in human myelogenous leukemic K562 cells. Cancer Res. 1993;53:2344–2348. [PubMed] [Google Scholar]
- Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, Lorincz AT, Hedrick L, Cho KR. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993;90:3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda SM, Sherman ML, Kufe DW. Effects of tiazofurin on guanine nucleotide binding regulatory proteins in HL-60 cells. Blood. 1990;75:583–588. [PubMed] [Google Scholar]
- Kiguchi K, Collart FR, Henning-Chubb C, Huberman E. Induction of cell differentiation in melanoma cells by inhibitors of IMPD dehydrogenase: altered patterns of IMPD dehydrogenase expression and activity. Cell Growth & Differ. 1990;1:259–270. [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Life (and death) in a malignant tumour. Nature. 1996;379:19–20. doi: 10.1038/379019a0. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS K, Tourtellotte WG, Brown GA J, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev, 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- Konno Y, Natsumeda Y, Nagai M, Yamaji Y, Ohno S, Suzuki K, Weber G. Expression of human IMP dehydrogenase types I and II in Escherichia coli and distribution in human normal lymphocytes and leukemic cell lines. J Biol Chem. 1991;266:506–509. [PubMed] [Google Scholar]
- Latham KM, Eastman SW, Wong A, Hinds PW. Inhibition of p53-mediated growth arrest by overexpression of cyclin-dependent kinases. Mol Cell Biol. 1996;16:4445–4455. doi: 10.1128/mcb.16.8.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Pawlak K, Nguyen BT, Robins RK, Sadée W. Biochemical differences among four inosinate dehydrogenase inhibitors, mycophenolic acid, ribavirin, tiazofurin, and selenazofurin, studied in mouse lymphoma cell culture. Cancer Res. 1985;45:5512–5520. [PubMed] [Google Scholar]
- Lehninger AL. Bioenergetics. 2nd ed. Menlo Park, CA: W.A. Benjamin; 1973. [Google Scholar]
- Lehninger AL. Biochemistry. 2nd ed. New York: Worth Publishers; 1975. [Google Scholar]
- Levine AJ, Momand J. Tumor suppressor genes: the p53 and retinoblastoma sensitivity genes and gene products. Biochim Biophys Acta. 1990;1032:119–136. doi: 10.1016/0304-419x(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Liang C, Weinreich M, Stillman B. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell. 1995;81:667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- Lightfoot T, Snyder FF. Gene amplification and dual point mutations of mouse IMP dehydrogenase associated with cellular resistance to mycophenolic acid. Biochim Biophys Acta. 1994;1217:156–162. doi: 10.1016/0167-4781(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Lin D, Fiscella M, O’Connor PM, Jackman J, Chen M, Luo LL, Sala A, Travali S, Appella E, Mercer WE. Constitutive expression of B-myb can bypass p53-induced Waf1/Cip1-mediated G1 arrest. Proc Natl Acad Sci USA. 1994;91:10079–10083. doi: 10.1073/pnas.91.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HJ, Eviner V, Prendergast GC, White E. Activated H-ras rescues E1A-induced apoptosis and cooperates with E1A to overcome p53-dependent growth arrest. Mol Cell Biol. 1995;15:4536–4544. doi: 10.1128/mcb.15.8.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke SP, Clarkin KC, Di Leonardo A, Tsou A, Wahl GM. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- Livingstone LR, White A, Sprouse J, Livanos E, Jacks T, Tlsty TD. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lui MS, Faderan MA, Liepnieks JJ, Natsumeda Y, Olah E, Jayaram HN, Weber G. Modulation of IMP dehydrogenase activity and guanylate metabolism by tiazofurin (2-β-d-ribofuranosylthiazole-4-carboxamide) J Biol Chem. 1984;259:5078–5082. [PubMed] [Google Scholar]
- Michael-Michalovitz D, Yehiely F, Gottlieb E, Oren M. Simian virus 40 can overcome the antiproliferative effect of wild-type p53 in the absence of stable large T antigen-p53 binding. J Virol. 1991;65:4160–4168. doi: 10.1128/jvi.65.8.4160-4168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Lieberman DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- Miyazaki M, Uritani M, Kagiyama H. The yeast peptide elongation factor 3 (EF-3) carries an active site for ATP hydrolysis which can interact with various nucleoside triphosphates in the absence of ribosomes. J Biochem. 1988;104:445–450. doi: 10.1093/oxfordjournals.jbchem.a122487. [DOI] [PubMed] [Google Scholar]
- Murphy M, Hinman A, Levine AJ. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996;10:2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- Nagai M, Natsumeda Y, Weber G. Proliferation-linked regulation of type II IMP dehydrogenase gene in human normal lymphocytes and HL-60 leukemic cells. Cancer Res. 1992;52:258–261. [PubMed] [Google Scholar]
- Natsumeda Y, Carr SF. Human type I and II IMP dehydrogenases as drug targets. Ann NY Acad Sci. 1993;696:88–93. doi: 10.1111/j.1749-6632.1993.tb17144.x. [DOI] [PubMed] [Google Scholar]
- Nelson JA, Rose LM, Bennett LL., Jr Effects of 2-amino-1,3,4,-thiadiazole on ribonucleotide pools of leukemia L1210 cells. Cancer Res. 1976;36:1375–1378. [PubMed] [Google Scholar]
- Nelson JA, Rose LM, Bennett LL., Jr Mechanism of action of 2-amino-1,3,4,-thiadiazole (NSC 4728) Cancer Res. 1977;37:182–187. [PubMed] [Google Scholar]
- Okayama H, Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983;3:280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Onozawa T, Danjoh I, Fujiyama A. Biochemical similarity of Schizosaccharomyces pombe ras1 protein with RAS2 protein of Saccharomyces cervisiae. Yeast. 1995;11:801–808. doi: 10.1002/yea.320110902. [DOI] [PubMed] [Google Scholar]
- Pall ML. GTP: A central regulator of cellular anabolism. Curr Top Cell Regul. 1985;25:1–20. doi: 10.1016/b978-0-12-152825-6.50005-9. [DOI] [PubMed] [Google Scholar]
- Pall ML, Robertson CK. Growth regulation by GTP. J Biol Chem. 1988;263:11168–11174. [PubMed] [Google Scholar]
- Peeper DS, Upton TM, Ladha MH, Neuman E, Zalvide J, Bernards R, DeCaprio JA, Ewen ME. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- Pelech SL, Meijer L, Krebs EG. Characterization of maturation-activated histone H1 and ribosomal S6 kinases in sea star oocytes. Biochemistry. 1987;26:7960–7968. doi: 10.1021/bi00398a062. [DOI] [PubMed] [Google Scholar]
- Proffitt RT, Pathak VK, Villacorte DG, Presant CA. Sensitive radiochemical assay for inosine-5′-monophosphate dehydrogenase and determination of activity in murine tumor tissue extracts. Cancer Res. 1983;43:1620–1623. [PubMed] [Google Scholar]
- Putnam-Evans CL, Harmon AC, Cormier MJ. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990;29:2488–2489. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Quartin RS, Cole CN, Pipas JM, Levine AJ. The amino-terminal functions of the simian virus 40 large T antigen are required to overcome wild-type p53-mediated growth arrest of cells. J Virol. 1994;68:1334–1342. doi: 10.1128/jvi.68.3.1334-1341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RR. G protein diversity and the regulation of signaling pathways. New Biol. 1990;2:957–960. [PubMed] [Google Scholar]
- Rizzo MT, Tricot G, Hoffman R, Jayaram HN, Weber G, Garcia JG N, English D. Inosine monophosphate dehydrogenase inhibitors. Probes for investigations of the functions of guanine nucleotide binding proteins in intact cells. Cell Signal. 1990;2:509–519. doi: 10.1016/0898-6568(90)90073-j. [DOI] [PubMed] [Google Scholar]
- Robins RK, Srivastava PC, Narayanan VL, Plowman J, Paull KD. 2-β-d-ribofuranosylthiazole-4-carboxamide, a novel potential antitumor agent for lung tumors and metastases. J Med Chem. 1982;25:107–108. doi: 10.1021/jm00344a002. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Segawa K, Minowa A, Sugasawa K, Takano T, Hanaoka F. Abrogation of p53-mediated transactivation by SV40 large T antigen. Oncogene. 1993;8:543–548. [PubMed] [Google Scholar]
- Selvakumaran M, Lin H-K, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Lieberman D. Immediate early up-regulation of bax expression by p53 but not TGFβ1: a paradigm for distinct apoptotic pathways. Oncogene. 1994;9:1791–1798. [PubMed] [Google Scholar]
- Shen Y, Shenk T. Relief of p53-mediated transcriptional repression by the adenovirus E1B 19-kDa protein or the cellular Bcl-2 protein. Proc Natl Acad Sci USA, 1994;91:8940–8944. doi: 10.1073/pnas.91.19.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley JL. Guanine nucleotide biosynthesis is regulated by the cellular p53 concentration. J Biol Chem. 1991;266:24815–24828. [PubMed] [Google Scholar]
- Sherley JL. The p53 tumor suppressor gene as regulator of somatic stem cell renewal division. Cope. 1996;12:9–10. [Google Scholar]
- Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988;263:8350–8358. [PubMed] [Google Scholar]
- Sherley JL, Stadler PB, Johnson DR. The p53 antioncogene induces guanine nucleotide-dependent stem cell division kinetics. Proc Natl Acad Sci USA. 1995a;92:136–140. doi: 10.1073/pnas.92.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley JL, Stadler PB, Stadler JS. A quantitative method for the analysis of mammalian cell proliferation in culture in terms of dividing and non-dividing cells. Cell Proliferation. 1995b;28:137–144. doi: 10.1111/j.1365-2184.1995.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Spitovsky D, Aengeneyndt F, Braspenning J, Doeberitz M vK. p53-independent growth regulation of cervical cancer cells by the papillomavirus E6 oncogene. Oncogene. 1996;13:1027–1035. [PubMed] [Google Scholar]
- Stadler PB, Pennacchi J, Sherley JL. Inosine-5′-monophosphate dehydrogenase activity is maintained in immortalized murine cells growth-arrested by serum deprivation. Adv Enzyme Regul. 1994;34:91–106. doi: 10.1016/0065-2571(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Stoehr SJ, Smolen JE. Human neutrophils contain a protein kinase C-like enzyme that utilizes guanosine triphosphate as a phosphate donor. Cofactor requirements, kinetics, and endogenous acceptor proteins. Blood. 1990;75:479–487. [PubMed] [Google Scholar]
- Streeter DG, Witkowski JT, Khare GP, Sidwell RW, Bauer RJ, Robins RK, Simon LN. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci USA. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres T, Fritzer M, Pillwein K, Felzmann T, Chiba P. Cell cycle dependent regulation of IMP dehydrogenase activity and effect of tiazofurin. Life Sci. 1992;51:1309–1315. doi: 10.1016/0024-3205(92)90021-g. [DOI] [PubMed] [Google Scholar]
- Tricot GJ, Jayaram HN, Lapis E, Natusmeda Y, Nichols CR, Kneebone P, Heerema N, Weber G, Hoffman R. Biochemically directed therapy of leukemia with tiazofurin, a selective blocker of inosine 5′-phosphate dehydrogenase activity. Cancer Res. 1989;49:3696–3701. [PubMed] [Google Scholar]
- Turka LA, Dayton J, Sinclair G, Thompson CB, Mitchell BS. Guanine ribonucleotide depletion inhibits T cell activation: Mechanism of action of the immunosuppressive drug mizoribine. J Clin Invest. 1991;87:940–948. doi: 10.1172/JCI115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman B. Characterization of mutant murine lymphoma cells with altered inosinate dehydrogenase activities. J Biol Chem. 1983;258:523–528. [PubMed] [Google Scholar]
- Uritani M, Miyazaki M. Characterization of the ATPase and GTPase activities of elongation factor 3 (EF-3) purified from yeasts. J Biochem. 1988;103:522–530. doi: 10.1093/oxfordjournals.jbchem.a122302. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Vojtesek B, Fisher C, Lane D. HPV-16 E7 or adenovirus E1A can overcome the growth arrest of cells immortalized with a temperature-sensitive p53. Oncogene. 1993;8:697–702. [PubMed] [Google Scholar]
- Wang Y, Okan I, Szekely L, Klein G, Wiman KG. Bcl-2 inhibits wild-type p53-triggered apoptosis but not G1 cell cycle arrest and transactivation of WAF1 and bax. Cell Growth & Differ. 1995;6:1071–1075. [PubMed] [Google Scholar]
- Weber G, Nakamura H, Natsumeda Y, Szekeres T, Nagai M. Regulation of GTP biosynthesis. Advan Enzyme Regul. 1992;32:57–69. doi: 10.1016/0065-2571(92)90008-n. [DOI] [PubMed] [Google Scholar]
- Williams RS, Shohet RV, Stillman B. A human protein related to yeast Cdc6p. Proc Natl Acad Sci USA. 1997;94:142–147. doi: 10.1073/pnas.94.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Fukada S, Yoshikuni M, Bulet P, Hirai T, Yamaguchi A, Yasuda H, Ohba Y, Nagahama Y. M-phase-specific histone H1 kinase in fish oocytes: Purification, components, and biochemical properties. Eur J Biochem. 1992;205:537–543. doi: 10.1111/j.1432-1033.1992.tb16810.x. [DOI] [PubMed] [Google Scholar]
- Yin Y, Tainsky MA, Bischoff FZ, Strong LC, Wahl GM. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992;70:937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zhen W, Jayaram HN, Weber G. Antitumor activity of tiazofurin in human colon carcinoma HT-29. Cancer Invest. 1992;10:505–511. doi: 10.3109/07357909209024812. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Rottjakob H-W, Kuntzel H. The Saccharomyces cerevisiae CDC6 gene is transcribed at late mitosis and encodes a ATP/GTPase controlling S phase initiation. J Biol Chem. 1994;269:23351–23356. [PubMed] [Google Scholar]