Abstract
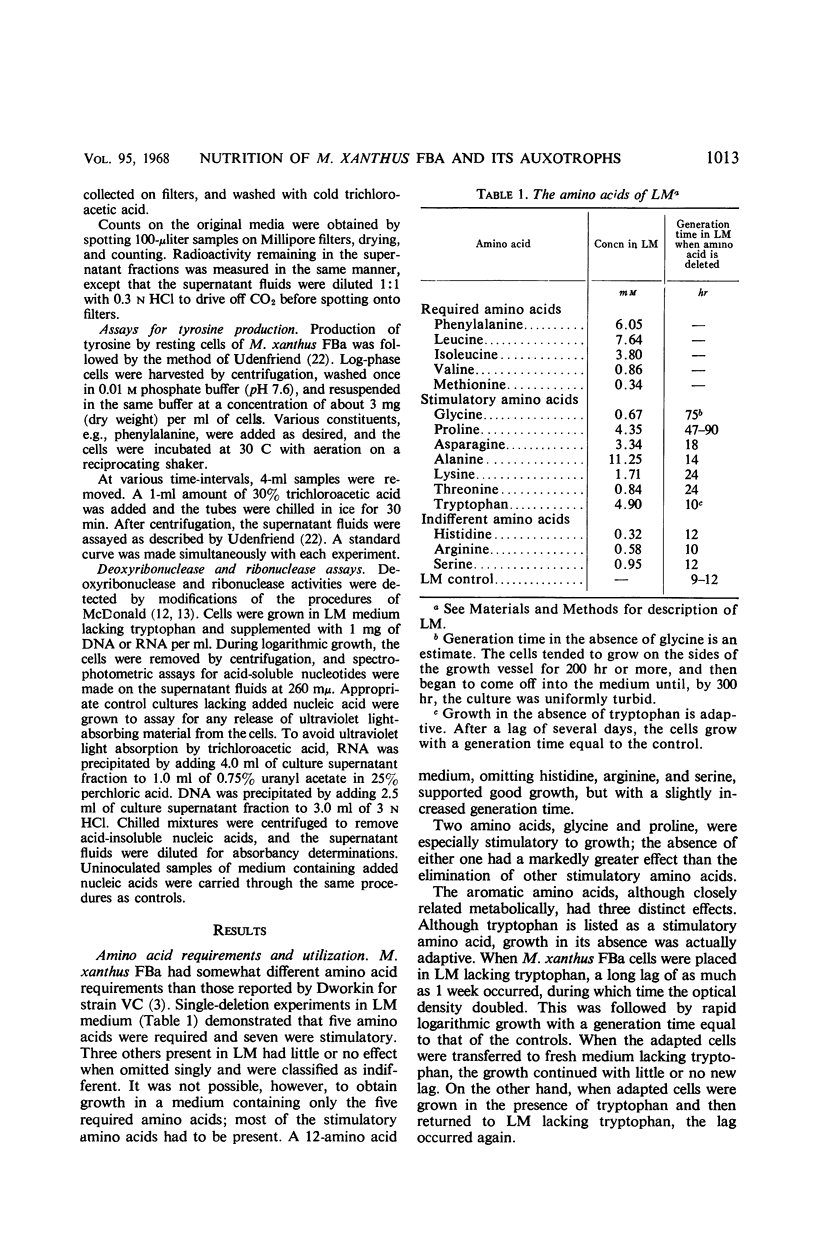
A defined medium containing 15 amino acids plus salts was used to study the nutrition of Myxococcus xanthus FBa. The amino acids phenylalanine, leucine, isoleucine, valine, and methionine were essential for growth, whereas glycine, proline, asparagine, alanine, lysine, and threonine stimulated growth. An unusual pattern of requirement was found in the aromatic amino acids. Phenylalanine was essential and served as the precursor of tyrosine. Growth in the absence of tryptophan was adaptive, with cells reaching a growth rate equal to that of controls after a lag of about a week. 14C-labeled ribose and glucose were not appreciably metabolized. Auxotrophs requiring purines and pyrimidines were isolated and were used to study the fate of externally supplied nucleic acid derivatives. Appropriate mutants could satisfy their requirements with free bases, nucleosides, and nucleotides, and could hydrolyze nucleic acids and use the products. However, studies using 14C-ribose-labeled uridine (isolated from a Salmonella typhimurium pyrimidine auxotroph) showed that externally supplied nucleic acid derivatives were incorporated almost solely into the nucleic acids of the myxobacters, with little used either for energy-yielding oxidations or other cell anabolism.
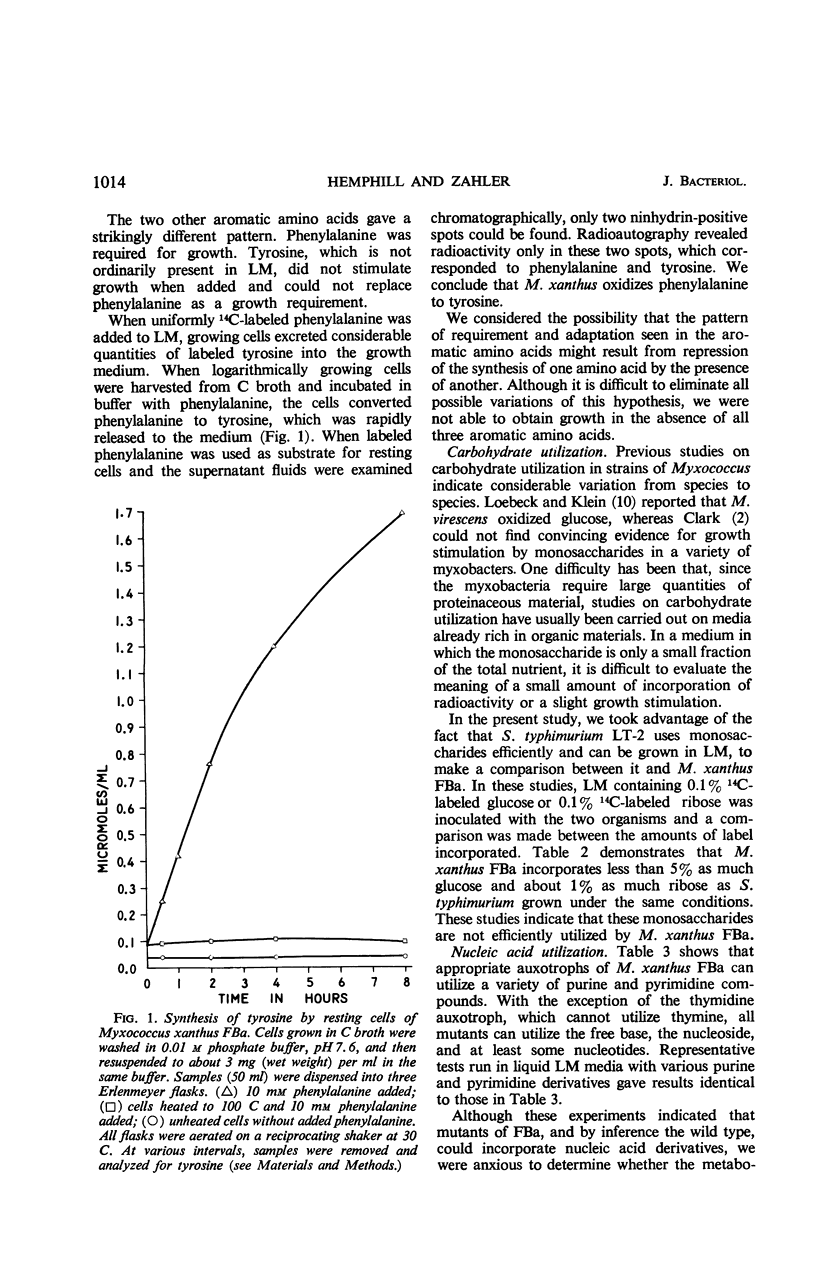
Full text
PDF


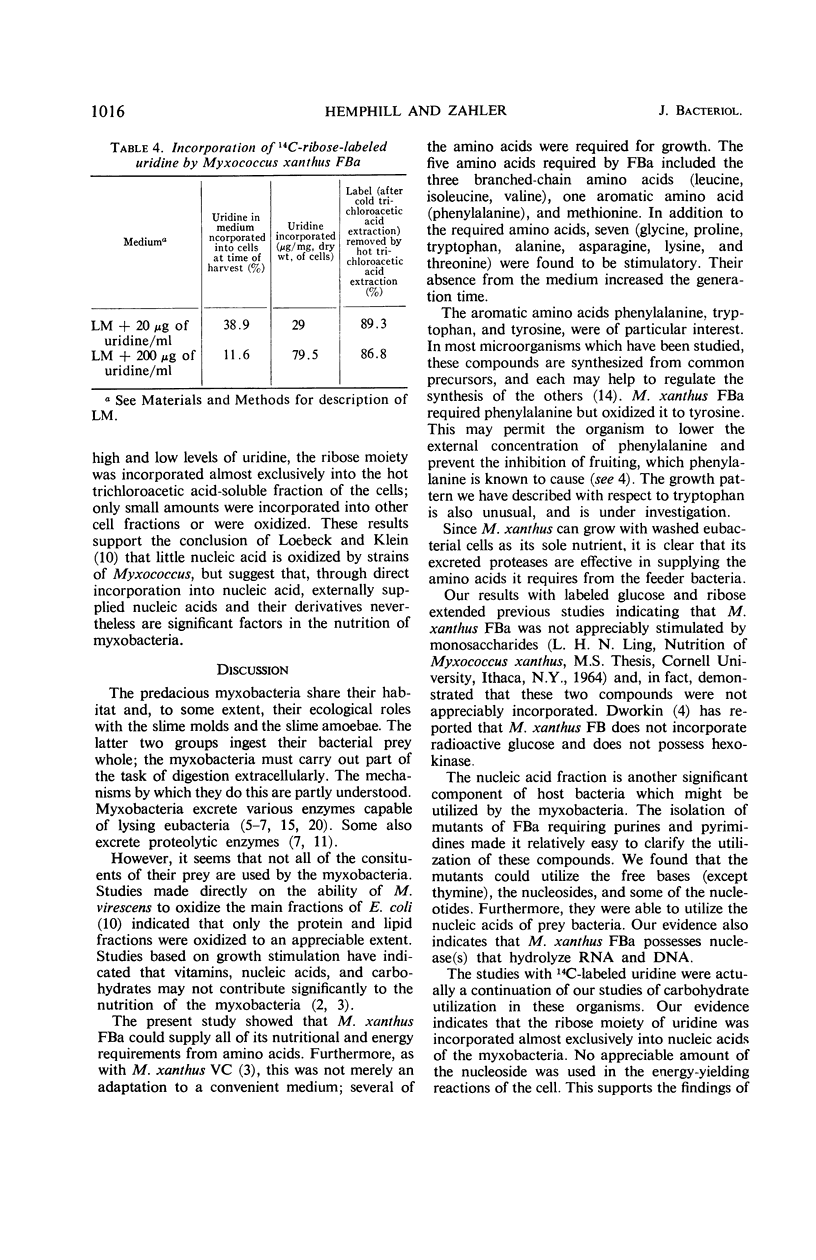

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSCH H., HURLBERT R. B., POTTER V. R. Anion exchange chromatography of acids of the citric acid cycle. J Biol Chem. 1952 May;196(2):717–727. [PubMed] [Google Scholar]
- CLARK W. A. Effect of carbohydrates on some Myxobacteria. J Bacteriol. 1954 May;67(5):589–592. doi: 10.1128/jb.67.5.589-592.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. LYSIS OF BACTERIAL CELL WALLS BY AN ENZYME ISOLATED FROM A MYXOBACTER. J Bacteriol. 1965 Aug;90:395–402. doi: 10.1128/jb.90.2.395-402.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Hart B. A., Zahler S. A. Lytic enzyme produced by Myxococcus xanthus. J Bacteriol. 1966 Dec;92(6):1632–1637. doi: 10.1128/jb.92.6.1632-1637.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem J. 1956 Nov;64(3):405–408. doi: 10.1042/bj0640405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEBECK M. E., KLEIN H. P. Substrates for Myxococcus virescens with special reference to eubacterial fractions. J Gen Microbiol. 1956 Apr;14(2):281–289. doi: 10.1099/00221287-14-2-281. [DOI] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science. 1966 Sep 2;153(3740):1119–1120. doi: 10.1126/science.153.3740.1119. [DOI] [PubMed] [Google Scholar]



