Abstract
Vascular endothelial growth factor (VEGF) is a key regulator of developmental, physiological, and tumor angiogenesis. Upregulation of VEGF expression by hypoxia appears to be a critical step in the neovascularization of solid cancers. The VEGF mRNA is intrinsically labile, but in response to hypoxia the mRNA is stabilized. We have systematically analyzed the regions in the VEGF mRNA that are responsible for its lability under normoxic conditions and for stabilization in response to hypoxia. We find that the VEGF mRNA not only contains destabilizing elements in its 3′ untranslated region (3′UTR), but also contains destabilizing elements in the 5′UTR and coding region. Each region can independently promote mRNA degradation, and together they act additively to effect rapid degradation under normoxic conditions. Stabilization of the mRNA in response to hypoxia is completely dependent on the cooperation of elements in each of the 5′UTR, coding region, and 3′UTR. Combinations of any of two of these three regions were completely ineffective in responding to hypoxia, whereas combining all three regions allowed recapitulation of the hypoxic stabilization seen with the endogenous VEGF mRNA. We conclude that multiple regions in the VEGF mRNA cooperate both to ensure the rapid degradation of the mRNA under normoxic conditions and to allow stabilization of the mRNA in response to hypoxia. Our findings highlight the complexity of VEGF gene expression and also reveal a mechanism of gene regulation that could become the target for strategies of therapeutic intervention.
INTRODUCTION
All tissue growth requires the establishment of an adequate vascular structure. In its absence the tissue becomes deprived of oxygen and nutrients and in response the cells produce angiogenic factors, which function to recruit new blood vessels into the undervascularized tissue (Folkman and Shing, 1992; Battegay, 1995). Of the angiogenic factors identified to date, vascular endothelial growth factor (VEGF) is likely to be a key regulator of angiogenesis (Shweiki et al., 1992; Kim et al., 1993; Millauer et al., 1994). VEGF is a secreted, endothelial cell-specific mitogen that is highly expressed in solid tumors and in areas of active vascularization (Leung et al., 1989; Plate et al., 1992; Shweiki et al., 1992). Upregulation of VEGF expression by hypoxia appears to be a critical step in the process of neovascularization of solid cancers (Shweiki et al., 1992; Kim et al., 1993; Shweiki et al., 1995). Inhibition of VEGF activity in vivo can block both tumor establishment and progression, by inhibiting vascularization (Kim et al., 1993; Millauer et al., 1994), and VEGF also appears to be required for the maintenance of tumor blood vessels, because withdrawal of VEGF leads to breakdown of the vascular structure and consequently tumor regression (Benjamin and Keshet, 1997).
The upregulation of VEGF expression by hypoxia is due to both transcriptional activation and a marked stabilization of the normally labile VEGF mRNA (Ikeda et al., 1995; Levy et al., 1995, 1996a,b; Shima et al., 1995; Stein et al., 1995; Damert et al., 1997). Transcriptional activation of VEGF is mediated in large part by the binding of hypoxia inducible factor-1 to an enhancer element in the 5′-flanking region of the VEGF gene (Ikeda et al., 1995; Levy et al., 1995; Forsythe et al., 1996); however, relatively little is known about the mechanism of stabilization of the VEGF mRNA in response to hypoxia. Previous studies on the regulation of VEGF mRNA stability have focused on the role of the 3′UTR, which is 1.9 kb in length and contains a number of elements likely to be important in regulating the stability of the mRNA, including several copies of the nonameric consensus for the AU-destabilizing element (Levy et al., 1995, 1996a,b, 1997; Claffey et al., 1998). Consistent with the presence of destabilizing elements, the VEGF 3′UTR can reduce the expression of a reporter mRNA in vivo (Shima et al., 1996). In vitro degradation studies have shown that the VEGF 3′UTR is stabilized in response to hypoxia (Levy et al., 1996a,b), and a number of studies have demonstrated the presence of binding sites for hypoxia-inducible binding proteins in the 3′UTR (Levy et al., 1996a,b, 1997; Claffey et al., 1998); however, it appears that the VEGF 3′UTR is not sufficient to confer hypoxic regulation on a reporter mRNA in vivo (Levy et al., 1997; Claffey et al., 1998), and recapitulation of hypoxic stabilization of the VEGF mRNA in a reporter system has not been achieved.
Apart from VEGF, the stability of a number of other mRNAs has also been found to be regulated by hypoxia, including the mRNAs for erythropoietin, tyrosine hydroxylase, and glut-1 (Goldberg et al., 1991; Czyzyk-Krzeska et al., 1994; Stein et al., 1995; McGary et al., 1997). Although the mechanistic details of the stabilization of these other mRNAs have yet to be elucidated, it is also likely that the binding of proteins to elements within the 3′ untranslated regions (3′UTRs) of these mRNAs will be important in the regulation of mRNA stability (Czyzyk-Krzeska and Beresh, 1996; Czyzyk-Krzeska et al., 1997; McGary et al., 1997). VEGF is encoded by a single gene that gives rise to four isoforms by alternative splicing (Tischer et al., 1991; Shima et al., 1996). Of these four isoforms, the 164 and 121 amino acid isoforms appear to be the primary mitogenic forms in vivo (Houck et al., 1991; Cohen et al., 1995). All four isoforms share the same 5′ UTR, which is unusually long and GC-rich (Levy et al., 1995; Shima et al., 1996; Tischer et al., 1991). The unusual structure of the 5′UTR suggests that elements regulating translation and/or stability of the mRNA may also reside within this region. As a first step toward elucidating the molecular mechanisms involved in the regulation of VEGF mRNA stability, we have systematically analyzed the regions in the VEGF mRNA that are responsible for its lability under normoxic conditions and the regions that are responsible for stabilization in response to hypoxia. We find that multiple regions in the VEGF mRNA cooperate both to ensure the rapid degradation of the mRNA under normoxic conditions and to allow stabilization of the mRNA in response to hypoxia.
MATERIALS AND METHODS
Cell Culture and Transfection
Mouse BALB/c3T3 lines were grown in DMEM with 7.5% FCS. Cells (2 × 106) were transfected with 20 μg of plasmid (linearized with ScaI) by CaPO4 precipitation, grown for 24–48 h, and selected in 400 μg/ml G418. After 10–12 d, the resulting colonies (containing at least 100 independent clones) were pooled and maintained in 200 μg/ml G418 sulfate (Life Technologies, Gaithersburg, MD). Hypoxic conditions (1% O2, 5–8% CO2) were generated either by ascorbic acid depletion of atmospheric oxygen in a sealed 2.5-l container (AnaeroGen AN25; Oxoid, Basingstoke, Hampshire, England) or by infusion of N2 gas using a Reming Bioinstruments (Redfield, NY) Oxyreducer unit on a standard CO2 incubator. Actinomycin D was used at 5 μg/ml to inhibit transcription of endogenous genes. Serum stimulation of cells was performed essentially as described previously (Lagnado et al., 1994), except that 1) hypoxic incubation and serum starvation were performed concurrently for 24 h and 2) FCS was added directly to the medium for serum stimulation.
Plasmid Constructions
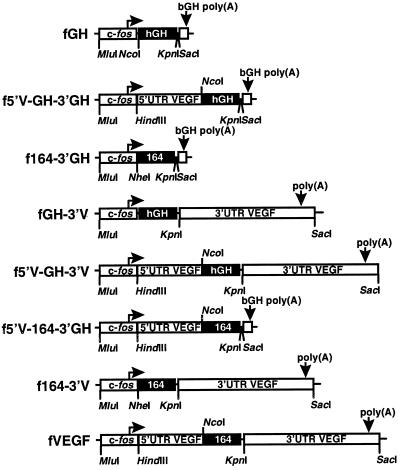
Plasmids were generated by insertion of murine VEGF sequences into the pfGH plasmid (see Figure 2). Plasmid pfGH-3′V contains the entire 3′UTR of VEGF (from the first nucleotide after the VEGF termination codon to 2.2 kb downstream) and was generated by cloning a 2.2 kb Pfu PCR fragment from p3′VEGF, a clone derived from a λ FIX II mouse genomic VEGF clone (Carmeliet et al., 1996; Breier, unpublished observations), between the KpnI and SacI sites of pfGH. Plasmid pf164–3′GH substitutes the coding region for human growth hormone (hGH) with the coding region for the VEGF 164 amino acid isoform. The plasmid was generated by cloning a XbaI–KpnI fragment from pVEGF1, a cDNA clone of the VEGF 164 amino acid isoform (Breier et al., 1992), into the NheI and KpnI sites of pfGH. Plasmid pf5′V-GH-3′GH contains the entire VEGF 5′UTR inserted into the 5′UTR of the fGH reporter gene and was constructed by incorporating a NcoI site at the VEGF initiation codon by PCR amplification with Pfu polymerase from pV5NBgl63 (Damert et al., 1997) and cloning a HindIII to NcoI fragment (sequence coordinates 1218 to 2234 [Shima et al., 1996]) into the same sites in pfGH. Plasmid pf164–3′V, which contains the 164 amino acid coding region linked to the VEGF 3′UTR, was created by cloning a MluI to KpnI fragment from pf164–3′GH into the same sites in plasmid pfGH-3′V. Plasmid pf5′V-GH-3′V contains the VEGF 5′UTR linked to the hGH coding region and VEGF 3′UTR and was constructed by cloning a MluI to KpnI fragment from pf5′V-GH-3′GH into the same sites in pfGH-3′V. Plasmid pf5′V-164–3′GH contains the VEGF 5′UTR and 164 amino acid coding region and was created by incorporating a NcoI site at the VEGF initiation codon by PCR amplification with Pfu polymerase and cloning the NcoI to KpnI PCR fragment containing the VEGF coding region into the same sites in pf5′V-GH-3′GH. To create a reconstructed version of the entire VEGF mRNA, tagged at the coding/3′UTR junction to distinguish it from the endogenous VEGF mRNA, the MluI to KpnI fragment from pf5′V-164–3′GH was cloned into the same sites in pfGH-3′V. The resulting plasmid was termed pfVEGF and contains 48 bp of pBluescript polylinker between the VEGF termination codon and the start of the 3′UTR.
Figure 2.
The fGH reporter gene and the chimeric genes constructed in this work. Boxes indicate segments of the gene derived from the chicken c-fos gene, human growth hormone cDNA (hGH), 3′UTR and poly(A) signal from bovine growth hormone (bGH), or the 5′UTR, 164 amino acid coding region or 3′UTR from the murine VEGF gene. Translated regions are indicated by shading.
Plasmid pGH/VEGF was created by cloning a 389 bp BglII–SmaI fragment from pfGH-3′V into the BamHI and SmaI sites of pBluescriptSK+. This plasmid contains junction sequence between the hGH gene and the VEGF 3′UTR and was used to detect the endogenous VEGF mRNA and also to detect the chimeric fGH-3′V mRNA. pGEM164/3′ was created by cloning a 260 bp EcoRI to SmaI fragment from pf164–3′V into the same sites in pGEM4Z. This plasmid contains the 164/3′UTR junction present in the reconstructed fVEGF mRNA and was used to generate probes to allow discrimination between the reconstructed fVEGF mRNA and the endogenous VEGF mRNA.
RNA Isolation and Analysis
Total RNA was isolated either from untransfected BALB/c3T3 cells or from polyclonal pools of transfected cells by guanidine thiocyanate extraction (Chomczynski and Sacchi, 1987). Specific transcripts were detected either by Northern analysis (Sambrook et al., 1989) or by RNase protection analysis (Lagnado et al., 1994). The GAPDH1 mRNA and chimeric mRNAs containing the hGH coding region were detected as described previously (Lagnado et al., 1994). For detection of the endogenous VEGF mRNA, a T7 synthesized transcript from pGH/VEGF digested with XbaI was used. For detection of mRNAs containing the VEGF 164 amino acid coding region, an SP6 synthesized transcript from plasmid pf164–3′GH digested with HinfI was used. The reconstructed fVEGF mRNA was detected by use of a T7 synthesized transcript from plasmid pGEM164/3′ digested with EcoRI. The neomycin phosphotransferase (neo) mRNA was detected by use of plasmid pGNEO digested with RsaI and T7 RNA polymerase. β-actin mRNA was detected by use of pS4Hβ-actin (a subclone of pHF-A1 [Ponte et al., 1983]) digested with PvuII and SP6 RNA polymerase.
Quantitation and Analysis of Specific mRNAs
The amount of specific mRNA was quantitated by PhosphorImager (Molecular Dynamics, Sunnyvale, CA) analysis of RNase protection gels. The level of each mRNA was normalized with respect to either GAPDH or neo mRNA, or was plotted without normalization, as indicated in the figure legends. Because the amount of GAPDH mRNA increased linearly during the 8 h after serum stimulation of normoxic cells, when GAPDH was used for normalization a regression line was fitted to the pooled GAPDH mRNA levels for each experiment and used to calculate the expected GAPDH level for each sample, as has been described previously (Lagnado et al., 1994). Levels of the fGH mRNA and its variants were then normalized by multiplying the ratio (expected GAPDH/observed GAPDH). This correction avoided overestimating the instability of chimeric mRNAs but did not change relative destabilizing effects. The prestimulation level of GAPDH mRNA was found to be elevated by hypoxia (Graven et al., 1994), but the level of GAPDH mRNA showed no significant change for the period of the 8-h time course after serum stimulation. To demonstrate that the serum-induced highest mRNA values were similar between experiments and with each reporter gene, the maximum levels of the reporter mRNA are given in each figure legend. To allow comparison between experiments (for which the times of phosphorimage exposure may differ), the maximum value was expressed relative to the normoxic GAPDH level. Transcriptional induction and turn-off of the fos promoter were unaffected by hypoxia.
The half-life of the endogenous VEGF mRNA was calculated using GraphPad (San Diego, CA) Prism (version 1.03) software and fitting the data to an equation for single-phase exponential decay. Apparent half-lives (t1/2) and decay rates (k) determined from serum stimulation data were calculated after subtraction of the background level from the unstimulated sample (t = 0) and fitting the data to an equation for single-phase exponential decay. For independently functioning destabilizing activities, the rate of decay of a mRNA containing more than one destabilizing element (kobs) should be equal to the sum of the individual decay rates (k1, k2, etc.).
RESULTS
VEGF mRNA Stability Is Regulated by Hypoxia in BALB/c3T3 Fibroblasts
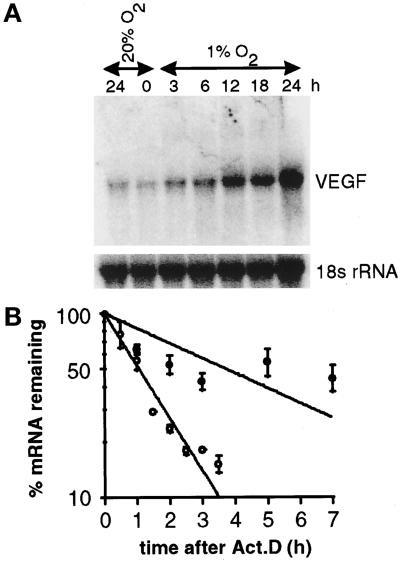
The stability of the VEGF mRNA has been shown to be regulated by hypoxia in a number of cell lines, although in some tumor-derived cell lines the VEGF mRNA is constitutively stabilized (White et al., 1995). We initially chose to use NIH3T3 cells to study the regulation of VEGF mRNA stability, because these cells are not tumor-derived and because we have previously used these cells to identify mRNA destabilizing elements (Lagnado et al., 1994; Brown et al., 1996); however, the VEGF mRNA was only modestly induced by hypoxia in NIH3T3 cells (Dibbens, unpublished observations). In contrast, in BALB/c3T3 fibroblasts we found that the VEGF mRNA was induced ∼10-fold by 24 h of hypoxia (Figure 1A), and we therefore chose this cell line for subsequent experiments.
Figure 1.
Time course of hypoxic induction of the VEGF mRNA and determination of VEGF mRNA half-life under normoxia and hypoxia. (A) Northern analysis of total RNA from BALB/c3T3 cells incubated under normoxia (20% O2) or hypoxia (1% O2) for the indicated times. The VEGF mRNA was detected by probing with a 529 bp SmaI–BamHI fragment from the VEGF 3′UTR. An oligonucleotide probe specific for 18s rRNA was used as an internal standard. (B) Quantitative analysis of VEGF mRNA stability using pooled data from two independent experiments that used actinomycin D. The VEGF and β-actin mRNAs were detected by RNase protection analysis and quantitated by PhosphorImager analysis. The level of VEGF mRNA was normalized with respect to β-actin mRNA. Open circles represent cells incubated under normoxia; closed circles represent cells incubated under hypoxia. The half-life of the VEGF mRNA was 1.1 h under normoxia and 4.0 h under hypoxia.
In BALB/c3T3 cells incubated under normoxic conditions (20% O2) and treated with actinomycin D to inhibit transcription, the VEGF mRNA decayed with a half-life of 65 min (Figure 1B). We observed a gradual stabilization of the VEGF mRNA with extended times of exposure to actinomycin D, a phenomenon that has been observed for a number of other mRNAs (Shaw et al., 1988; Wisdom and Lee, 1991; Zubiaga et al., 1995). Nevertheless, in cells incubated under hypoxic conditions (1% O2) for 24 h, the half-life of the VEGF mRNA was estimated to be 3.7 h, demonstrating stabilization of the mRNA in response to hypoxia (Figure 1B). The half-life of the VEGF mRNA under normoxia and the degree of stabilization in response to hypoxia determined in this way were in good agreement with similar previous studies (Ikeda et al., 1995; Stein et al., 1995; Levy et al., 1996a,b).
The VEGF mRNA Contains Destabilizing Elements in the 5′UTR, Coding Region, and 3′UTR
On the basis of the results of a previous in vitro study (Levy et al., 1996a,b), our expectation was that the VEGF 3′UTR would be sufficient to confer correct regulation of stability on a reporter mRNA. To investigate the role of the VEGF 3′UTR in regulating mRNA stability, we inserted the entire murine VEGF 3′UTR into the 3′UTR of the fGH reporter gene, creating the hybrid gene fGH-3′V (Figure 2). This construct was transfected into BALB/c3T3 cells, and a polyclonal population of G418 resistant cells was established. Transcription of the normally stable fGH mRNA is driven from the c-fos promoter, from which a brief pulse of transcription can be generated by serum stimulation, allowing subsequent degradation of the mRNA to be monitored (Kabnick and Housman, 1988; Shyu et al., 1989; Lagnado et al., 1994). This system allows a direct means of determining the stability of mRNAs rather than an indirect means, such as changes in the steady-state levels of reporter enzymes or the use of nonspecific inhibitors of transcription.
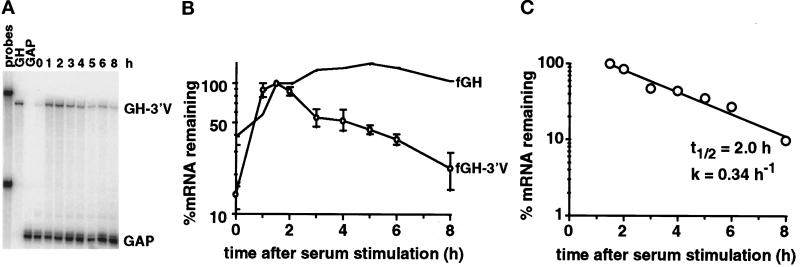
Insertion of the VEGF 3′UTR into the reporter gene significantly destabilized the fGH reporter mRNA under normoxic conditions (Figure 3, A and B), demonstrating the presence of destabilizing elements in the VEGF 3′UTR; however, the half-life of the fGH-3′V mRNA under normoxic conditions was ∼2 h (Figure 3C), significantly more stable than the half-life of the endogenous VEGF mRNA (65 min) determined using actinomycin D, and suggests that elements outside the 3′UTR may contribute to the lability of the VEGF mRNA under normoxic conditions.
Figure 3.
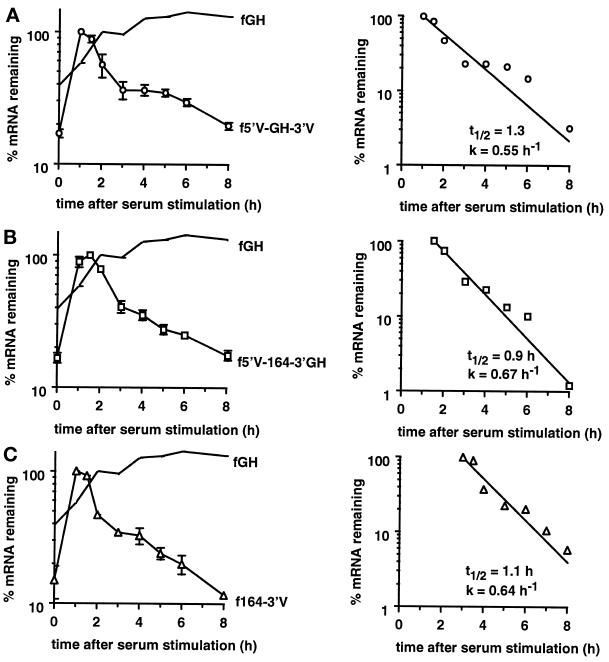
The VEGF 3′UTR destabilizes a reporter mRNA under normoxic conditions. Total RNA was isolated after serum stimulation of cells expressing either the fGH transcript or the fGH-3′V transcript. (A) PhosphorImage of RNase protection gel. The fGH-3′V mRNA and the GAPDH mRNA protection products are indicated. The undigested probes and each probe used individually (GH, GAP) are also shown. (B) Time course of degradation of the fGH mRNA and the fGH-3′V mRNA (○) under normoxia. The graph shows the data as mean ± SEM for four independent experiments, normalized with respect to GAPDH. The levels of serum induction observed for each experiment were 7.5-, 4.4-, 7.5-, and 14-fold. The maximal levels of induction of the reporter gene (expressed as a ratio to the GAPDH level) for each experiment were 0.33, 0.36, 0.48, and 0.23. (C) Decay kinetics of the fGH-3′V mRNA. The graph shows mean data taken from B, with the background level of mRNA from the unstimulated samples subtracted. Regression line, calculated half-life (t1/2), and decay rate (k) are indicated.
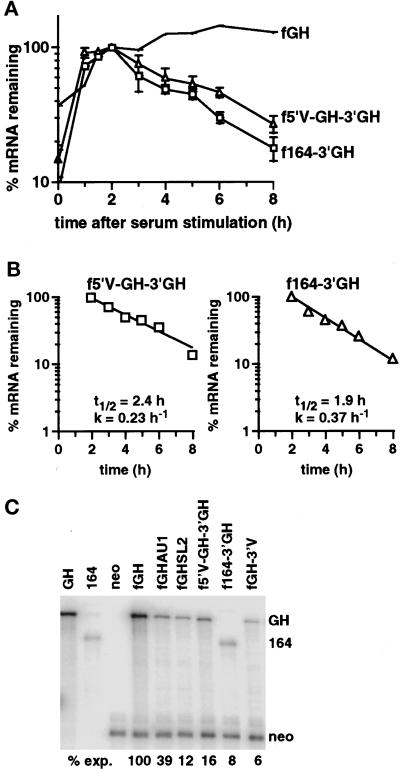
The murine VEGF mRNA comprises an unusually long 5′UTR of 1014 bases and a coding region of variable size, depending on the particular VEGF isoform expressed (Shima et al., 1996). To assess whether the 5′UTR contributed to the normoxic instability of the VEGF mRNA, we inserted the entire VEGF 5′UTR into the 5′UTR of the fGH reporter gene, yielding f5′V-GH-3′GH (Figure 2). The effect of the VEGF coding region on regulation of mRNA stability was tested by replacing the growth hormone coding region in the fGH gene with the coding region for the 164 amino acid isoform, yielding f164–3′GH (Figure 2).
The decay of the hybrid mRNAs was measured after serum stimulation of the c-fos promoter under normoxic conditions. Both the 5′UTR and the coding region destabilized the reporter mRNA (Figure 4, A and B), demonstrating that each region contained independently functioning destabilizing elements. Comparison of the decay profiles (Figures 3C and 4B) indicated that the 3′UTR and coding region destabilized the reporter mRNA to a similar extent (t1/2 = 2.0 and 1.9 h, respectively), whereas the 5′UTR was less destabilizing (t1/2 = 2.4 h).
Figure 4.
The VEGF 5′UTR and 164 amino acid coding region destabilize the reporter mRNA. (A) Time courses of degradation from cells expressing the fGH mRNA, the f5′V-GH-3′GH mRNA (▵), or the f164–3′GH mRNA (□) grown under normoxic conditions. The graph shows data as mean ± SEM for at least three independent experiments, normalized with respect to GAPDH. The levels of serum induction observed for each experiment were 9.6-, 4.4-, and 8.6-fold for the f5′V-GH-3′GH reporter gene and 23-, 16-, 12-, and 14-fold for the f164–3′GH reporter gene. The maximal levels of induction (expressed as a ratio to the GAPDH level) were 0.35, 0.29, and 0.32 for the f5′V-GH-3′GH reporter gene and 0.28, 0.22, 0.31, and 0.28 for the f164–3′GH reporter gene. (B) Decay kinetics of the f5′V-GH-3′GH and f164–3′GH mRNAs. The graph shows mean data taken from A, with the background level of mRNA from the unstimulated samples subtracted. Regression lines, calculated half-lives (t1/2), and decay rates (k) are indicated. (C) RNase protection analysis of RNA isolated from stably transfected BALB/c3T3 cells expressing the indicated chimeric genes. Cells were grown in 7.5% FCS under normoxic conditions. The first threelanes show the protection products obtained with growth hormone (GH), 164 amino acid coding region (164), or neo probes alone. The remaining lanes show protection products found with cells expressing the stable fGH mRNA, the fGH mRNA with AU-destabilizing elements (fGHAU1) (Brown et al., 1996), the fGH mRNA with the stem-loop destabilizing element from G-CSF (fGHSL2) (Brown et al., 1996), or the f5′V-GH-3′GH, f164–3′GH, or fGH-3′V mRNAs. The level of each mRNA was normalized with respect to the neo mRNA. The expression level relative to fGH is indicated below each lane.
We confirmed the destabilizing potential of each of the three regions by determining the relative expression level of each of the mRNAs expressed constitutively from the fos promoter under normoxic conditions (Figure 4C). To account for any differences in transfection efficiencies between plasmids in each of the polyclonal populations, we used the mRNA from the linked neo gene for normalization. Variants of the fGH mRNA containing known destabilizing elements (fGHAU1; fGHSL2) gave reduced levels of the mRNA as expected (Figure 4C), and all three regions of the VEGF mRNA reduced the level of the reporter mRNA (Figure 4C), confirming the presence of destabilizing elements.
We conclude that regions outside the 3′UTR are important for the regulation of VEGF mRNA stability and that the VEGF mRNA contains destabilizing elements not only in the 3′UTR but also in the 5′UTR and coding region.
Multiple Elements Are Required for the Lability of the VEGF mRNA under Normoxic Conditions
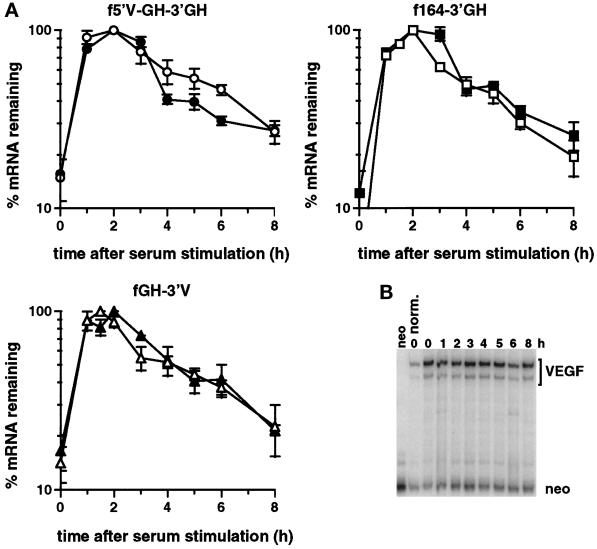
The half-lives of the hybrid mRNAs bearing either the 5′UTR, coding region, or 3′UTR were all significantly greater than that found for the endogenous VEGF mRNA. This indicated that the lability of the endogenous mRNA under normoxic conditions may be caused by the combined action of multiple instability elements. We therefore tested the effect of each pairwise combination of the 5′UTR, coding region, and 3′UTR on degradation of the reporter mRNA (Figure 2). Each pairwise combination was more unstable than each of the contributing single regions (Figure 5). The rate of decay (k) for each of the pairwise combinations (Figure 5) was approximately the sum of the decay rates for each individual region (Figures 3 and 4), indicating that each region acts independently to destabilize the reporter mRNA. This additivity also suggests that each destabilizing activity acts to promote a common, rate-limiting step in the overall degradation pathway of the VEGF mRNA. The half-life of each combination was similar to that of the endogenous VEGF mRNA, indicating that any two of the three regions suffices to recapitulate the lability of the mRNA. We conclude that the VEGF mRNA contains multiple, independently functional destabilizing elements and that the rapid degradation of the VEGF mRNA under normoxia is due to the combined action of these elements.
Figure 5.
Degradation of the reporter mRNA containing pairwise combinations of the VEGF 5′UTR, coding region, and 3′UTR. Time courses of degradation of the f5′V-GH-3′V (A), f5′V-164–3′GH (B), and f164–3′V (C) mRNAs from cells incubated under normoxic conditions. The graphs show data as mean ± SEM for two independent experiments, normalized with respect to GAPDH. The levels of serum induction observed for each experiment were 6.4- and 5.5-fold for the f5′V-GH-3′V reporter gene, 5.7- and 6.3-fold for the f5′V-164–3′GH reporter gene, and 5.2- and 9.3-fold for the f164–3′V reporter gene. The maximal levels of induction (expressed as a ratio to the GAPDH level) were 0.22 and 0.25 for the f5′V-GH-3′V reporter gene, 0.17 and 0.20 for the f5′V-164–3′GH reporter gene, and 0.27 and 0.11 for the f164–3′V reporter gene. Decay kinetics of the mRNAs are also shown and were calculated from mean data with the background levels of mRNA from the unstimulated samples subtracted. Regression lines, calculated half-lives (t1/2), and decay rates (k) are indicated.
The VEGF 5′UTR, Coding Region, or 3′UTR Do Not Independently Confer Hypoxic Stabilization
Having found that each of the three regions in the VEGF mRNA can independently destabilize a reporter mRNA, we tested whether each of the regions responds to hypoxia; however, maintaining the cells in a hypoxic environment did not result in any significant stabilization of any of the hybrid mRNAs (Figure 6A). To verify that the cells had been adequately exposed to hypoxia, we measured the levels of the mRNA from the endogenous VEGF gene. We found that the endogenous VEGF mRNA was induced approximately sevenfold by hypoxia and remained constant throughout the 8-h time course after serum stimulation (Figure 6B), indicating that the cells were responding appropriately to hypoxia and that serum stimulation was unlikely to affect mRNAs already stabilized in response to hypoxia.
Figure 6.
The VEGF 5′UTR, coding region, and 3′UTR do not individually confer hypoxic stabilization on a reporter mRNA. (A) Time courses of degradation from cells expressing the f5′V-GH-3′GH, the f164–3′GH, or the f164–3′V mRNAs grown under normoxia (open symbols) or hypoxia (closed symbols) for 24 h. The graph shows the data as mean ± SEM for at least two independent experiments, normalized with respect to GAPDH. The levels of serum induction observed for the f5′V-GH-3′GH reporter gene were 9.6-, 4.4-, and 8.6-fold under normoxia and 6.2- and 6.6-fold under hypoxia. For the f164-GH-3′GH reporter gene the serum induction was 23-, 16-, and 11-fold under normoxia and 12- and 6.2-fold under hypoxia. For the fGH-3′V reporter gene the serum induction was 7.5-, 7.5-, 4.4-, and 14-fold under normoxia and 7.8-, 5.0-, and 6.6-fold under hypoxia. The maximal levels of induction (expressed as a ratio to the GAPDH level) for the f5′V-GH-3′GH reporter gene were 0.30 and 0.29 under normoxia and 0.23 and 0.18 under hypoxia. The maximal levels of induction for the f164–3′GH reporter gene were 0.23 and 0.22 under normoxia and 0.24 and 0.28 under hypoxia. The maximal levels of induction for the fGH-3′V reporter gene were 0.32, 0.49, and 0.22 under normoxia and 0.26, 0.16, and 0.16 under hypoxia. (B) RNase protection gel of the endogenous VEGF mRNA and the neo mRNA from serum stimulated cells incubated under hypoxia for 24 h before serum stimulation and for the duration of the time course after serum stimulation. RNA from cells grown under normoxic conditions before serum stimulation is shown for comparison. The neo mRNA was used for normalization because the gene is expressed from the SV40 early promoter, the transcription of which is unaffected by hypoxia (Semenza and Wang, 1992).
The inability of the 5′UTR, coding region, and 3′UTR to independently confer hypoxic stabilization on the reporter mRNA indicated that multiple elements are required for stabilization and that the determinants for stabilization are distinct from those that act to destabilize the mRNA under normoxic conditions.
Hypoxic Stabilization of the VEGF mRNA Requires the Cooperation of RNA Elements from Multiple Regions of the mRNA
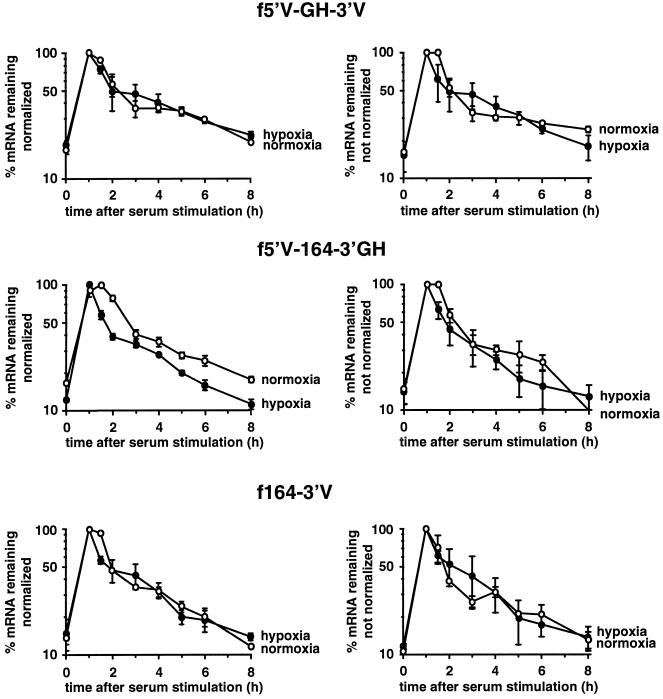
The inability of the 5′UTR, coding region, or 3′UTR to confer hypoxic stabilization suggested that stabilization may require the participation of two or more regions in the VEGF mRNA. We therefore tested the ability of each of the pairwise combinations of the 5′UTR, coding region, and 3′UTR to be stabilized in response to hypoxia. Although combining regions resulted in enhanced degradation rates in normoxic cells, there was no stabilization in response to hypoxia (Figure 7). Because the level of GAPDH mRNA, which is used as an internal standard, is increased as a result of hypoxia, we present the data with and without normalization, to show that there is no apparent bias of the decay rate as a result of the normalization procedure.
Figure 7.
Analysis of the effect of hypoxia on chimeric mRNAs containing pairwise combinations of the VEGF 5′UTR, coding region, and 3′UTR. Time courses of degradation of the f5′V-GH-3′V, f5′V-164–3′GH, or f164–3′V mRNAs from cells incubated under normoxia (open symbols) or hypoxia (closed symbols) for 24 h. The graphs show data as mean ± SEM for two independent experiments, either normalized for GAPDH (left panels) or without normalization (right panels). The levels of serum induction observed for the f5′V-GH-3′V reporter gene were 6.4- and 5.5-fold under normoxia and 4.9- and 6.0-fold under hypoxia. For the f5′V-164–3′GH reporter gene the serum induction was 5.7- and 6.3-fold under normoxia and 8.4- and 8.0-fold under hypoxia. For the f164–3′V reporter gene the serum induction was 5.2- and 9.3-fold under normoxia and 6.6- and 8.3-fold under hypoxia. The maximal levels of induction (expressed as a ratio to the GAPDH level) for the f5′V-GH-3′V reporter gene were 0.22 and 0.27 under normoxia and 0.24 and 0.21 under hypoxia. The maximal levels of induction for the f5′V-164–3′GH reporter gene were 0.18 and 0.22 under normoxia and 0.18 and 0.30 under hypoxia. The maximal levels of induction for the f164–3′V reporter gene were 0.22 and 0.36 under normoxia and 0.19 and 0.21 under hypoxia.
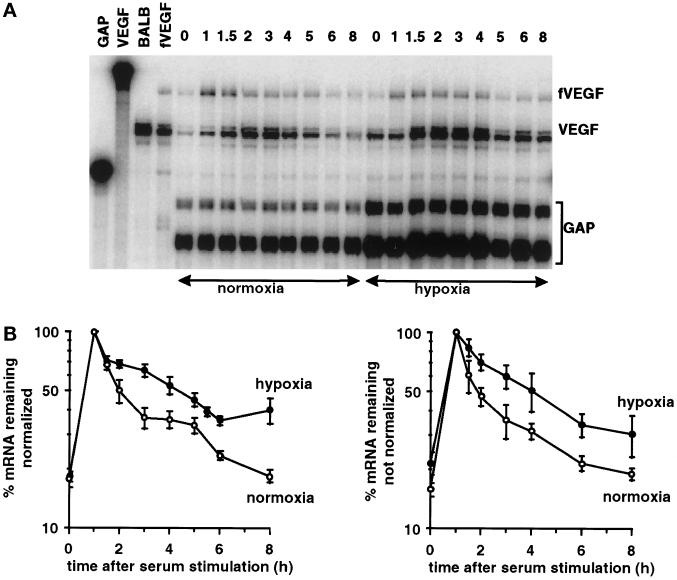
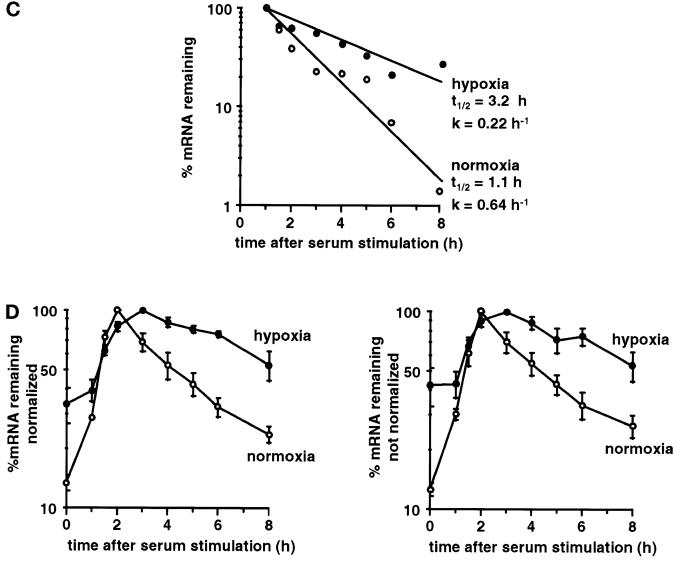
Because there was no regulation of stability with the pairwise combinations, we combined all three regions, essentially regenerating the entire VEGF mRNA, but with a 48-base sequence tag inserted between the coding region and the 3′UTR. This allowed the discrimination between the reconstructed mRNA, termed fVEGF (Figure 2), and the endogenous VEGF mRNA. In contrast to the results obtained with the pairwise combinations, we found that the reconstructed fVEGF mRNA was stabilized in response to hypoxia (Figure 8, A and B). Again, the data are shown with and without normalization, to show that normalization with GAPDH did not significantly alter the rate of decay of the fVEGF mRNA. We observed stabilization of the reconstructed fVEGF mRNA in each of three repetitions of the experiment, including experiments performed simultaneously with pairwise combinations that showed no hypoxic stabilization (Figure 7). The half-life of the reconstructed mRNA was 65 min under normoxic conditions, and the mRNA was stabilized 2.9-fold in response to hypoxia (Figure 8C), essentially recapitulating the stabilization found with the endogenous VEGF mRNA (Figure 1). The stabilization of the reconstructed fVEGF mRNA, but not any of the pairwise combinations, demonstrates that hypoxic stabilization of the VEGF mRNA requires the cooperation of elements in all three regions of the mRNA.
Figure 8.
A reconstructured version of the VEGF mRNA is stabilized in response to hypoxia. RNA was isolated after serum stimulation of cells expressing the fVEGF mRNA grown under normoxia or hypoxia for 24 h. (A) PhosphorImager analysis of representative RNase protection gel. The fVEGF mRNA, the endogenous VEGF mRNA, and the GAPDH mRNA protection products are indicated. Also shown are the undigested GAPDH and VEGF probes and use of the VEGF probe alone on RNA isolated from cells not expressing the fVEGF mRNA (BALB) or from cells expressing the fVEGF mRNA (fVEGF). (B) Time course of degradation of the fVEGF mRNA under normoxia and hypoxia. The graph shows the data as mean ± SEM for three independent experiments, either normalized for GAPDH (left panel) or without normalization (right panel). The levels of serum induction observed for the fVEGF reporter gene were 6.6-, 5.8-, and 4.8-fold under normoxia and 6.2-, 5.3-, and 5.2-fold under hypoxia. The maximal levels of induction (expressed as a ratio to the GAPDH level) for the fVEGF reporter gene were 0.28, 0.26, and 0.20 under normoxia and 0.24, 0.23, and 0.20 under hypoxia. (C) Time course of degradation of the fVEGF mRNA under normoxia and hypoxia. The graph shows mean data taken from B, with the background level of mRNA from the unstimulated samples subtracted. Regression lines for the decay of the mRNA under normoxia and hypoxia, the calculated half-lives (t1/2), and decay rates (k) are shown. (D) Time course of induction and degradation of the endogenous VEGF mRNA under normoxia and hypoxia. The graph shows the data as mean ± SEM for three independent experiments, either normalized for GAPDH (left panel) or without normalization (right panel). The levels of serum induction observed for the endogenous VEGF gene were 7.8-, 9.6-, and 7.0-fold under normoxia and 3.7-, 2.8-, and 2.5-fold under hypoxia. The maximal levels of induction (expressed as a ratio to the GAPDH level) for the endogenous VEGF gene were 0.55, 0.67, and 038 under normoxia and 1.4, 0.67, and 0.58 under hypoxia.
The endogenous VEGF mRNA also showed a pattern of serum stimulation and decay under normoxic conditions (Figure 8D), suggesting that the VEGF promoter is subject to a pulse of transcription in response to serum stimulation. The apparent half-life of the endogenous mRNA under these conditions was ∼100 min. Under hypoxia, the kinetics of VEGF expression were consistent with stabilization of the mRNA, with an apparent half-life of 210 min (Figure 8D).
We conclude that the VEGF mRNA contains multiple, independently functional destabilizing elements, and the cooperation of RNA elements from three different regions is essential for stabilization of the mRNA in response to hypoxia.
DISCUSSION
The regulation of VEGF mRNA stability is of particular interest, not only because VEGF is a member of the class of hypoxia-stabilized mRNAs, but also because of its importance as a potential target for antiangiogenic therapy (Kim et al., 1993; Millauer et al., 1994). The design of agents to inhibit or promote the activity of VEGF requires elucidation of the specific mechanisms controlling its expression. For this reason there is considerable interest in identifying the determinants in the VEGF mRNA involved in its lability under normoxic conditions and its stabilization in response to hypoxia.
In this study we investigated the role of various regions in the VEGF mRNA in regulating its lability in vivo. The use of a reporter mRNA driven from the fos promoter allowed us to determine the effect of different regions of the VEGF mRNA on the stability of a reporter gene, rather than using indirect means such as changes in the steady-state levels of reporter enzymes or the use of nonspecific inhibitors of transcription. In all of our constructs we maintained the positional and translational context of the inserted region in the reporter mRNA. We found that the VEGF mRNA contains multiple, independently functional destabilizing elements and that the rapid degradation of the VEGF mRNA under normoxia is due to the combined action of these elements. The normoxic half-life of the entire reconstructed mRNA was not significantly different from those calculated for each pairwise combination of the three regions, suggesting that any two of the three regions suffices to recapitulate the lability of the mRNA; however, this finding could reflect the fact that the fos promoter, which produces a transcriptional pulse of ∼30 min duration (Rivera et al., 1990), cannot resolve half-lives significantly <1 h.
A number of other mRNAs have been shown to contain destabilizing elements in both the 3′UTR and coding region, including the fos and myc mRNAs (Kabnick and Housman, 1988; Shyu et al., 1989; Wisdom and Lee, 1991; Herrick and Ross, 1994); however, the ability of the VEGF 5′UTR to destabilize the reporter mRNA in our studies suggests that a destabilizing element is also present in the 5′UTR. The presence of a destabilizing element in the 5′UTR is highly unusual, although we note that the first 40 bases of the fos mRNA are able to weakly destabilize a globin reporter mRNA (Kabnick and Housman, 1988). We found that the VEGF 5′UTR, coding region, and 3′UTR were also potently destabilizing in NIH3T3 cells, indicating that the destabilizing activity of each region was not restricted to the BALB/c3T3 cells used in our study. Although the mechanism of destabilization promoted by each of the destabilizing elements in the VEGF mRNA remains to be determined, the existence of multiple destabilizing elements in the VEGF mRNA indicates that multiple degradation pathways have evolved to ensure rapid turnover of the mRNA under normoxic conditions; however, it has also become apparent that a number of tumor-derived cell lines constitutively express elevated levels of VEGF caused by persistent stabilization of the mRNA (White et al., 1995; Iliopoulos et al., 1996; Levy et al., 1996a,b). The existence of multiple instability elements in the VEGF mRNA and the ability of any two of the three regions to recapitulate the lability of the mRNA suggests that the persistent stabilization of the VEGF mRNA in these tumor-derived cells may be due to the constitutive activation of a stabilizing pathway rather than the loss of function of two distinct degradation pathways.
In contrast to the additive effects of individual regions in destabilizing the mRNA, we found that hypoxic stabilization of the VEGF mRNA occurs only if the 5′UTR, coding region, and 3′UTR are all present in the mRNA. Based on the results of a previous study in which hypoxic stabilization of the VEGF 3′UTR was observed in a cell-free extract system (Levy et al., 1996a,b), our expectation was that the 3′UTR would be sufficient to confer correct regulation on a reporter mRNA in vivo; however, our observation in intact cells demonstrates that the regulation of VEGF mRNA stability is more complex than previous studies have suggested and involves considerable complexity in the molecular interactions. This could be due to the requirement for three distinct RNA binding proteins each interacting with a discrete element, or it could reflect the need to form a particular RNA structure from various RNA sequences that is recognized by a single protein.
The ability to recapitulate hypoxic stabilization is significant for a number of reasons. It will allow further delineation of the elements required for stabilization and therefore provide a route to the isolation of factors involved in stabilization of the mRNA. It also provides the ability to study the effect of various conditions or factors on mRNA stability directly, without the complication of associated changes in transcription from the VEGF promoter. Furthermore, our demonstration of the need for the cooperation of multiple elements for hypoxic stabilization of the VEGF mRNA could have important implications for antiangiogenic therapy. Inhibition of the function of any of the cooperating elements should prevent accumulation of the mRNA in response to hypoxia.
Although the VEGF 3′UTR is not sufficient to confer hypoxic stabilization, our results show that elements in the 3′UTR are essential for stabilization. This is supported by other studies that show that RNA elements in the 3′UTR play a role in hypoxic stabilization. Antisense expression of HuR, a protein that binds to a distal AU-rich region in the VEGF 3′UTR, prevents hypoxic stabilization of the VEGF mRNA (Levy et al., 1998). HuR is a member of the Elav-like family of binding proteins and has been implicated in the function of AU-destabilizing elements (Myer et al., 1997). Additionally, one of the hypoxia-inducible proteins found to bind to a pyrimidine-rich element in the VEGF 3′UTR in vitro also binds to a similar element found in the erythropoietin and tyrosine hydroxylase mRNAs (Czyzyk-Krzeska and Beresh, 1996; Iliopoulos et al., 1996; Levy et al., 1996a,b; McGary et al., 1997). Mutation of this element in the erythropoietin mRNA destabilizes the mRNA in vivo under normoxic conditions and prevents hypoxic stabilization of the mRNA (McGary et al., 1997).
We found that the endogenous VEGF mRNA showed a pattern of serum stimulation and decay under normoxic conditions, indicating that the VEGF promoter is also subject to a pulse of transcription in response to serum stimulation (Figure 8). A similar pattern of VEGF transcription in response to serum has also been seen in human fibroblasts (Enholm et al., 1997). The pattern of decay of the endogenous mRNA under hypoxia was consistent with stabilization of the mRNA, although nuclear run-on experiments are required to verify that the shut-off of transcription under hypoxia and normoxia occurs at the same rate. Nevertheless, the degree of hypoxic stabilization (twofold) of the endogenous mRNA determined from these apparent half-lives was similar to that determined from the reconstructed mRNA. If the pulse of transcription generated from the VEGF promoter in response to serum stimulation is similar under normoxia and hypoxia, this method may allow the direct determination of the effect of various agents on stabilization of the endogenous VEGF mRNA.
The degree of complexity of the molecular interactions required for the regulation of stability of some mRNAs is only now becoming apparent. Our finding that multiple regions are required for VEGF mRNA stabilization parallels similar findings with the interleukin (IL)-2 and IL-11 mRNAs. Stabilization of the IL-2 mRNA in T cells by the c-Jun amino-terminal kinase (JNK) pathway requires elements at the junction of the 5′UTR and coding region as well as elements in the 3′UTR (Chen et al., 1998), and stabilization of the IL-11 mRNA in bone marrow stromal cells in response to phorbol ester requires all three regions of the mRNA (Yang et al., 1996); however, the VEGF mRNA appears to be unique in the complexity of the interactions required for both its lability and stabilization. Such complexity most likely reflects the need for expression of the molecule to be very tightly regulated and is most apparent in the failure in vascular development in mice carrying a single defective VEGF gene (Carmeliet et al., 1996; Ferrara et al., 1996). This heterozygous lethality is unprecedented in an autosomal gene and indicates that the level of VEGF is critical for correct embryonic vascular development. The ability to regulate the stability of the VEGF mRNA allows a level of control in addition to regulation of transcription, and this may be important for coordinating expression of VEGF with the many other factors that also play a role in the angiogenic process (Folkman and Shing, 1992).
ACKNOWLEDGMENTS
We thank T. Gonda for critical reading of this manuscript. This work was supported by grants from the AntiCancer Foundation of South Australia and the Kathleen Cunningham Foundation. Initial postdoctoral support for J.A.D. was provided by an F.T.T. Fricker Fellowship from the Department of Medicine, University of Adelaide.
Abbreviations used:
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- neo
neomycin phosphotransferase
- RNase
ribonuclease
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
Footnotes
Dedicated to the memory of Werner Risau (December 18, 1953–December 13, 1998).
REFERENCES
- Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995;73:333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Brown CY, Lagnado CA, Goodall GJ. A cytokine mRNA-destabilizing element that is structurally and functionally distinct from A+U-rich elements. Proc Natl Acad Sci USA. 1996;93:13721–13725. doi: 10.1073/pnas.93.24.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chen CY, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claffey KP, Shih SC, Mullen A, Dziennis S, Cusick JL, Abrams KR, Lee SW, Detmar M. Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell. 1998;9:469–481. doi: 10.1091/mbc.9.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T, Gitay-Goren H, Sharon R, Shibuya M, Halaban R, Levi BZ, Neufeld G. VEGF121, a vascular endothelial growth factor (VEGF) isoform lacking heparin binding ability, requires cell-surface heparan sulfates for efficient binding to the VEGF receptors of human melanoma cells. J Biol Chem. 1995;270:11322–11326. doi: 10.1074/jbc.270.19.11322. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Beresh JE. Characterization of the hypoxia-inducible protein binding site within the pyrimidine-rich tract in the 3′-untranslated region of the tyrosine hydroxylase mRNA. J Biol Chem. 1996;271:3293–3299. doi: 10.1074/jbc.271.6.3293. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem. 1994;269:760–764. [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Paulding WR, Beresh JE, Kroll SL. Posttranscriptional regulation of tyrosine hydroxylase gene expression by oxygen in PC12 cells. Kidney Int. 1997;51:585–590. doi: 10.1038/ki.1997.84. [DOI] [PubMed] [Google Scholar]
- Damert A, Machein M, Breier G, Fujita MQ, Hanahan D, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor expression in a rat glioma is conferred by two distinct hypoxia-driven mechanisms. Cancer Res. 1997;57:3860–3864. [PubMed] [Google Scholar]
- Enholm B, et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MA, Gaut CC, Bunn HF. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991;77:271–277. [PubMed] [Google Scholar]
- Graven KK, Troxler RF, Kornfeld H, Panchenko MV, Farber HW. Regulation of endothelial cell glyceraldehyde-3-phosphate dehydrogenase expression by hypoxia. J Biol Chem. 1994;269:24446–24453. [PubMed] [Google Scholar]
- Herrick DJ, Ross J. The half-life of c-myc mRNA in growing and serum-stimulated cells: influence of the coding and 3′ untranslated regions and role of ribosome translocation. Mol Cell Biol. 1994;14:2119–2128. doi: 10.1128/mcb.14.3.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabnick KS, Housman DE. Determinants that contribute to cytoplasmic stability of human c-fos and β-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988;8:3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Lagnado CA, Brown CY, Goodall GJ. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A) Mol Cell Biol. 1994;14:7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998;273:6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- Levy NS, Goldberg MA, Levy AP. Sequencing of the human vascular endothelial growth factor (VEGF) 3′ untranslated region (UTR): conservation of five hypoxia-inducible RNA protein binding sites. Biochim Biophys Acta. 1997;1352:167–173. doi: 10.1016/s0167-4781(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Goldberg MA. Posttranscriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996a;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Goldberg MA. Hypoxia-inducible protein binding to vascular endothelial growth factor mRNA and its modulation by the von Hippel-Lindau protein. J Biol Chem. 1996b;271:25492–25497. doi: 10.1074/jbc.271.41.25492. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- McGary EC, Rondon IJ, Beckman BS. Posttranscriptional regulation of erythropoietin mRNA stability by erythropoietin mRNA-binding protein. J Biol Chem. 1997;272:8628–8634. doi: 10.1074/jbc.272.13.8628. [DOI] [PubMed] [Google Scholar]
- Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- Myer VE, Fan XC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997;16:2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Ponte P, Gunning P, Blau H, Kedes L. Human actin genes are single copy for α-skeletal and α-cardiac actin but multicopy for β- and gamma-cytoskeletal genes: 3′ untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983;3:1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera VM, Sheng M, Greenberg ME. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990;4:255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J, Meerovitch K, Bleackley RC, Paetkau V. Mechanisms regulating the level of IL-2 mRNA in T lymphocytes. J Immunol. 1988;140:2243–2248. [PubMed] [Google Scholar]
- Shima DT, Deutsch U, D’Amore PA. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D’Amore PA. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and posttranscriptional regulatory sequences. J Biol Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu AB, Greenberg ME, Belasco JG. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- White FC, Carroll SM, Kamps MP. VEGF mRNA is reversibly stabilized by hypoxia and persistently stabilized in VEGF-overexpressing human tumor cell lines. Growth Factors. 1995;12:289–301. doi: 10.3109/08977199509028967. [DOI] [PubMed] [Google Scholar]
- Wisdom R, Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991;5:232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]
- Yang L, Steussy CN, Fuhrer DK, Hamilton J, Yang YC. Interleukin-11 mRNA stabilization in phorbol ester-stimulated primate bone marrow stromal cells. Mol Cell Biol. 1996;16:3300–3307. doi: 10.1128/mcb.16.7.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiaga AM, Belasco JG, Greenberg ME. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol Cell Biol. 1995;15:2219–2230. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]