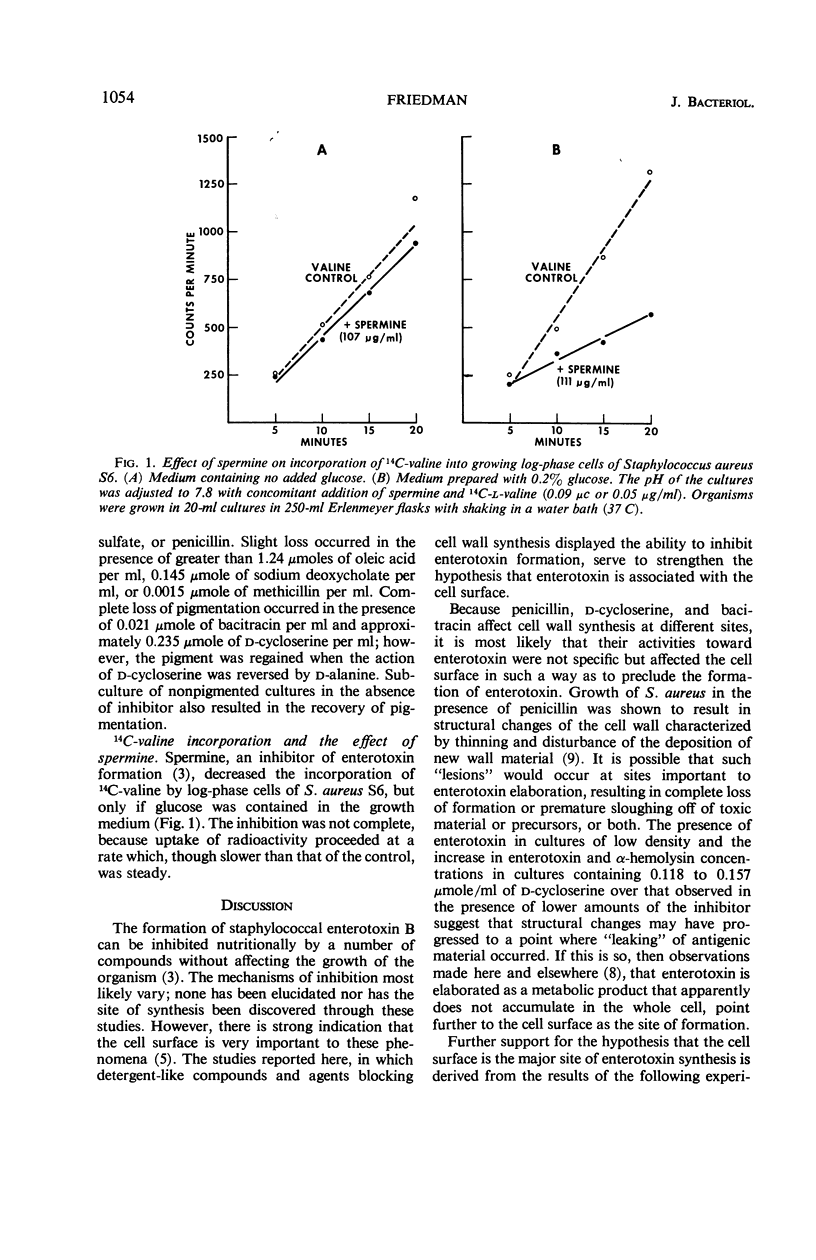
Abstract
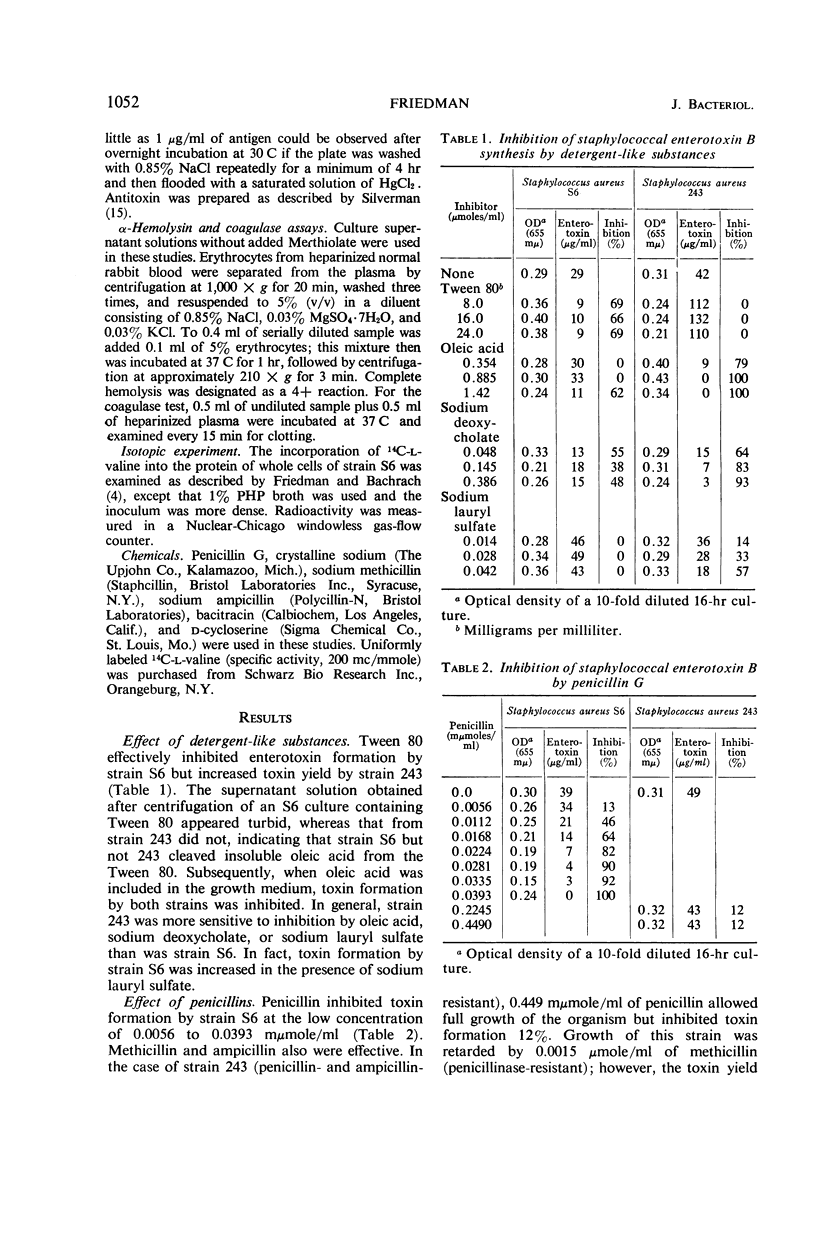
Enterotoxin B formation by Staphylococcus aureus S6 was inhibited by Tween 80, oleic acid, sodium deoxycholate, penicillin, d-cycloserine, or bacitracin. Toxin formation by strain 243 was sensitive to oleic acid, sodium deoxycholate, sodium lauryl sulfate, d-cycloserine, or bacitracin. The effect of d-cycloserine was reversed by d-alanine with strain 243 but not with strain S6. Neither penicillin nor bacitracin inhibited α-hemolysin or coagulase activity of strain S6; however, 0.118 μmoles of d-cycloserine per ml increased the α-hemolysin titer more than eightfold. Pigmentation of strain 243 was reduced by oleic acid, sodium deoxycholate, or methicillin, and was completely inhibited by d-cycloserine or bacitracin. Glucose was required for the inhibition by spermine of 14C-valine incorporation into cellular protein of strain S6. These data indicate that the cell surface may contain sites important to the synthesis of enterotoxin B.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASMAN E. P., BERGDOLL M. S., ROBINSON J. DESIGNATION OF STAPHYLOCOCCAL EXTEROTOXINS. J Bacteriol. 1963 Mar;85:715–716. doi: 10.1128/jb.85.3.715-716.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN M. E., WHITE J. D. IMMUNOFLUORESCENT DEMONSTRATION OF CELL-ASSOCIATED STAPHYLOCOCCAL ENTEROTOXIN B. J Bacteriol. 1965 Apr;89:1155–1155. doi: 10.1128/jb.89.4.1155-1155.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. E., Bachrach U. Inhibition of protein synthesis by spermine in growing cells of Staphylococcus aureus. J Bacteriol. 1966 Jul;92(1):49–55. doi: 10.1128/jb.92.1.49-55.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. E. Inhibition of staphylococcal enterotoxin B formation in broth cultures. J Bacteriol. 1966 Jul;92(1):277–278. doi: 10.1128/jb.92.1.277-278.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., FRANCOMBE W. H., MAYALL B. H. The effect of penicillin on the structure of staphylococcal cell walls. Can J Microbiol. 1959 Dec;5:641–648. doi: 10.1139/m59-078. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Marcus S. Relation of pyrogenic and emetic properties of enterobacteriaceal endotoxin and of staphylococcal enterotoxin. J Bacteriol. 1964 May;87(5):1019–1026. doi: 10.1128/jb.87.5.1019-1026.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S., ROZANSKY R. Mechanism of the antibacterial action of spermine. Arch Biochem Biophys. 1959 Mar;81(1):36–54. doi: 10.1016/0003-9861(59)90173-0. [DOI] [PubMed] [Google Scholar]
- SILVERMAN S. J. SEROLOGICAL ASSAY OF CULTURE FILTRATES FOR STAPHYLOCOCCUS ENTEROTOXIN. J Bacteriol. 1963 Apr;85:955–956. doi: 10.1128/jb.85.4.955-956.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURGALLA M. J., BERGDOLL M. S., DACK G. M. Some observations on the assay of staphylococcal enterotoxin by the monkey-feeding test. J Lab Clin Med. 1953 May;41(5):782–788. [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Wagman J., Spero L., Dunnery D. A., Bergdoll M. S. Purification of staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1011–1016. doi: 10.1021/bi00882a005. [DOI] [PubMed] [Google Scholar]
- Schlenk F., Dainko J. L. Effects of ribonuclease and spermine on yeast cells. Arch Biochem Biophys. 1966 Jan;113(1):127–133. doi: 10.1016/0003-9861(66)90165-2. [DOI] [PubMed] [Google Scholar]


