Abstract
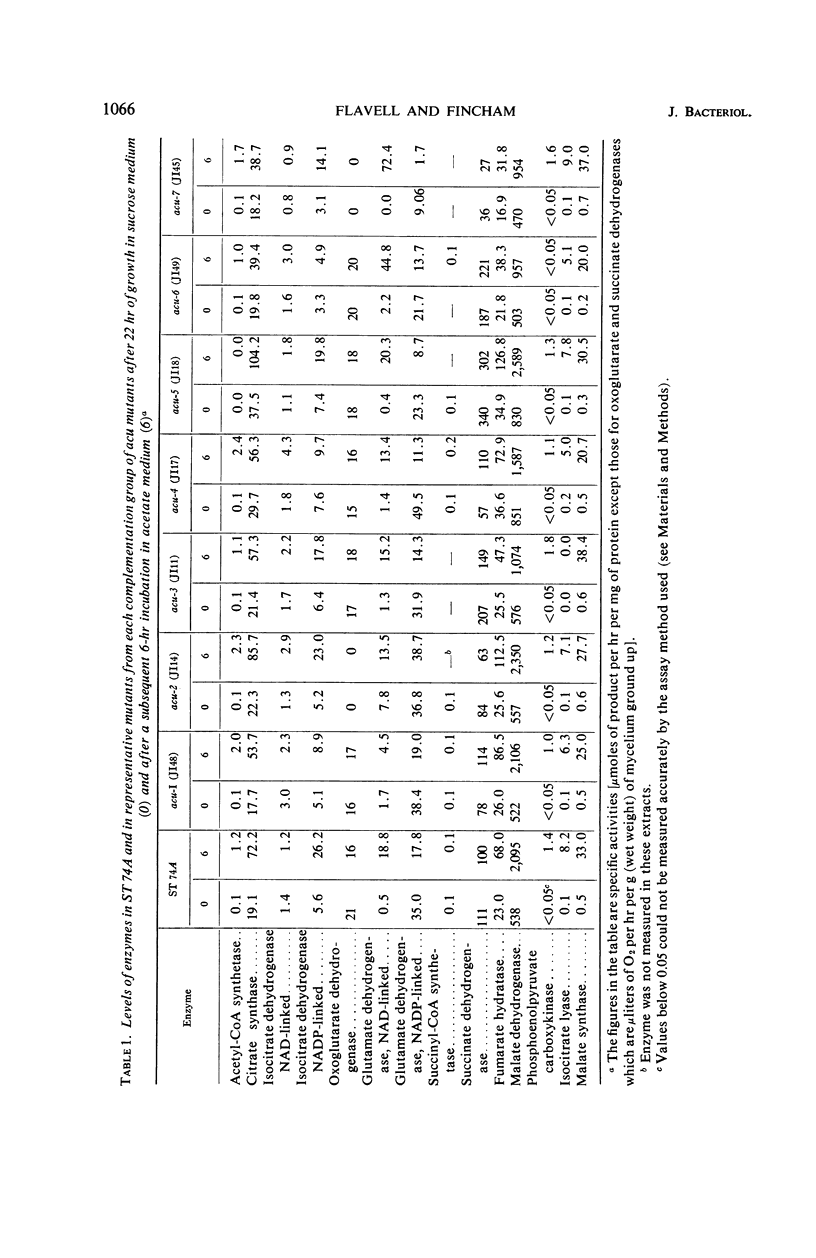
The levels of Krebs cycle, glyoxylate cycle, and certain other enzymes were measured in a wild-type strain and in seven groups of acetate-nonutilizing (acu) mutants of Neurospora crassa, both after growth on a medium containing sucrose and after a subsequent 6-hr incubation in a similar medium, containing acetate as the sole source of carbon. In the wild strain, incubation in acetate medium caused a rise in the levels of isocitrate lyase, malate synthase, phosphoenolpyruvate carboxykinase, acetyl-coenzyme A synthetase, nicotinamide adenine dinucleotide phosphate-linked isocitrate dehydrogenase, citrate synthase, and fumarate hydratase. Isocitrate lyase activity was absent in acu-3 mutants; acu-5 mutants lacked acetyl-coenzyme A synthetase activity; and no oxoglutarate dehydrogenase activity (or only low levels) could be detected in acu-2 and acu-7 mutants. In acu-6 mutants, phosphoenolpyruvate carboxykinase activity was either very low or absent. No specific biochemical deficiencies could be attributed to the acu-1 and acu-4 mutations. The role of several of these enzymes during growth on acetate is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., APPEL S. H., TOMKINS G. M. A SPECTROPHOTOMETRIC ASSAY FOR THIOGALACTOSIDE TRANSACETYLASE. J Biol Chem. 1965 Jan;240:10–13. [PubMed] [Google Scholar]
- CODDINGTON A., FINCHAM J. R. PROOF OF HYBRID ENZYME FORMATION IN A CASE OF INTER-ALLELIC COMPLEMENTATION IN NEUROSPORA CRASSA. J Mol Biol. 1965 May;12:152–161. doi: 10.1016/s0022-2836(65)80289-3. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-onutilizing mutants of Neurospora crassa. I. Mutant isolation, complementation studies, and linkage relationships. J Bacteriol. 1968 Mar;95(3):1056–1062. doi: 10.1128/jb.95.3.1056-1062.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Oliver R. M., Reed L. J. Alpha-Keto acid dehydrogenase complexes, V. Macromolecular organization of pyruvate and alpha-ketoglutarate dehydrogenase complexes isolated from beef kidney mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):534–541. doi: 10.1073/pnas.56.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munkres K. D., Woodward D. O. On the genetics of enzyme locational specificity. Proc Natl Acad Sci U S A. 1966 May;55(5):1217–1224. doi: 10.1073/pnas.55.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTE D., KLINGENBERG M., BUECHER T. Comparable and specific proportions in the mitochondrial enzyme activity pattern. Biochem Biophys Res Commun. 1962 Jun 4;7:425–429. doi: 10.1016/0006-291x(62)90328-5. [DOI] [PubMed] [Google Scholar]
- PETTE D., LUH W., BUECHER T. A constant-proportion group in the enzyme activity pattern of the Embden-Meyerhof chain. Biochem Biophys Res Commun. 1962 Jun 4;7:419–424. doi: 10.1016/0006-291x(62)90327-3. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Amil M., De Torrontegui G., Palacián E., Catalina L., Losada M. Properties and function of yeast pyruvate carboxylase. J Biol Chem. 1965 Sep;240(9):3485–3492. [PubMed] [Google Scholar]
- SANWAL B. D., ZINK M. W., STACHOW C. S. NICOTINAMIDE ADENINE DINUCLEOTIDE-SPECIFIC ISOCITRIC DEHYDROGENASE. A POSSIBLE REGULATORY PROTEIN. J Biol Chem. 1964 May;239:1597–1603. [PubMed] [Google Scholar]
- SHEPHERD C. J. The enzymes of carbohydrate metabolism in Neurospora. 1. Succinic dehydrogenase. Biochem J. 1951 Apr;48(4):483–486. doi: 10.1042/bj0480483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS B. S. Oxalacetic carboxylase deficiency of the succinate-requiring mutants of Neurospora crassa. J Biol Chem. 1957 Mar;225(1):535–544. [PubMed] [Google Scholar]
- TURIAN G. SUR LE M'ECANISME DE L'INDUCTION ISOCITRATASIQUE CHEZ ALLOMYCES ET NEUROSPORA. Pathol Microbiol (Basel) 1963;26:553–563. [PubMed] [Google Scholar]



