Abstract
We noted previously that certain aminoglycoside antibiotics inhibit the binding of coatomer to Golgi membranes in vitro. The inhibition is mediated in part by two primary amino groups present at the 1 and 3 positions of the 2-deoxystreptamine moiety of the antibiotics. These two amines appear to mimic the ε-amino groups present in the two lysine residues of the KKXX motif that is known to bind coatomer. Here we report the effects of 1,3-cyclohexanebis(methylamine) (CBM) on secretion in vivo, a compound chosen for study because it contains primary amino groups that resemble those in 2-deoxystreptamine and it should penetrate lipid bilayers more readily than antibiotics. CBM inhibited coatomer binding to Golgi membranes in vitro and in vivo and inhibited secretion by intact cells. Despite depressed binding of coatomer in vivo, the Golgi complex retained its characteristic perinuclear location in the presence of CBM and did not fuse with the endoplasmic reticulum (ER). Transport from the ER to the Golgi was also not blocked by CBM. These data suggest that a full complement of coat protein I (COPI) on membranes is not critical for maintenance of Golgi integrity or for traffic from the ER to the Golgi but is necessary for transport through the Golgi to the plasma membrane.
INTRODUCTION
Coat protein I (COPI) is a protein complex concentrated on membranes of the intermediate compartment (also called vesicular tubular clusters, the endoplasmic reticulum-Golgi–intermediate compartment, the salvage compartment, and the 15°C compartment) and the Golgi complex in mammalian cells (Oprins et al., 1993; Pind et al., 1994; Griffiths et al., 1995). Surprisingly, COPI was also found recently on peroxisomes (Passreiter et al., 1998). COPI consists of ADP-ribosy-lation factor 1 (ARF1)1 and coatomer (for reviews, see Kreis and Pepperkok, 1994; Kreis et al., 1995; Rothman and Wieland, 1996). ARF1, a small GTP-binding protein, is implicated in recruiting coatomer to membranes. Coatomer is a soluble macromolecular protein complex that contains seven protein subunits (α, β, β′, γ, δ, ε, and ζ). The precise role of COPI-coated vesicles in secretory membrane traffic is under debate (for reviews, see Bannykh and Balch, 1997; Cosson and Letourneur, 1997; Schekman and Mellman, 1997). Strong genetic and biochemical evidence indicates that COPI-coated vesicles function in retrograde transport, conveying membrane from the Golgi complex back to the endoplasmic reticulum (Letourneur et al., 1994; Cosson et al., 1996; Lewis and Pelham, 1996). Biochemical and morphological evidence also suggests that COPI-coated vesicles function in anterograde transport, conveying membrane through the Golgi complex toward the plasma membrane (Fiedler et al., 1996; Orci et al., 1997). Thus, COPI may have a dual function and participate in both anterograde and retrograde membrane transport.
Coatomer binds peptides containing the carboxy-terminal motif KKXX in which K is lysine and X is any amino acid (Cosson and Letourneur, 1994). This motif, or a closely related sequence, is present in the cytoplasmic domains of many type I transmembrane proteins that are residents of the endoplasmic reticulum and is a signal for returning the proteins to the endoplasmic reticulum should they escape to the Golgi complex (Jackson et al., 1990, 1993). The fact that coatomer binds this retrieval motif is part of the evidence that COPI-coated vesicles function in retrograde transport from the Golgi to the endoplasmic reticulum. Coatomer was also recently reported to bind peptides containing a diphenylalanine motif, and it was suggested that this interaction may mediate anterograde transport by COPI (Fiedler et al., 1996; Fiedler and Rothman, 1997). The basis for anterograde or retrograde selectivity of COPI might be related to sequence motifs in transmembrane cargo proteins.
Coatomer recruitment to membranes involves the GTPase cycle of ARF1, including the action of a guanine nucleotide exchange factor that promotes the exchange of GDP for GTP and results in the association of cytoplasmic ARF1 with membranes. How the binding of ARF1 to membranes recruits coatomer is not well understood, but ARF1 can be cross-linked to the β subunit of coatomer (Zhao et al., 1997), suggesting that coatomer could bind ARF1 directly on the membrane. Alternatively, ARF1 may promote coatomer binding to another receptor on the membrane. In addition, ARF1 stimulates phospholipase D (Ktistakis et al., 1995), and there is evidence that ARF1 need not be present for coatomer binding provided phospholipase D is activated (Ktistakis et al., 1996).
Studies with the drug brefeldin A have strongly influenced models of coatomer function. Brefeldin A dissociates COPI from membranes of intact cells and induces the Golgi apparatus and the endoplasmic reticulum (ER) to fuse, suggesting that removal of the COPI coat might induce membrane fusion. However, the molecular mechanism of brefeldin A action is not well understood, and recent work of Mironov et al. (1997) suggests that the dissociation of coatomer from Golgi membranes induced by brefeldin A may not be sufficient to cause unregulated membrane fusion. It would be useful to have alternative agents that interfere with coatomer binding in intact cells to help investigate coatomer function and to compare the consequences with those of brefeldin A treatment. We recently found that certain aminoglycoside antibiotics bind to coatomer in vitro (Hudson and Draper, 1997). The features of this interaction suggested that the antibiotics were binding to the same site on coatomer that binds the KKXX sequence and that there were at least two such sites per coatomer. Guided by the structure of the antibiotics, we studied compounds that might retain the ability to interact with coatomer and also more readily pass through the plasma membrane so that the effects of the compounds on the secretory process could be analyzed with intact cells. We found that 1,3-cyclohexanebis(methylamine) (CBM) inhibited coatomer binding to membranes in vitro and in vivo and inhibited secretion by intact cells. However, the drug did not induce the Golgi apparatus to fuse with the ER and did not inhibit transport from the ER to the Golgi.
MATERIALS AND METHODS
Reagents
l-1-Tosylamide-2-phenylethylchloromethyl-trypsin, soybean trypsin inhibitor, protein A-Sepharose CL-4B, endoglycosidase H, HEPES, sucrose, magnesium acetate, potassium chloride, ATP, GTP, creatine phosphate, creatine phosphokinase, protease inhibitors, dilysine, and superfibronectin were purchased from Sigma Chemical (St. Louis, MO). Brefeldin A was purchased from Calbiochem (La Jolla, CA), dissolved in DMSO to 1 mg/ml, and stored at −20°C before use. Tran35S label was from ICN Radiochemical (Irvine, CA). CBM, 1,2-diaminocyclohexane, and 1,3-diaminopropane were from Acros Organics (Pittsburgh, PA). Fluoromount G was from Fisher Scientific (Pittsburgh, PA).
Antibodies
Mouse anti-vesicular stomatitis virus (VSV) G protein (monoclonal P5D4) and horseradish peroxidase conjugated either to goat anti-mouse IgG or to protein A were from Sigma Chemical. Goat anti-hemagglutinin of influenza virus (-HA) (strain X31) antiserum was from Dr. R. Webster (Saint Jude Children’s Hospital, Memphis, TN). Rabbit anti-mannosidase II was from Dr. K. Moremen (The University of Georgia, Athens, GA). Anti-β-COP monoclonal antibody M3A5 was obtained from the supernatant of hybridoma cells. The cells were provided by Dr. Thomas Kreis (Universite de Geneve, Geneva, Switzerland). Mouse anti-trans-Golgi network (-TGN)38 monoclonal antibody (mAb2F 7.1) was from Affinity Bioreagents (Golden, CO). Mouse anti-γ-adaptin monoclonal antibody (mAb 88) was from Transduction Laboratories (Lexington, KY). Rabbit polyclonal anti-VSV G protein was prepared with G protein as the immunogen.
Cells and Virus
The propagation and use of recombinant influenza virus X31 were as described by Wang et al. (1990). Cells were cultured as described previously (Kao and Draper, 1992; Bau and Draper, 1993).
Analysis of Influenza Virus HA Protein Transport
To study the effect of diamino compounds on the transport of influenza virus HA protein from the ER to the Golgi and then to the cell surface, we grew Chinese hamster ovary (CHO) or normal rat kidney (NRK) cells to confluence in 24-well plates 1 d before an experiment. Cells were infected with influenza virus at 37°C for 45 min, washed, and incubated for a further 3.5–4 h. The cells were then incubated for 30 min in assay medium (DMEM lacking methionine and sodium bicarbonate and supplemented with 10% dialyzed fetal bovine serum and diamino compounds, pH 8.8). Subsequently, cells were incubated for 5 min with assay medium containing 100 μCi/ml Tran35S label. The radioactive medium was then removed, and 0.2 ml of fresh assay medium was added for further incubation.
To assess the acquisition of endoglycosidase H resistance by HA and the sensitivity of HA to extracellular trypsin, the labeled cells were rinsed once with PBS and treated on ice with PBS containing 100 μg/ml l-1-tosylamide-2-phenylethylchloromethyl-trypsin for 30 min. Ten microliters of 10 mg/ml soybean trypsin inhibitor were added 15 min before the cells were lysed with lysis buffer (1% NP-40, 50 mM Tris, pH 8.0, 150 mM NaCl, 10 mM EDTA, 1 mM PMSF, 0.1 μg/ml aprotinin, and 0.1 mg/ml soybean trypsin inhibitor). HA was immunoprecipitated with goat anti-HA antibody and protein A-Sepharose 4B. The immunoprecipitated complex was boiled for 5 min in 50 mM sodium citrate, pH 5.5, containing 0.1% SDS. Two 10-μl aliquots were taken from each sample. One aliquot was mixed with 10 μl of 50 mM sodium citrate, pH 5.5, containing 0.5 mU of endoglycosidase H, whereas the other one was mixed with citrate buffer only. After incubation at 37°C for 16–20 h, each sample was mixed with Laemmli sample buffer and electrophoresed in a 10.5% SDS-polyacrylamide gel. Radiolabeled protein bands were scanned and quantitated with the STORM PhosphorImager system from Molecular Dynamics (Sunnyvale, CA). The following formula was used to determine the fraction of HA proteins processed to the endoglycosidase H–resistant form (Endo HR) or the trypsin-sensitive form (TrypsinS): Endo HR (%) = 100 × [(HA0 Endo HR + HA1 + HA2)/HA total]; TrypsinS (%) = 100 × [(HA1 + HA2)/HA total]; HA total = HA0 Endo HS + HA0 Endo HR + HA1 + HA2
Binding of Coatomer to Golgi-enriched Membranes
Golgi-enriched membranes and cytosol were prepared as detailed previously (Hudson and Draper, 1997). The binding of coatomer in cytosol to Golgi-enriched membranes was also measured as described by Hudson and Draper (1997). Briefly, membranes (5 μg) and cytosol (60 μg) were incubated with 1 mM ATP, 1 mM GTP, 5 mM creatine phosphate, 8 U of creatine phosphokinase, and protease inhibitors in a total volume of 100 μl for 20 min at 34°C. Binding reactions were stopped by chilling to 4°C, and membranes were collected by centrifugation at 16,000 × g for 10 min at 4°C in a microfuge. The pelleted membranes were dissolved in sample buffer and electrophoresed in 7% polyacrylamide gels with SDS. Proteins were transferred to nitrocellulose by electrophoresis, and the nitrocellulose was incubated with 0.1% Tween 20 as a blocking agent, followed by incubation with monoclonal antibody M3A5 to β-COP. The secondary antibody for detecting β-COP was horseradish peroxidase–conjugated goat anti-mouse. The blots were developed with the Pierce Chemical (Rockford, IL) Supersignal ECL system. Films were digitized using an LKB (Piscataway, NY) densitometer, and the intensities of bands were quantitated using ImageQuant (Molecular Dynamics, Sunnyvale, CA) analysis software.
Analysis of G Protein Glycosylation
CHO cells were infected with the ts045 strain of VSV at 40°C and then radiolabeled as described for influenza virus. To determine the acquisition of endoglycosidase H resistance of the G protein, we rinsed the labeled cells once with PBS and then lysed the cells with lysis buffer (1% Nonidet P-40, 50 mM Tris, pH 8.0, 150 mM NaCl, 10 mM EDTA, 1 mM PMSF, 0.1 μg/ml aprotinin, and 0.1 mg/ml soybean trypsin inhibitor). G protein was immunoprecipitated with rabbit anti-G protein antibody and protein A-Sepharose 4B. The immunoprecipitated complex was then treated with endoglycosidase H and electrophoresed with SDS in an 8.0% polyacrylamide gel.
Immunofluorescence Microscopy
NRK cells were plated on glass coverslips in the presence of 1 μg/ml superfibronectin 1 d before the experiments. The presence of superfibronectin was important because CBM had a tendency to reduce adherence of cells to surfaces. In all of the indirect immunofluorescence experiments, cells were fixed and permeabilized in cold methanol for 15 min, rinsed three times with PBS, and then blocked for 10 min with 1% BSA in PBS. Cells were incubated with primary antibody for 30 min at room temperature followed by rinsing and blocking procedures as just described. The same incubation and washing procedure was applied to secondary antibody. Rabbit antisera were detected with an FITC goat anti-rabbit IgG, and mouse antisera were detected with TRITC goat anti-mouse IgG. Coverslips were mounted in Fluoromount G and viewed with a Zeiss (Thornwood, NY) photoscope equipped with epifluorescence illumination and a 40× Apochromat lens. Photography was with Kodak (Rochester, NY) TMAX 400 film.
Protein Synthesis
The effect of diphtheria toxin on protein synthesis was measured by assessing the incorporation of radioactivity from Tran35S label into acid insoluble protein as described by Bau and Draper (1993) with modifications. Cells were plated at 105 cells per well in 48-well culture dishes the day before an experiment. The cells were incubated in DMEM, pH 8.8, lacking methionine and bicarbonate in the presence of the desired concentration of CBM for 15 min at 37°C. Diphtheria toxin was added, and the cells were incubated for another 90 min. Tran35S label was added for 15 min, and the cells were washed, lysed, and spotted on filter paper. The filter paper was incubated in 5% trichloroacetic acid containing 0.5 mg/ml methionine for 30 min at room temperature and dried, and the radioactivity associated with each cell lysate was measured with the PhosphorImager.
RESULTS
The Effect of Antibiotics and Structural Analogues on Secretion
We noted previously that certain aminoglycoside antibiotics precipitated coatomer from cytosol and also prevented the binding of coatomer to Golgi membranes in vitro (Hudson and Draper, 1997). The functional groups on the antibiotics that interacted with coatomer were pairs of amino groups that appeared to mimic the ε-amino groups of the lysine residues in the KKXX retention motif. It follows that the antibiotics might interfere with secretion by intact cells if they block coatomer binding to membranes in the cytosol, and we tested this idea by studying the effect of Geneticin on secretion. We observed a 50% inhibition of secretion in the presence of 25 mM Geneticin; however, this high concentration of Geneticin also strongly inhibited protein synthesis (our unpublished observations). We therefore turned our attention to identifying drugs that should maintain the structural features necessary to interact with coatomer but that would enter cells more readily and also not strongly inhibit protein synthesis.
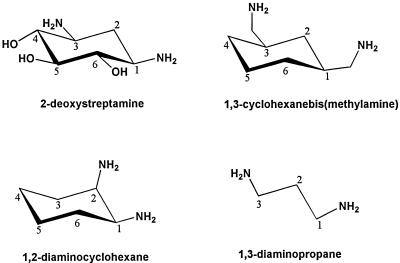
A core component of aminoglycoside antibiotics, including Geneticin, is 2-deoxystreptamine, whose structure is shown in Figure 1. 2-Deoxystreptamine contains a pair of amino groups and also appears to interact with coatomer via these groups in vitro (Hudson and Draper, 1997). We studied three compounds structurally similar to 2-deoxystreptamine: CBM, 1,2-diaminocyclohexane, and 1,3-diaminopropane (Figure 1). CBM and 1,2-diaminocyclohexane contain two primary amino groups and also retain a cyclohexyl ring structure; however, they lack hydroxyl groups, which should facilitate passage through membranes. 1,3-Diaminopropane is the simplest analogue of 2-deoxystreptamine, but the amino groups are not constrained by the cyclohexyl structure and should have more conformational flexibility.
Figure 1.
Structures of diamino compounds used in this study.
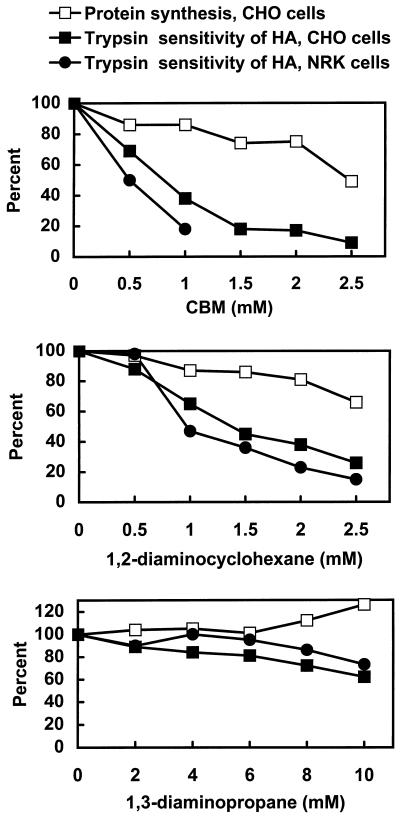
The HA protein of influenza virus was used as the model system to monitor secretion quantitatively. Cells were infected with influenza virus and exposed to different concentrations of the three drugs. The pH of the medium during exposure to drugs (and in control cells not exposed to drugs) in this and subsequent experiments was 8.8 to facilitate the entry of the basic drugs into cells. After a pulse and chase with radioactive methionine, the amount of the HA protein on the cell surface was assessed by exposing intact cells to trypsin. Only HA transported to the cell surface is cleaved by trypsin in intact cells to produce the products HA1 and HA2, which can be separated from uncleaved, intact HA by electrophoresis in polyacrylamide gels with SDS. Thus, the percent of total HA sensitive to trypsin digestion is a measure of secretion. Both CHO and NRK cells were included in the study, and we also measured the effects of the drugs on protein synthesis with CHO cells. CBM strongly inhibited HA secretion in both CHO and NRK cells in the range of 1–1.5 mM (Figure 2, top). The drug also had a dose-dependent effect on protein synthesis, but it is evident that the inhibition of secretion occurred at low concentrations of drug that had little effect on protein synthesis. The effect of CBM on protein synthesis varied with different lots and was sometimes as high as 80% inhibition at 2 mM and above; nevertheless, all lots of CBM strongly inhibited secretion at concentrations at which there was a minimal effect on protein synthesis. 1,2-Diaminocyclohexane also inhibited secretion, but not as effectively as did CBM (Figure 2, middle). 1,3-Diaminopropane had little effect on either protein synthesis or secretion even at the highest concentration tested of 10 mM (Figure 2, bottom). The remainder of this study focused on CBM, the most effective drug.
Figure 2.
Effects of diamino compounds on protein synthesis and HA transport in CHO and NRK cells. To measure the extent of secretion by the trypsin sensitivity of HA, cells were infected with influenza virus and exposed to the indicated concentrations of drugs for 30 min at 37°C. The cells were incubated with Tran35S label for 5 min and then incubated for 1 h in the absence of radioactive medium. The sensitivity of HA to trypsin was assessed as described in MATERIALS AND METHODS. To measure protein synthesis, uninfected cells were exposed to drugs and incubated for 1.5 h at 37°C. Tran35S label was added during the last 30 min. The cells were lysed, and acid-insoluble radioactivity was measured.
CBM Inhibits Coatomer Binding to Golgi Membranes In Vitro and In Vivo
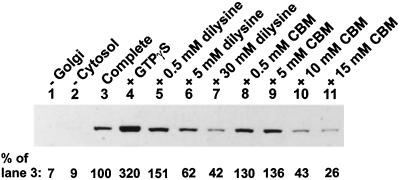
CBM contains two primary amino groups that are predicted to specify interaction with the dilysine-binding sites on coatomer. If so, CBM should inhibit the binding of coatomer to Golgi membranes in vitro, analogous to the effects of dilysine (Hudson and Draper, 1997). To test this, Golgi membranes and cytosol were incubated together under conditions to promote coating, and the effects of dilysine (a positive control) and CBM on the extent of coating were measured. Coating was assessed by immunoblotting for the β-COP subunit of coatomer associated with pelleted membranes. The immunoblot is shown in Figure 3 with a quantitation of the β-COP bands given below the blot. In the absence of either Golgi membranes or cytosol, there was very little β-COP detected, demonstrating the necessity of these components for the coating reaction (Figure 3, lanes 1 and 2). The level of coating with the complete reaction mixture is shown in Figure 3, lane 3, and this reaction is taken as 100% in the quantitation below the blot. Enhanced coating was observed in the presence of GTPγS, a nonhydrolyzable analogue of GTP (Figure 3, lane 4), as expected. Dilysine stimulated coating at 0.5 mM but then inhibited coating at higher concentrations (Figure 3, lanes 5–7). The stimulation of coating by low concentrations of a diamino compound, followed by inhibition at higher concentrations, is an effect we have frequently observed and is also evident in our previously published data (Hudson and Draper, 1997). CBM also stimulated coating at low concentrations but then strongly inhibited coating at 10 mM and above (Figure 3, lanes 8–11). We conclude that CBM is similar to dilysine and aminoglycoside antibiotics that interact with coatomer via dilysine-binding sites and in so doing CBM interferes with coatomer binding to Golgi membranes.
Figure 3.
Effect of CBM on the binding of β-COP to Golgi-enriched membranes. Golgi-enriched membranes and cytosol were incubated together (Complete) under conditions to promote coatomer binding as described in MATERIALS AND METHODS. Omissions (−) and additions (+) to the reaction mixtures are indicated. Membrane-associated β-COP was detected by immunoblotting. The numbers below the bands represent a quantitative analysis of the band intensities as a percent of the complete reaction mixture (lane 3).
We also checked whether 1,3-diaminopropane inhibited coatomer binding to Golgi membranes in vitro and found no inhibition up to 20 mM, the highest concentration tested (our unpublished observations). This is consistent with the observation that 1,3-diaminopropane did not inhibit HA secretion and suggests that there is something specific to the structure of CBM beyond its dibasic nature that is critical for the interference of coatomer binding to Golgi membranes.
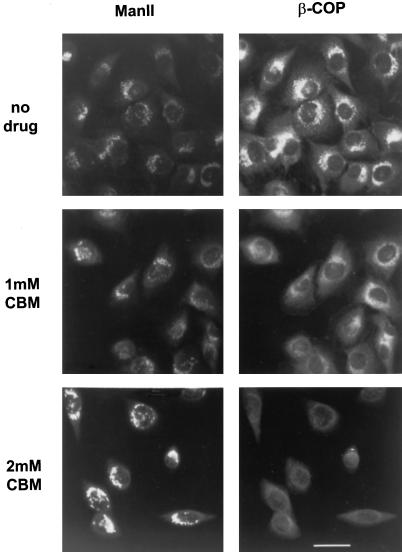
To determine whether CBM reduced coatomer binding to the Golgi complex of intact cells, NRK cells were treated with CBM for 90 min and prepared for double immunofluorescence using antibodies to β-COP and to a marker for the medial Golgi, mannosidase II. Figure 4, right, demonstrates that the fluorescent signal associated with COPI on Golgi membranes was diminished at 1 mM CBM and strongly reduced at 2 mM CBM. Thus, CBM appears to reduce coatomer binding to the Golgi both in vitro and in intact cells. 1,3-Diaminopropane did not reduce coatomer binding to the Golgi complex of NRK cells (our unpublished observations). It is interesting, however, that the reduced COPI signal was not accompanied by a disappearance of the Golgi complex, as occurs in response to brefeldin A. There was a swelling and disorganization of the fine reticular structure of the Golgi complex, but mannosidase II retained a definite perinuclear location (Figure 4, left). This result suggests that the Golgi complex can maintain a morphologically identifiable structure despite a strong reduction in the amount of bound COPI.
Figure 4.
Distribution of mannosidase II and β-COP in CBM-treated cells. NRK cells grown on glass coverslips were incubated with the indicated concentration of CBM at 37°C for 90 min. The cells were then fixed and double-labeled with antibodies to mannosidase II (ManII; left) and β-COP (right) followed by FITC- and TRITC-coupled secondary antibodies as described in MATERIAL AND METHODS. Bar, 10 μm.
CBM is a weak base and has the potential to elevate the mildly acidic pH within compartments of the Golgi complex. Such an increase in pH could conceivably cause COPI to dissociate from Golgi membranes. To check this, NRK cells were incubated with 10 mM ammonium chloride, which is known to raise the pH within acidic organelles (de Duve et al., 1974), and the distribution of β-COP was assessed by immunofluorescence microscopy. Despite an obvious swelling of lysosomes caused by ammonium ion accumulation, there was no reduction in the β-COP signal coincident with the Golgi complex (our unpublished observations). This suggests that the effect of CBM on COPI dissociation from the Golgi complex is not correlated with changes in luminal Golgi pH. Additionally, incubating the cells with 10 mM 1,3-diaminopropane also had no effect on the distribution of β-COP (our unpublished observations).
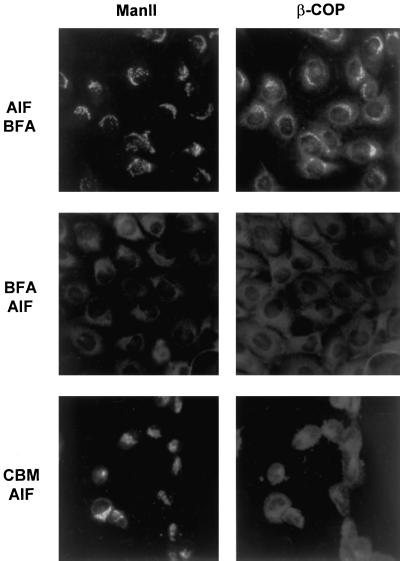
It is difficult to quantitate from immunofluorescence micrographs such as Figure 4 the extent to which CBM removes COPI from Golgi membranes in vivo. The difficulty is exacerbated by the fact that the Golgi complex remains in place and is a source of background fluorescence. To approach the question of how effectively CBM removed COPI from Golgi membranes, we examined the influence of AlF4− on COPI distribution in the presence of CBM. AlF4− stabilizes coatomer binding to Golgi membranes in vitro and in vivo and renders COPI highly resistant to removal by brefeldin A, apparently by inhibiting COPI dissociation (Donaldson et al., 1991a; Finazzi et al., 1994). The mechanism by which AlF4− stabilizes COPI binding is not well understood but probably involves trimeric G proteins (Donaldson et al., 1991a; Helms et al., 1998). If coatomer retains significant ability to bind Golgi membranes in the presence of CBM, then COPI should accumulate on the Golgi in the presence of AlF4−, because of inhibition of COPI dissociation. In a control experiment to assess the effectiveness of AlF4−, cells were treated with AlF4− for 15 min, followed by brefeldin A for 15 min (Figure 5, top). As noted by Donaldson et al. (1991a), AlF4− stabilized COPI association with the Golgi, and brefeldin A was unable to promote β-COP dissociation or the disappearance of the Golgi. When cells were first treated with brefeldin A for 15 min, followed by AlF4− for 15 min (Figure 5, middle), there was no significant staining for either mannosidase II or β-COP, verifying that the Golgi complex was gone (fused with the ER) and that AlF4− did not reverse this action. After cells were treated for 45 min with CBM, followed by the addition of AlF4− for 45 min, the Golgi was still evident, but there was still strongly reduced staining of β-COP associated with the Golgi (Figure 5, bottom). The fact that AlF4− could not induce β-COP to accumulate on Golgi membranes in the presence of CBM argues that CBM is highly effective in blocking coatomer binding to membranes.
Figure 5.
The influence of AlF4− on the distribution of mannosidase II and β-COP in the presence of CBM. AlF4− was prepared by adding 30 mM NaF to medium (pH 8.8) followed by the addition of 100 μM AlCl3. Top, cells were treated with medium containing AlF4− for 15 min, and brefeldin A (BFA, 1 μg/ml) was added for 15 min in the presence of AlF4−. Middle, cells were treated with brefeldin A (1 μg/ml) for 15 min, and AlF4− was added for 15 min in the presence of brefeldin A. Bottom, cells were incubated with CBM (2 mM) for 45 min, and AlF4− was added for 45 min in the presence of CBM. Samples were prepared for immunofluorescence microscopy with antibodies to mannosidase II (left) and β-COP (right) as described in Figure 4.
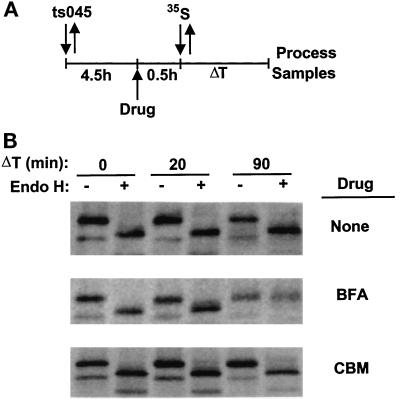
CBM Does Not Induce Golgi Enzymes to Enter the ER
CBM and brefeldin A have in common that both impair coatomer binding to Golgi membranes, although by different mechanisms, and also block secretion. In addition, brefeldin A induces the fusion of Golgi membranes with the ER, which results in the absence of an identifiable Golgi complex in cells. However, the results in Figures 4 and 5 suggest that CBM does not induce the Golgi complex to enter the ER despite the reduction in bound COPI. To verify the latter finding, we assessed whether or not Golgi enzymes entered the ER in the presence of CBM by whether the N-linked carbohydrate chains of a glycoprotein in the ER showed evidence of modification by Golgi enzymes. CHO cells were infected with the ts045 strain of VSV at the restrictive temperature to retain the G protein within the ER. The cells were then treated either with no drugs, with brefeldin A (a positive control), or with 1.5 mM CBM. Thirty minutes after drug addition, the cells were pulsed with radioactive methionine, and the sensitivity of the G protein in the ER to digestion by endoglycosidase H was determined at times after the pulse (Figure 6A). In the absence of drugs, the G protein remained sensitive to digestion by endoglycosidase H, as expected for a protein within the ER (Figure 6B, top). In the presence of brefeldin A, the G protein was fully resistant to digestion, indicating that enzymes required for carbohydrate processing of glycoproteins in the Golgi complex had been relocated to the ER under the influence of brefeldin A (Figure 6B, middle) (Doms et al., 1989). In contrast, the G protein remained sensitive to endoglycosidase H in cells treated with 1.5 mM CBM (Figure 6B, bottom). Similar results were obtained with CBM at 1.0 and 2.0 mM (our unpublished observations). These data support the conclusion that CBM does not cause the Golgi complex to fuse with the ER, implying that CBM and brefeldin A operate in fundamentally different ways, despite evidence that both drugs prevent coatomer binding to the Golgi.
Figure 6.
CBM does not cause redistribution of Golgi proteins to the ER. (A) A summary of the procedure. Cells were infected with the ts045 strain of VSV at 40°C and then were exposed for 30 min either to no drugs, to brefeldin A (0.5 μg/ml), or to CBM (1.5 mM). Tran35S label was added for 15 min, and the incubation with or without drugs was continued for the indicated times. The cells were then lysed, and the G protein was immunoprecipitated, treated with endoglycosidase H, and electrophoresed as described in MATERIALS AND METHODS. (B) The migration of the G protein under the various conditions of the experiment.
CBM Blocks Transport from the Golgi to the Plasma Membrane but Not from the ER to the Golgi
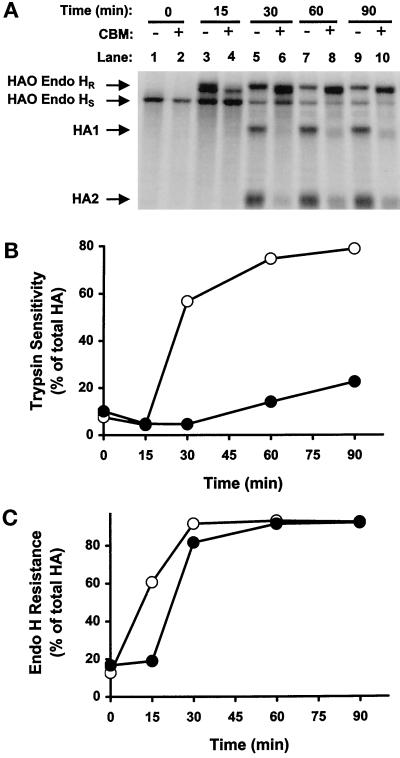
Considering that CBM inhibits secretion in a way that appears to be different from that of brefeldin A, we determined whether the block in secretion by CBM was at the level of the ER or the Golgi. Cells were infected with influenza virus and exposed either to no drug or to CBM. After a pulse with radioactive methionine, intact cells were treated with trypsin to cleave secreted HA on the cell surface. Total HA was then extracted from cells and treated with endoglycosidase H to determine the percent of HA that was resistant to digestion and therefore in the Golgi complex. Figure 7A shows the various radioactive HA bands in the gel under the conditions of the experiment. A quantitative analysis of these bands with the PhosphorImager was used to calculate the data presented in Figure 7, B and C. The rate of HA secretion in the presence and absence of CBM is given by the rate at which HA acquires sensitivity to trypsin (Figure 7B), and the data verify that CBM inhibits transport to the cell surface. The rate of HA export from the ER to the Golgi is given by the rate at which HA acquires resistance to endoglycosidase H (Figure 7C). This is a valid measurement of HA export because the data in Figure 6 demonstrate that the acquisition of endoglycosidase H resistance is not caused by the return of Golgi enzymes to the ER. It is evident that CBM slightly delays export from the ER but does not impair the complete export of HA from the ER to the Golgi.
Figure 7.
The effects of CBM on the transport of HA from the ER to the Golgi and from the Golgi to the plasma membrane in CHO cells. Influenza virus–infected CHO cells were either untreated (B and C, ○) or treated with CBM (B and C, ●), radiolabeled, and then chased for the indicated times. After trypsin treatment, the cells were solubilized, and HA molecules were immunoprecipitated, treated with endoglycosidase H, and resolved in 10.5% SDS-polyacrylamide gels (A). Transport to the plasma membrane (B) and to the Golgi (C) was determined by trypsin sensitivity and endoglycosidase H resistance, respectively, as described in MATERIALS AND METHODS.
In another approach to address where CBM blocks secretion, the effect of the drug on transport of the G protein of VSV strain ts045 was assessed by immunofluorescence microscopy. Cells were infected with virus for 4 h at 41°C to trap the temperature-sensitive G protein in the ER. Medium at pH 8.8 was added with or without 2 mM CBM, and the cells were incubated for an additional 10 min at 41°C, followed by a 2 h incubation at 32°C to activate secretion of the G protein. Immunofluorescence microscopy revealed that the G protein was primarily on the cell surface in the absence of CBM but was retained in a perinuclear location coincident with the Golgi complex in the presence of CBM (our unpublished observations). Thus, the G protein left the ER in the presence of CBM but was unable to exit the Golgi region, consistent with data on HA.
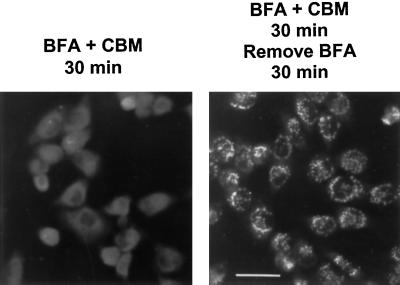
Data presented so far indicate that CBM does not block transport from the ER to the Golgi and that the morphology of the Golgi complex is also maintained in the presence of the drug. To verify these results in a different experiment, we incubated NRK cells with both brefeldin A and 2 mM CBM. After 30 min in the presence of both drugs, the Golgi was no longer evident (Figure 8, left), indicating that CBM did not inhibit the ability of brefeldin A to induce fusion of the Golgi and the ER. The brefeldin A was removed, but the CBM maintained, to see whether the Golgi complex could reform in the presence of CBM. Thirty minutes after removal of the brefeldin A, mannosidase II had left the ER and was present in perinuclear structures that resembled the Golgi complex despite the presence of CBM (Figure 8, right). These morphological data support the conclusion that CBM does not block export from the ER; otherwise the Golgi would not have reformed.
Figure 8.
Reformation of the Golgi complex in cells first treated with brefeldin A and CBM followed by removal of the brefeldin A but retention of the CBM. NRK cells grown on glass coverslips were incubated with 2 mM CBM and 5 μg/ml brefeldin A for 30 min. Brefeldin A was then removed, but 2 mM CBM was retained. After a further 30 min incubation, the cells were fixed and prepared for immunofluorescence microscopy with anti-mannosidase II antibody.
CBM Inhibits the Association of γ-Adaptin with the TGN
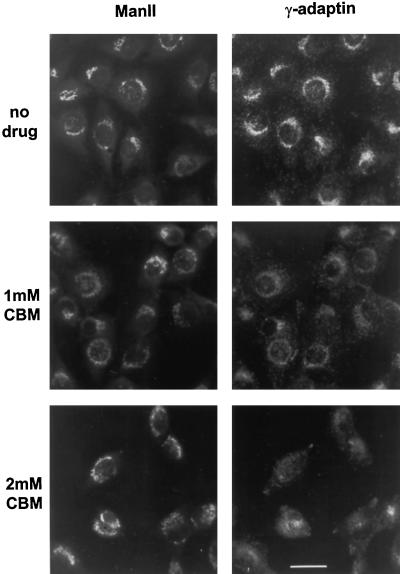
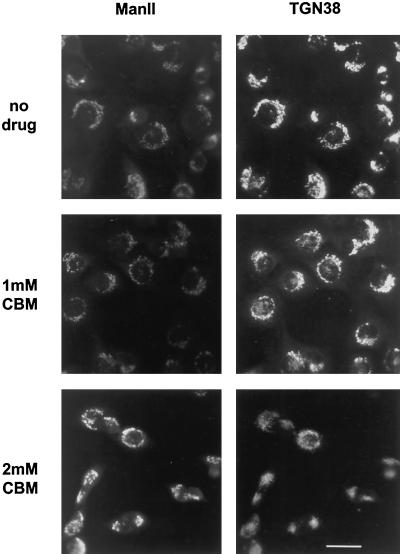
We also studied the effect of CBM on the distribution of γ-adaptin on the TGN in cells by immunofluorescence microscopy. CBM significantly reduced the intensity of γ-adaptin staining associated with the Golgi complex (Figure 9, right) and again had little effect on the morphology of the Golgi as indicated by mannosidase II staining. To see whether the reduction in γ-adaptin stain was accompanied by a change in the morphology of the TGN itself, we measured the effect of CBM on the staining of TGN38, a marker for the TGN. There appeared to be a slight disorganization of the TGN at 2 mM CBM, but an identifiable TGN was still evident (Figure 10, right). Thus, it seems that CBM treatment reduces the amount of γ-adaptin on the TGN, but there is no gross change in TGN morphology.
Figure 9.
Distribution of mannosidase II and γ-adaptin in CBM-treated cells. NRK cells were prepared for immunofluorescence microscopy as described in Figure 4 except that the cells were double-labeled with antibodies to mannosidase II (left) and γ-adaptin (right).
Figure 10.
Distribution of mannosidase II and TGN38 in CBM-treated cells. NRK cells were prepared for immunofluorescence microscopy as described in Figure 4 except that the cells were double-labeled with antibodies to mannosidase II (left) and TGN38 (right).
CBM Does Not Inhibit the Action of Diphtheria Toxin
To assess what other aspects of membrane traffic might be affected by CBM, we measured the effect of CBM on the sensitivity of CHO cells to diphtheria toxin. Diphtheria toxin kills cells by a mechanism that requires three major steps (Middlebrook and Dorland, 1984; Eidels and Draper, 1988). 1) Receptor-mediated endocytosis of the toxin bound to a cell surface receptor initially brings the toxin into the cell inside a vesicle. 2) Insertion of the toxin into the vesicle membrane and transfer of the catalytic fragment of the toxin across the membrane into the cytosol occur. The insertion is initiated by a conformational change induced upon exposure of the toxin to an acidic pH within an endocytic vesicle. 3) The catalytic fragment inactivates elongation factor 2, arresting protein synthesis. The susceptibility of cells to diphtheria toxin is a sensitive indicator of the function of those aspects of membrane traffic required by the toxin for activity. The toxin is particularly sensitive to increases in vacuolar pH caused by lysosomotropic amines such as ammonium chloride. The data in Table 1 show that CBM had no consistent effect on the concentration of diphtheria toxin required to reduce protein synthesis by 50% (IC50). This is evidence that CBM does not significantly impair receptor-mediated endocytosis of the toxin or the acidification of intracellular compartments.
Table 1.
Effect of CBM on the sensitivity of CHO cells to diphtheria toxin
| CBM (mM)
|
|||||
|---|---|---|---|---|---|
| 0 | 0.1 | 0.5 | 1.0 | 1.5 | |
| IC50 (μg/ml)a | 39 | 51 | 25 | 32 | 53 |
The IC50 is the concentration of diphtheria toxin required to reduce protein synthesis by 50%. Each value is the average of three or more independent determinations at each CBM concentration.
DISCUSSION
We arrived at the study of CBM by its structural similarity to aminoglycoside antibiotics that are believed to interact with the dilysine-binding sites on coatomer (Hudson and Draper, 1997). Like the aminoglycoside antibiotic Geneticin, as well as dilysine itself, CBM inhibited the binding of coatomer to Golgi membranes in vitro. CBM also strongly reduced the immunofluorescence signal of β-COP associated with the Golgi complex of intact cells, suggesting that CBM inhibits coatomer binding to Golgi membranes in vivo as well as in vitro. This inhibition is presumably mediated by the two strategically placed primary amino groups in CBM, similar to those in 2-deoxystreptamine, that appear to mimic the amino groups of dilysine. The simplest model for the effect of dibasic compounds on coating is that they occupy the dilysine-binding sites on coatomer and impair the binding of coatomer to membranes. In vivo, the steady-state extent of COPI association with membranes is likely a balance between coating and uncoating so that a block in the coating step eventually depletes Golgi membranes of COPI.
CBM inhibited transport to the cell surface of both influenza HA and the G protein of vesicular stomatitis virus, suggesting that the drug is a general inhibitor of secretion. The concentration of CBM that impaired secretion was in the range of 1–2 mM, similar to the concentration that reduced coatomer binding to the Golgi of intact cells. It is reasonable, therefore, that the block in coating could cause the block in secretion. Alternatively, CBM is a weak base and has the potential to raise the pH in acidic compartments such as the Golgi complex by ion trapping (de Duve et al., 1974), which could conceivably cause COPI dissociation and block secretion. However, several lines of evidence argue that CBM does not interfere with secretion by altering proton gradients. First, at concentrations that impaired secretion, CBM did not appear to elevate the pH within acidic compartments. This is indicated by the observation that the cytotoxic activity of diphtheria toxin, which is very sensitive to the action of weak bases on pH (Middlebrook and Dorland, 1984; Eidels and Draper, 1988), was not affected by CBM. Second, intentionally elevating the pH inside acidic compartments with ammonium chloride did not alter the distribution of COPI on the Golgi complex of NRK cells. Thus, even if CBM subtly raised the luminal pH of the Golgi compartment in a way undetected by diphtheria toxin, this alone would not affect COPI association with Golgi membranes. Third, 1,3-diaminopropane, a linear dibasic compound, did not impair secretion of HA, even at 10 mM (Figure 2). 1,3-Diaminopropane also had no effect on coatomer binding to Golgi membranes in vitro or in vivo. This further suggests that it is not simply the weakly basic nature of CBM that inhibits secretion; rather, a more specific feature of CBM is involved, probably the conformation of the amino groups constrained by the cyclohexane ring.
Previous work with brefeldin A, which dissociates COPI from Golgi membranes and causes the Golgi and ER membranes to fuse, led to the suggestion that the COPI coat was required for the structural integrity of the Golgi complex (Donaldson et al., 1991b; Klausner et al., 1992). We found, however, that the Golgi apparatus retained a recognizable morphology in the presence of CBM, even though the COPI normally bound to Golgi membranes was absent. Also, Golgi carbohydrate-modifying enzymes could not be biochemically detected within the ER after CBM treatment, indicating that CBM did not induce the Golgi and ER membranes to fuse. This is consistent with the maintenance of Golgi integrity in the presence of CBM and also suggests that dissociation of COPI is not sufficient in itself to induce the fusion of Golgi and ER membranes.
The mechanism of brefeldin A action is not well understood. However, an advance was made with the discovery that brefeldin A stimulates a mono-ADP-ribosylation activity that may underlie some effects of the drug on membrane traffic (De Matteis et al., 1994; Di Girolamo et al., 1995). This work has recently been extended to demonstrate that inhibiting the brefeldin A-dependent ADP-ribosylation activity results in dissociation of COPI from Golgi membranes upon brefeldin A addition, but the morphology of the Golgi complex is retained, and fusion with the ER does not occur (Mironov et al., 1997; Weigert et al., 1997). Our results with CBM are consistent with the work of Mironov et al. (1997) suggesting that dissociation of COPI from Golgi membranes is insufficient alone to cause a loss of Golgi complex morphology and fusion with the ER.
Three lines of evidence indicate that CBM does not inhibit secretion by impairing the transport of material from the ER to the Golgi. First, the acquisition of endoglycosidase H resistance by HA was only slightly delayed by CBM, indicating that HA was exposed to N-acetylglucosaminidase I and α-mannosidase II, enzymes necessary to process glycoproteins to endoglycosidase H resistance. Because CBM did not induce fusion of the Golgi with the ER, resistance to endoglycosidase H could not have resulted from the processing of HA by Golgi enzymes that had been returned to the ER. The fact that the Golgi and ER remained separate under the influence of CBM also implies that dissociation of COPI did not cause massive unregulated membrane fusion. These data strongly suggest that HA was transported from the ER, through the intermediate compartment, to at least the cis/medial Golgi where the two processing enzymes are present. Second, upon shifting the temperature from 41 to 32°C in the presence of CBM, the G protein of VSV was transported out of the ER to a compartment that was identifiable as the Golgi complex by immunofluorescence microscopy. Third, when Golgi membranes were induced to fuse with the ER by brefeldin A, followed by removal of brefeldin A in the presence of CBM, a Golgi complex with a perinuclear morphology recognizable by immunofluorescence microscopy was reformed. Thus, at least α-mannosidase II, the antigen used in these immunofluorescence experiments, exited the ER to become part of a perinuclear Golgi complex in the presence of CBM. Other Golgi proteins presumably left the ER as well, although fine structural details of the reformed Golgi membranes are not available.
CBM strongly reduced the fluorescence signal from β-COP associated with Golgi membranes in vivo, even in the presence of AlF4−, which normally stabilizes COPI binding to the Golgi complex. This suggests that CBM is very effective in preventing coatomer binding to the Golgi complex. However, the possibility cannot be dismissed that residual membrane-bound COPI could still be present and serve functions necessary for transport from the ER to the Golgi, although transport from the Golgi to the plasma membrane is blocked. This prospect is particularly important in light of evidence that anterograde transport by coatomer may be mediated by binding to diphenylalanine motifs (Fiedler et al., 1996; Fiedler and Rothman, 1997), an interaction that might not be impaired by CBM. Nevertheless, our results suggest that a full complement of COPI on membranes is not essential for normal ER-to-Golgi transport in mammalian cells, which seems to differ from the interpretation of several lines of evidence in the literature. Microinjection into cells of an antibody to β-COP (anti-EAGE) inhibits by 50% the acquisition of resistance to endoglycosidase H by VSV G protein (Pepperkok et al., 1993) and inhibits by 50% the reformation of the Golgi upon removal of brefeldin A (Scheel et al., 1997). However, recent work indicates that anti-EAGE does not inhibit the binding of coatomer to membranes; rather, it inhibits the dissociation of COPI from membranes (Scheel et al., 1997). Other agents that block COPI dissociation, GTPγS (Scales et al., 1997) and the GTP-restricted form of ARF1 (Aridor et al., 1995), also impair transport from the intermediate compartment to the Golgi. These results suggest that the dissociation of COPI from certain membranes may be necessary for transport to the Golgi, but they do not seem to contradict the possibility that ER-to-Golgi transport can occur in the absence of bound COPI.
Balch and coworkers examined the role of COPI in transport from the ER to the Golgi with semi-intact cells (Peter et al., 1993; Aridor et al., 1995; Rowe et al., 1996; Tisdale et al., 1997) and concluded that COPI was necessary for the recycling of membrane from the intermediate compartment back to the ER but was not needed for vesicle formation from the ER. However, there were conflicting data on whether coatomer recruitment to membranes was directly needed for transport from the intermediate compartment to the Golgi. ARF1(T31N), the GDP-restricted form of ARF1 that blocks coatomer binding, inhibited ER-to-Golgi transport, suggesting that COPI recruitment was essential to reach the Golgi (Aridor et al., 1995; Rowe et al., 1996). In contrast, depleting cytosol of coatomer and ARF1 did not inhibit transport from the intermediate compartment to the Golgi, leaving open the question of whether coatomer binding was directly needed to complete transport to the Golgi (Rowe et al., 1996). A role for COPI in ER-to-Golgi transport has also been inferred from studies of the temperature-sensitive ldlF mutant of CHO cells that are defective in ε-COP (Guo et al., 1994; Scales et al., 1997). However, not all consequences of the lesion are consistent with such a role. For example, the Golgi complex is disorganized within 6 h of placing ldlF cells at 40°C (Guo et al., 1994), but at 7 h after the temperature shift, low density lipoprotein is still converted to an intermediate form that is resistant to digestion by endoglycosidase H, suggesting that it has reached the cis/medial Golgi (Hobbie et al., 1994). In addition, ldlF cells do not exhibit a brefeldin A-like phenotype at the restrictive temperature despite the absence of ε-COP (Daro et al., 1997). Several hours or more are required to induce the phenotype in ldlF cells, and it is difficult to separate primary defects in ER-to-Golgi transport from secondary consequences of the lesion. Altogether, there is no conclusive evidence that COPI is directly required for ER-to-Golgi transport in mammalian cells, and our results with CBM may reflect that COPI is not critical for this process.
CBM-induced dissociation of COPI from membranes correlated with defective transport from the Golgi to the plasma membrane, but it is not clear what transport event is blocked. The Golgi complex as seen by immunofluorescence microscopy appeared distended and swollen, which might be expected if membrane from the ER had entered the cis side of the Golgi but had not exited the trans side. Two popular models to explain the anterograde transport of material through the Golgi apparatus are the vesicular transport model and the maturation model. The vesicular transport model proposes that vesicles, presumed to use a COPI coat, carry material from one stack to the next in the trans direction. The maturation model proposes that one stack matures into the succeeding stack without anterograde vesicular movement, but the maturation process does imply retrograde vesicular transport in the trans-to-cis direction by COPI-coated vesicles to recover material from succeeding stacks. Thus, the dissociation of COPI from Golgi membranes induced by CBM should have adverse effects on secretion regardless of the model.
An unexpected consequence of CBM treatment was reduced γ-adaptin binding to the TGN. It is not clear why CBM should impair γ-adaptin binding to membranes, but a common factor required for binding of both coatomer and γ-adaptin to membranes is ARF1. Conceivably, the depletion of COPI from Golgi membranes could trap the GTP-bound form of ARF1 on Golgi membranes in a futile attempt to restore COPI. This could exhaust the cytosolic pool of ARF1 and thereby interfere with γ-adaptin binding. Further work is needed to explore this issue.
ACKNOWLEDGMENTS
We thank C. Mikoryak and P. Colbaugh for reading the manuscript. This work was supported in part by grants from the National Institutes of Health (GM-34297) and the National Science Foundation (MCB-9513244). Work by T. Hu partially fulfills the requirements for the Ph.D. degree in Molecular and Cell Biology.
Abbreviations used:
- ARF1
ADP-ribosylation factor 1
- BFA
brefeldin A
- CBM
1,3-cyclohexanebis(methylamine)
- CHO
Chinese hamster ovary
- COPI
coat protein I
- Endo H
endoglycosidase H
- ER
endoplasmic reticulum
- HA
hemagglutinin of influenza virus
- ManII
mannosidase II
- NRK
normal rat kidney
- TGN
trans-Golgi network
- VSV
vesicular stomatitis virus
REFERENCES
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between CopII and CopI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bau M-Y, Draper RK. Ricin intoxicates End4 mutants that have an aberrant Golgi complex. J Biol Chem. 1993;268:19939–19942. [PubMed] [Google Scholar]
- Cosson P, Démollière C, Hennecke S, Duden R, Letourneur F. δ- and ζ-COP, two coatomer subunits homologous to clathrin-associated proteins, are involved in ER retrieval. EMBO J. 1996;15:1792–1798. [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer (COPI)-coated vesicles: role in intracellular transport and protein sorting. Curr Opin Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component ε-COP. J Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C, de Barsey T, Poole B, Trouet A, Tulkens P, van Hoff F. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, et al. Stimulation of endogenous ADP-ribosylation by brefeldin A. Proc Natl Acad Sci USA. 1994;91:1114–1118. doi: 10.1073/pnas.91.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo M, Silletta MG, De Matteis MA, Braca A, Colanzi A, Pawlak D, Rasenick MM, Luini A, Corda D. Evidence that the 50-kDa substrate of brefeldin A-dependent ADP-ribosylation binds GTP and is modulated by the G-protein βγ subunit complex. Proc Natl Acad Sci USA. 1995;92:7065–7069. doi: 10.1073/pnas.92.15.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Kahn RA, Lippincott-Schwartz J, Klausner RD. Binding of ARF and β-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991a;254:1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J, Klausner RD. Guanine nucleotides modulate the effects of brefeldin A in semipermeable cells: regulation of the association of a 110-kDa peripheral membrane protein with the Golgi apparatus. J Cell Biol. 1991b;112:579–588. doi: 10.1083/jcb.112.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L, Draper RK. Handbook of Natural Toxins. M.C. Hardegree and A.T. Tu, New York: Marcel Dekker; 1988. Diphtheria toxin; pp. 217–247. [Google Scholar]
- Fiedler K, Rothman JE. Sorting determinants in the transmembrane domain of p24 proteins. J Biol Chem. 1997;272:24739–24742. doi: 10.1074/jbc.272.40.24739. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Finazzi D, Cassel D, Donaldson JG, Klausner RD. Aluminum fluoride acts on the reversibility of ARF1-dependent coat protein binding to Golgi membranes. J Biol Chem. 1994;269:13325–13330. [PubMed] [Google Scholar]
- Griffiths G, Pepperkok R, Locker JK, Kreis TE. Immunocytochemical localization of β-COP to the ER-Golgi boundary and the TGN. J Cell Sci. 1995;108:2839–2856. doi: 10.1242/jcs.108.8.2839. [DOI] [PubMed] [Google Scholar]
- Guo QG, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by ε-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Helms-Brons D, Brugger B, Gkantiragas I, Eberle H, Nickel W, Nurnberg B, Gerdes HH, Wieland FT. A putative heterotrimeric G protein inhibits the fusion of COPI-coated vesicles. Segregation of heterotrimeric G proteins from COPI-coated vesicles. J Biol Chem. 1998;273:15203–15208. doi: 10.1074/jbc.273.24.15203. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Fisher AS, Lee S, Flint A, Krieger M. Isolation of three classes of conditional lethal Chinese hamster ovary cell mutants with temperature-dependent defects in low density lipoprotein receptor stability and intracellular membrane transport. J Biol Chem. 1994;269:20958–20970. [PubMed] [Google Scholar]
- Hudson RT, Draper RK. Interaction of coatomer with aminoglycoside antibiotics: evidence that coatomer has at least two di-lysine binding sites. Mol Biol Cell. 1997;8:1901–1910. doi: 10.1091/mbc.8.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Nilsson T, Peterson PA. Retrieval of transmembrane proteins to the endoplasmic reticulum. J Cell Biol. 1993;121:317–333. doi: 10.1083/jcb.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C-Y, Draper RK. Retention of secretory proteins in an intermediate compartment and disappearance of the Golgi complex in an END4 mutant of Chinese hamster ovary cells. J Cell Biol. 1992;117:701–715. doi: 10.1083/jcb.117.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Lowe M, Pepperkok R. COPs regulating membrane traffic. Annu Rev Cell Dev Biol. 1995;11:677–706. doi: 10.1146/annurev.cb.11.110195.003333. [DOI] [PubMed] [Google Scholar]
- Kreis TE, Pepperkok R. Coat proteins in intracellular membrane transport. Curr Opin Cell Biol. 1994;6:533–537. doi: 10.1016/0955-0674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Ktistakis N, Brown A, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc Natl Acad Sci USA. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Démolliére C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Middlebrook JL, Dorland RB. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984;48:199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov A, et al. Role of NAD+ and ADP-ribosylation in the maintenance of the Golgi structure. J Cell Biol. 1997;139:1109–1118. doi: 10.1083/jcb.139.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprins A, Duden R, Kreis TE, Geuze HJ, Slot JW. β-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Passreiter M, Anton M, Lay D, Frank R, Harter C, Wieland FT, Gorgas K, Just WW. Peroxisome biogenesis: involvement of ARF and coatomer. J Cell Biol. 1998;141:373–383. doi: 10.1083/jcb.141.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis T. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Peter F, Plutner H, Zhu H, Kreis TE, Balch WE. β-COP is essential for transport of protein from the endoplasmic reticulum to the Golgi in vitro. J Cell Biol. 1993;122:1155–1167. doi: 10.1083/jcb.122.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Rowe R, Aridor M, McCaffery JM, Plutner H, Nuoffer C, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Scheel J, Pepperkok R, Lowe M, Griffiths G, Kreis TE. Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum. J Biol Chem. 1997;137:319–333. doi: 10.1083/jcb.137.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Mellman I. Does COPI go both ways? Cell. 1997;90:197–200. doi: 10.1016/s0092-8674(00)80326-8. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-H, Colbaugh PA, Kao C-Y, Rutledge EA, Draper RK. Impaired secretion and fluid-phase endocytosis in the End4 mutant of Chinese hamster ovary cells. J Biol Chem. 1990;265:20179–20187. [PubMed] [Google Scholar]
- Weigert R, et al. Characterization of chemical inhibitors of brefeldin A-activated mono-ADP-ribosylation. J Biol Chem. 1997;272:14200–14207. doi: 10.1074/jbc.272.22.14200. [DOI] [PubMed] [Google Scholar]
- Zhao L, Helms BJ, Brügger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland FT. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit β. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]