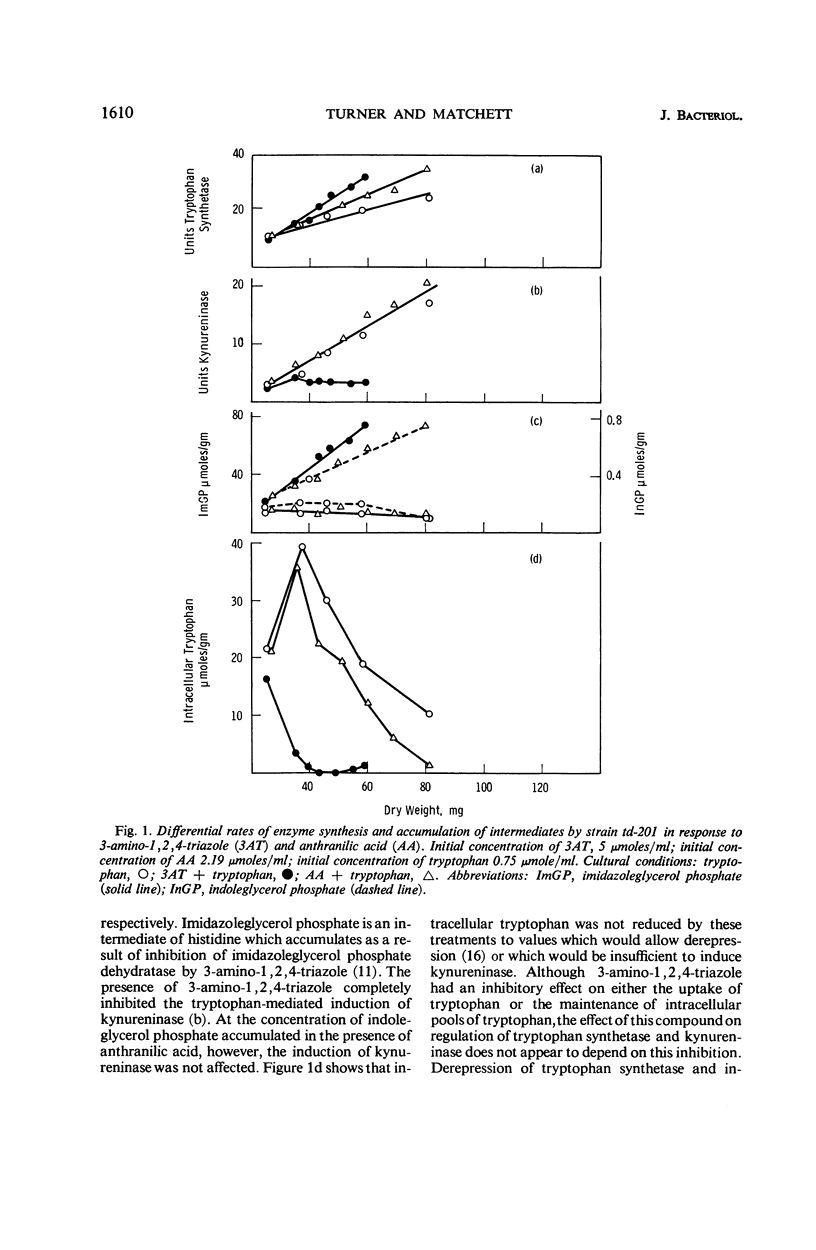
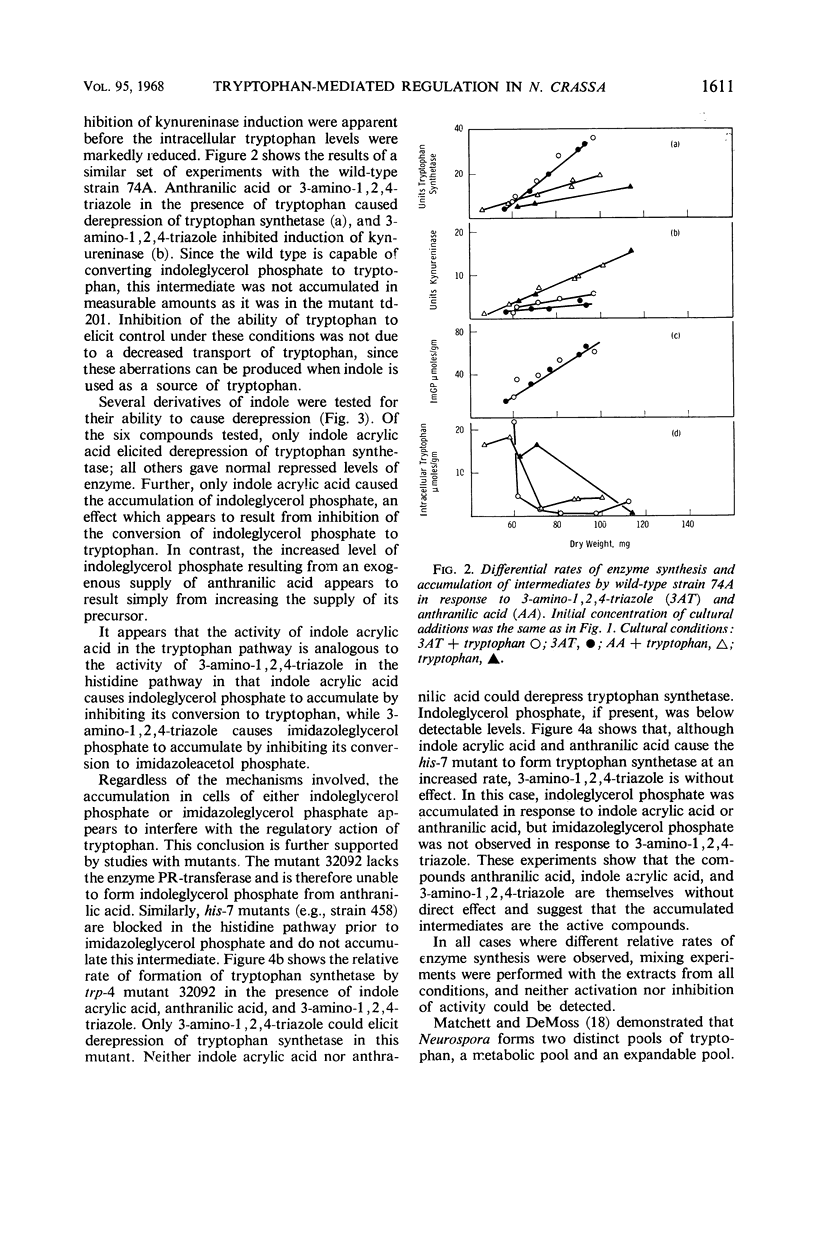
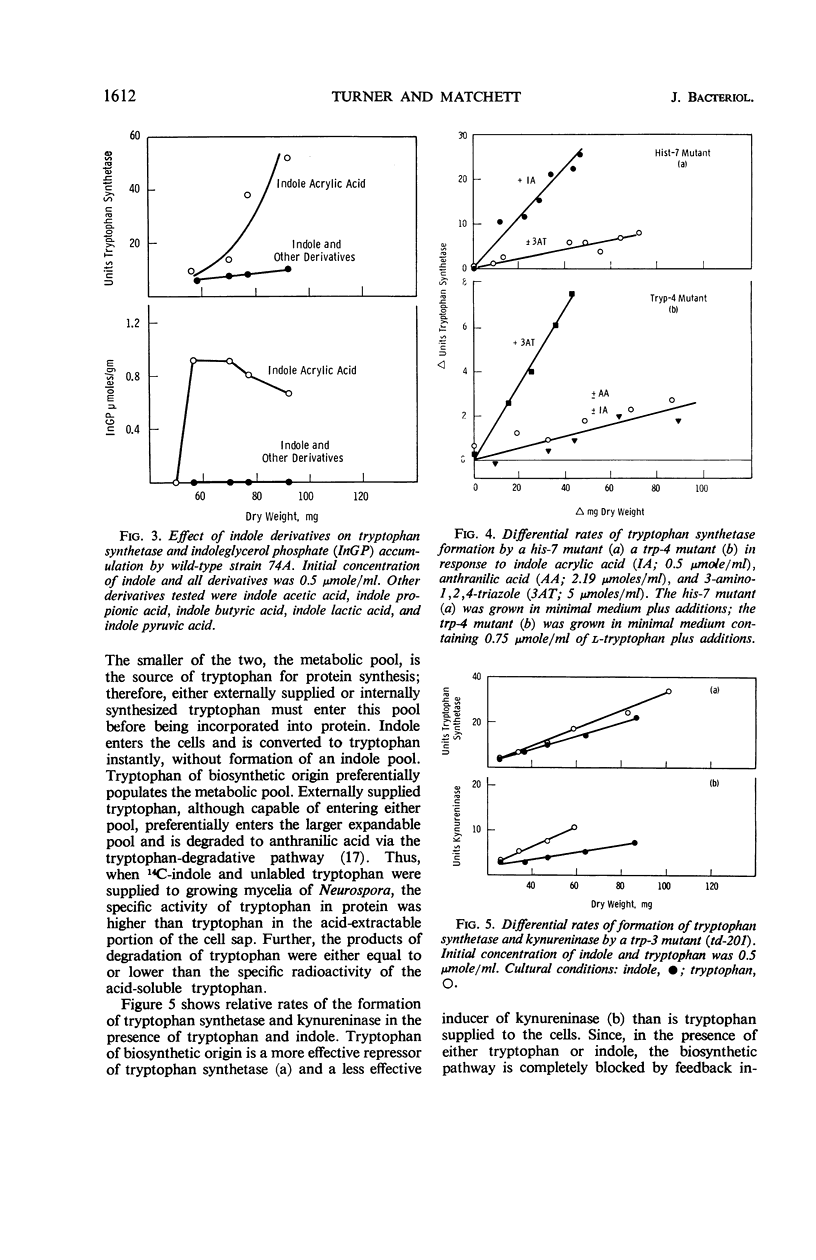
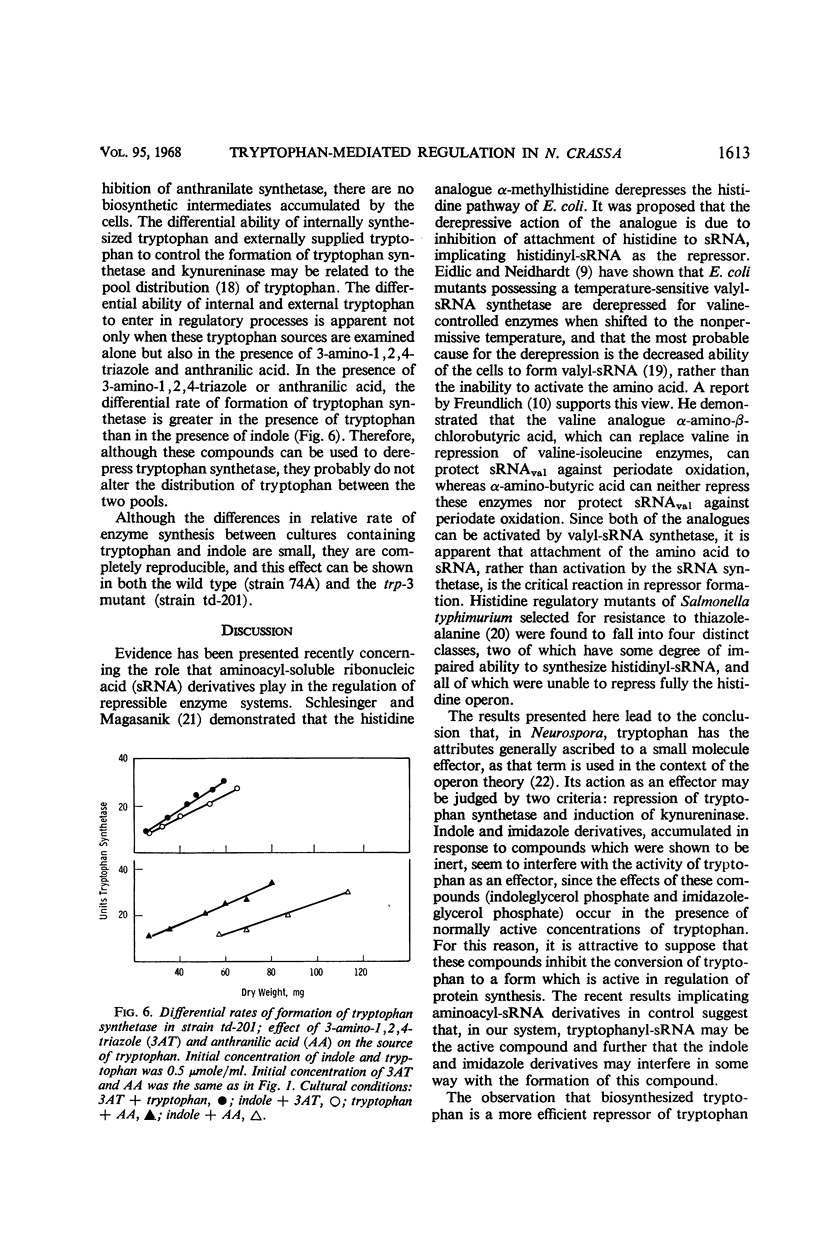
Abstract
The accumulation of imidazoleglycerol phosphate during growth of Neurospora crassa in the presence of 3-amino-1,2,4-triazole was found to cause derepression of tryptophan synthetase and to inhibit the induction of kynureninase. Accumulation of indoleglycerol phosphate in response to growth in the presence of indole acrylic acid or anthranilic acid was also accompanied by derepressed synthesis of tryptophan synthetase. Enzyme synthesis in mutants (his-7 and trp-4) unable to form these intermediates was not altered under similar conditions. The rate of formation of tryptophan synthetase and kynureninase was found to differ in the presence of tryptophan and indole.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N. The biosynthesis of histidine; D-erythro-imidazoleglycerol phosphate dehydrase. J Biol Chem. 1957 Sep;228(1):131–143. [PubMed] [Google Scholar]
- BARRATT R. W., NEWMEYER D., PERKINS D. D., GARNJOBST L. Map construction in Neurospora crassa. Adv Genet. 1954;6:1–93. doi: 10.1016/s0065-2660(08)60127-3. [DOI] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOSS J. A. Studies on the mechanism of the tryptophan synthetase reaction. Biochim Biophys Acta. 1962 Aug 13;62:279–293. doi: 10.1016/0006-3002(62)90041-0. [DOI] [PubMed] [Google Scholar]
- DEMOSS J. A. THE CONVERSION OF SHIKIMIC ACID TO ANTHRANILIC ACID BY EXTRACTS OF NEUROSPORA CRASSA. J Biol Chem. 1965 Mar;240:1231–1235. [PubMed] [Google Scholar]
- DeMoss J. A., Wegman J. An enzyme aggregate in the tryptophan pathway of Neurospora crassa. Proc Natl Acad Sci U S A. 1965 Jul;54(1):241–247. doi: 10.1073/pnas.54.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich M. Valyl-Transfer RNA: Role in Repression of the Isoleucine-Valine Enzymes in Escherichia coli. Science. 1967 Aug 18;157(3790):823–825. doi: 10.1126/science.157.3790.823-a. [DOI] [PubMed] [Google Scholar]
- Hilton J. L., Kearney P. C., Ames B. N. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys. 1965 Dec;112(3):544–547. doi: 10.1016/0003-9861(65)90093-7. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., BONNER D. M. Kynureninase from Neurospora: purification and properties. J Biol Chem. 1953 Dec;205(2):699–707. [PubMed] [Google Scholar]
- LESTER G. Regulation of early reactions in the biosynthesis of tryptophan in Neurospora crassa. J Bacteriol. 1963 Feb;85:468–475. doi: 10.1128/jb.85.2.468-475.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Direct evidence for a trytophan-anthranilic acid cycle in Neurospora. Biochim Biophys Acta. 1963 Jun 4;71:632–642. doi: 10.1016/0006-3002(63)91136-3. [DOI] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Factors affecting increased production of tryptophan synthetase by a TD mutant of Neurospora crassa. J Bacteriol. 1962 Jun;83:1294–1300. doi: 10.1128/jb.83.6.1294-1300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. PHYSIOLOGICAL CHANNELING OF TRYPTOPHAN IN NEUROSPORA CRASSA. Biochim Biophys Acta. 1964 Apr 4;86:91–99. doi: 10.1016/0304-4165(64)90162-x. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- WAINWRIGHT S. D., BONNER D. M. On the induced synthesis of an enzyme required for biosynthesis of an essential metabolite: induced kynureninase synthesis in Neurospora crassa. Can J Biochem Physiol. 1959 Jun;37(6):741–750. [PubMed] [Google Scholar]



