Abstract
The importance of cholesterol for endocytosis has been investigated in HEp-2 and other cell lines by using methyl-β-cyclodextrin (MβCD) to selectively extract cholesterol from the plasma membrane. MβCD treatment strongly inhibited endocytosis of transferrin and EGF, whereas endocytosis of ricin was less affected. The inhibition of transferrin endocytosis was completely reversible. On removal of MβCD it was restored by continued incubation of the cells even in serum-free medium. The recovery in serum-free medium was inhibited by addition of lovastatin, which prevents cholesterol synthesis, but endocytosis recovered when a water-soluble form of cholesterol was added together with lovastatin. Electron microscopical studies of MβCD-treated HEp-2 cells revealed that typical invaginated caveolae were no longer present. Moreover, the invagination of clathrin-coated pits was strongly inhibited, resulting in accumulation of shallow coated pits. Quantitative immunogold labeling showed that transferrin receptors were concentrated in coated pits to the same degree (approximately sevenfold) after MβCD treatment as in control cells. Our results therefore indicate that although clathrin-independent (and caveolae-independent) endocytosis still operates after removal of cholesterol, cholesterol is essential for the formation of clathrin-coated endocytic vesicles.
INTRODUCTION
Endocytosis occurs by clathrin-dependent as well as clathrin-independent mechanisms (van Deurs et al., 1989; Sandvig and van Deurs, 1994, 1996). Clathrin-dependent endocytosis is the best-defined process so far and is responsible for the rapid uptake of, e.g., hormones, growth factors, and transport molecules such as EGF and transferrin. The interaction of the molecules involved in this process has been investigated both in vivo and in vitro, resulting in characterization of a number of important proteins such as clathrin, adaptors, and dynamin (Schmid, 1997). Less is known about clathrin-independent endocytosis, but different forms seem to exist; for instance, both dynamin dependent and dynamin-independent mechanisms have been reported (Damke et al., 1994, 1995; Deirdre et al., 1998; Henley et al., 1998). Clathrin-independent endocytosis can clearly be different from uptake by invaginated caveolae. Thus, clathrin-independent endocytosis exists both in lymphocytes (Subtil et al., 1994) and on the apical side of polarized Madin–Darby canine kidney (MDCK) cells (Eker et al., 1994), which do not contain invaginated caveolae (Vogel et al., 1998).
Earlier studies have suggested a role for cholesterol in endocytosis (Heiniger et al., 1976); however, the type of endocytosis affected by cholesterol depletion was not investigated. Cholesterol present in the membrane of mammalian cells is required for normal cellular function (Bloch, 1991) and either is delivered from low-density lipoproteins internalized by receptor-mediated endocytosis via clathrin-coated pits followed by subsequent hydrolysis in lysosomes (Brown and Goldstein, 1980) or is synthesized in the endoplasmic reticulum (Reinhart et al., 1987). A major fraction of cholesterol is present in the plasma membrane (Lange, 1991), and cholesterol is important for various processes at the cell surface. For instance, there seems to be a direct interaction between the oxytocin receptor and cholesterol (Klein et al., 1995), and also the acetylcholine receptor was found to have a functional requirement for cholesterol (Criado et al., 1982). Furthermore, it has been proposed that annexin II, which is involved in the endocytic pathway (Creutz, 1992; Gruenberg and Emans, 1993), serves as an interface between membranes containing a high amount of cholesterol and the actin cytoskeleton (Harder et al., 1997), and it is clear that cholesterol is essential for the structure and function of invaginated caveolae, including the caveolae-dependent endocytosis that has been reported in some cell types (Rothberg et al., 1990; Parton et al., 1994; Parton, 1996; Schnitzer and Oh, 1994; Smaby et al., 1996; Chang et al., 1998; Hailstones et al., 1998).
In the present article, we have studied the role of cholesterol in clathrin-dependent and clathrin-independent endocytosis by using methyl-β-cyclodextrin (MβCD)1 to remove cholesterol from the plasma membrane. It has been shown that both β-cyclodextrin and MβCD remove cholesterol from cultured cells (Ohtani et al., 1989; Kilsdonk et al., 1995; Klein et al., 1995), and we have here used the methylated form because it was found to be more efficient than β-cyclodextrin (Klein et al., 1995). Cyclodextrins are cyclic oligomers of glucose that have the capacity to sequester lipophiles in their hydrophobic core (Pitha et al., 1988). The water-soluble MβCD is known to form soluble inclusion complexes with cholesterol, thereby enhancing its solubility in aqueous solution (Pitha et al., 1988; Irie et al., 1992). In this article we demonstrate that cholesterol plays an important role in the invagination of clathrin-coated pits and therefore in clathrin-dependent endocytosis.
MATERIALS AND METHODS
Materials
MβCD (average degree of substitution: 10.5–14.7 methyl groups per molecule), β-cyclodextrin, α-cyclodextrin, γ-cyclodextrin, water-soluble cholesterol (with ∼40 mg of cholesterol per gram solid; balance MβCD), lovastatin, pronase, HEPES, geneticin, lactose, BSA, ricin, and holo-transferrin (iron-saturated) were obtained from Sigma Chemical Co. (St. Louis, MO). [3H]leucine was obtained from the Radiochemical Center (Amersham, Buckinghamshire, UK). Na 125I and 125I-EGF were purchased from DuPont (Brussels, Belgium). Ricin and transferrin were 125I-labeled according to Fraker and Speck (1978) to a specific activity of 2 × 104–6 × 104 cpm/ng.
Cells
MDCK cells (strain II) transfected with the human transferrin receptor (TfR) (MDCK II hTfR) (from Dr. W. Hunziker, Lausanne, Switzerland) were maintained in DMEM (Flow Laboratories, Irvine, Scotland) supplemented with 5% FCS (Life Technologies, Paisly, Scotland), 100 μg/ml streptomycin, 100 U/ml penicillin, 2 mM l-glutamine (Life Technologies), and 0.25 mg/ml geneticin. HEp-2, A431, and NIH/3T3 cells (American Tissue Type Collection, Rockville, MD) were maintained in the same medium without the addition of geneticin and with different amounts of serum (5% FCS, 10% FCS, and 10% NCS, respectively). The HeLa cell line (tTA-HeLa), stably transfected with the cDNA for dynK44A, was cultured as described previously (Damke et al., 1995). For experiments, cell lines were seeded into 24-well plates (Falcon, Franklin Lakes, NJ) at a density of 105 cells/well, or in Falcon 25 cm2 flasks at a density of 1.25 × 106 1 or 2 d before the experiment. The experiments were performed in HEPES-buffered medium.
Measurement of Binding and Endocytosis of 125I-Ricin
Binding of ricin was measured as the amount of cell-associated 125I-ricin after a 30 min incubation at 0° or 37°C with 125I-ricin (40 ng/ml) and four washes with cold HEPES-buffered medium. Endocytosed 125I-labeled ricin was measured after 15 min at 37°C as the amount of toxin that could not be removed after one rapid wash, a 5-min incubation at 37°C, followed by three rapid washes, all with 0.1 M lactose in HEPES-buffered medium at 37°C (Sandvig and Olsnes, 1979). The endocytosed ricin was measured in a gamma counter (1261 Multigamma, Wallac, Gaithersburg, MD).
Measurement of Binding and Endocytosis of 125I-Transferrin
Binding of transferrin was measured as the amount of cell-associated 125I-transferrin after a 30 min incubation at 0° or 37°C with 125I-transferrin (100 ng/ml) and four washes with cold HEPES-buffered medium. Endocytosed 125I-labeled transferrin was measured after 5 min at 37°C and calculated as the percentage of total cell-associated (endocytosed and surface-bound) transferrin (Ciechanover et al., 1983). Surface-bound transferrin was removed by 1-h incubation with HEPES medium containing 2 mg/ml pronase on ice. After pronase treatment the medium containing the cells was centrifuged for 2 min in an Eppendorf centrifuge (Madison, WI) before the radioactive contents in the cell pellet (endocytosed) and in the supernatant (surface bound) were measured with a gamma counter.
Measurement of Binding and Endocytosis of 125I-EGF
Binding of EGF was measured as the amount of cell-associated 125I-EGF after a 30 min incubation at 0° or 37°C with 125I-EGF (3–4 μCi/ml) and four washes with cold HEPES. Endocytosed 125I-labeled EGF was measured after 10 min at 37°C and calculated as the percentage of total cell-associated EGF. Surface-bound EGF was measured as the amount of EGF that could be released by low pH after three rapid washes with PBS at 0°C. The cells were then incubated at 0°C for 6 min with low pH buffer (0.5 M NaCl and 0.2 M CH3COOH in HEPES, pH 2.5), followed by one rapid wash with the same buffer. Endocytosed EGF was measured as the amount of EGF that could not be removed by this treatment (Sandvig et al., 1987).
Measurement of the Potassium Content in Cells
The potassium content in cells was measured after the cells were washed three times with 100 mM MgCl2, air-dried, and dissolved in 0.1 M NaOH as described by Larkin et al. (1983). Potassium was then determined by ion selective electrodes (Vitros 250; Johnson-Johnson Clinica Diagnostics, Rochester, NY).
Measurement of Protein Synthesis
Protein synthesis was measured by incubating the cells for 10 min in a HEPES-buffered medium with 1 μCi/ml [3H]leucine. The medium was then removed, and 5% trichloroacetic acid was added. This solution was removed 10 min later, and the cells were washed twice with the same solution to remove free radioactive leucine. The precipitated protein was then dissolved in 0.1 M KOH, and the radioactivity associated with the cells was measured in a β-counter (MINAXI, TRI-CARB 4000 SERIES, United Technologies, Packard, Meriden, CT).
Electron Microscopy
HEp-2 cells for EM were preincubated with and without MβCD (10 or 15 mM) for 15 or 30 min at 37°C, washed with HEPES-buffered medium, and fixed with 2% formaldehyde and 0.1% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.2. The cells were then washed, scraped off the flasks, pelleted, and post-fixed with OsO4, contrasted en bloc with 1% uranyl acetate, dehydrated in a graded series of ethanols, and embedded in Epon. Sections were further contrasted with lead citrate and uranyl acetate and examined in a Philips CM 100 electron microscope (Philips, Eindhoven, the Netherlands). In some experiments, control cells and cells treated with MβCD were fixed, washed with PBS, and incubated with anti-human TfR antibody B3/25 (Boehringer Mannheim, Mannheim, Germany), 2 μg/ml PBS, for 1 h at room temperature. Then the cells remaining in monolayer in the culture flasks were washed with PBS and incubated for 2 h at room temperature with goat anti-mouse IgG coupled to 10 nm gold (Amersham), washed, scraped off, and processed for EM. Quantification of the immunogold labeling for TfRs was performed as described by Hansen et al. (1992).
Effect of MβCD on Cellular Cholesterol and Invaginated Caveolae
HEp-2 cells were preincubated in HEPES-buffered DMEM with 0.2% BSA for 10 min and then incubated for 15 min at 37°C in HEPES-buffered DMEM with 1 μCi (1α,1β(n)-[3H])-cholesterol (toluene solution; Amersham) to label the cell surface (Lange, 1991; Debry et al., 1997). To equilibrate with the cholesterol of intracellular compartments, cells were incubated for 20 h in DMEM containing 2.5% delipidated calf serum (Sigma C-1696) and 1 μCi [3H]cholesterol (Debry et al., 1997; Keller and Simons, 1998). In both instances, cells were subsequently washed thoroughly and incubated at 37°C with agitation in 1 ml DMEM without MβCD, or with 10 mM MβCD for 15, 30, and 60 min. After washing, the cells were lysed in 250 μl 2% NP-40, 0.2% SDS in distilled water, and the lysates were then centrifuged to remove insoluble material before aliquots were mixed with EcoLite+ scintillation mixture, 1:10, and counted in a scintillation counter (Beckman, Fullerton, CA).
In parallel experiments (i.e., same cell passage, same day, and same MβCD solution), HEp-2 cells were washed and incubated with 10 mM MβCD in DMEM for 0, 15, 30, and 60 min at 37°C, washed again, fixed, and further processed for electron microscopy as described above. The amount of characteristically invaginated caveolae on the plasma membrane (Vogel et al., 1998) was subsequently quantitated.
RESULTS
Effect of MβCD on Endocytosis of Transferrin, EGF, and Ricin
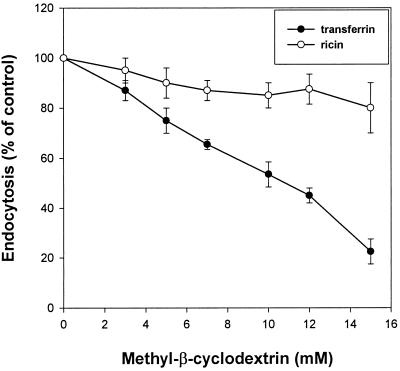
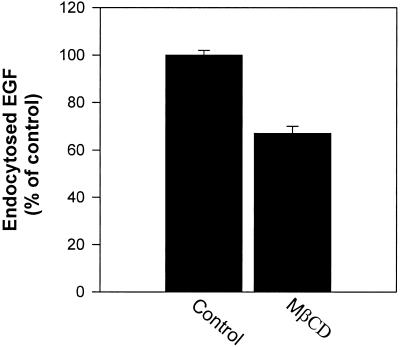
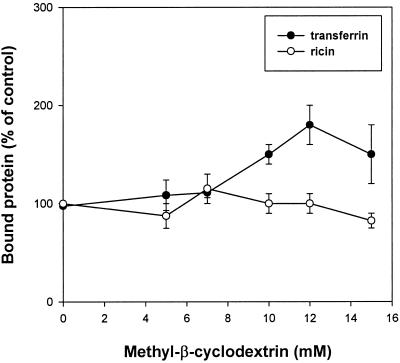
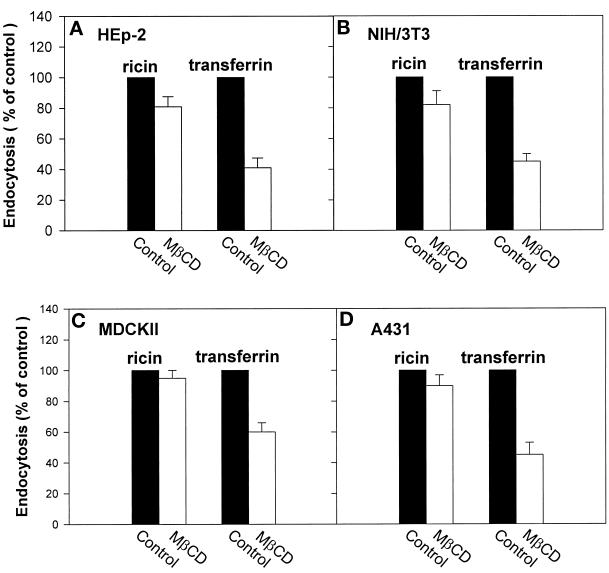
To study whether incubation with MβCD would affect endocytosis of transferrin and EGF, cells were preincubated for 15 min with MβCD at 37°C, and then 125I-transferrin or 125I-EGF was added. Endocytosis of transferrin was strongly reduced with increasing concentrations of MβCD (50% reduction at 10 mM MβCD) (Figure 1). As shown in Figure 2, 10 mM MβCD also gives a strong reduction of 125I-EGF endocytosis (∼40%). In agreement with the result that MβCD inhibits transferrin endocytosis, pretreatment of cells with 10 mM MβCD for 15 min at 37°C increased the cell surface binding of 125I-transferrin twofold (Figure 3). To further investigate the effect of MβCD on clathrin-dependent endocytosis, uptake of 125I-transferrin was measured in different cell lines preincubated with 10 mM MβCD for 15 min at 37°C. As shown in Figure 4, this concentration of MβCD inhibited endocytosis of transferrin in all cell lines by ∼50%.
Figure 1.
Effect of MβCD on transferrin (●) and ricin (○) endocytosis. MDCK II cells were washed briefly in HEPES-buffered medium before incubation with and without the indicated concentrations of MβCD for 15 min at 37°C. 125I-labeled transferrin or 125I-labeled ricin was then added to the cells, and the amounts of endocytosed transferrin or ricin were measured as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
Figure 2.
Effect of MβCD on endocytosis of EGF. HEp-2 cells were washed briefly in HEPES-buffered medium and incubated with and without MβCD (10 mM) for 15 min at 37°C. 125I-labeled EGF was then added to the cells, and the amounts of endocytosed EGF were measured as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
Figure 3.
Effect of MβCD on binding of transferrin (●) and ricin (○). MDCK II cells were incubated with and without the indicated concentrations of MβCD in HEPES-buffered medium at 37°C for 15 min. Then the cells were cooled down to 0°C, 125I-labeled transferrin or 125I-labeled ricin was added, and binding was measured after 30 min at 0°C as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
Figure 4.
Effect of MβCD on transferrin and ricin endocytosis in different cell lines. HEp-2, NIH/3T3, MDCK II, and A431 cells were washed briefly in HEPES-buffered medium and incubated with and without MβCD (10 mM) for 15 min at 37°C. 125I-labeled transferrin or 125I-labeled ricin was then added to the cells, and the amounts of endocytosed transferrin or ricin were measured as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
To test whether MβCD would affect all endocytic activity, we studied the uptake of a molecule that does not only enter by clathrin-dependent endocytosis. Experiments performed with increasing concentrations of MβCD showed that the endocytosis of the general membrane marker ricin (Sandvig and Olsnes, 1979; Sandvig and van Deurs, 1996) was less affected by increasing concentrations of MβCD than that of transferrin in MDCK II cells (Figure 1). In addition, there was no effect on the binding of 125I-ricin (Figure 3). Endocytosis of 125I-ricin was also measured in different cell lines preincubated with 10 mM MβCD for 15 min at 37°C. As shown in Figure 4, MβCD inhibited endocytosis of ricin in the various cell types by ∼20%. Because MβCD strongly decreased the number of invaginated caveolae (see below), the data suggest that there is another clathrin-independent form of endocytosis that is resistant to removal of cholesterol.
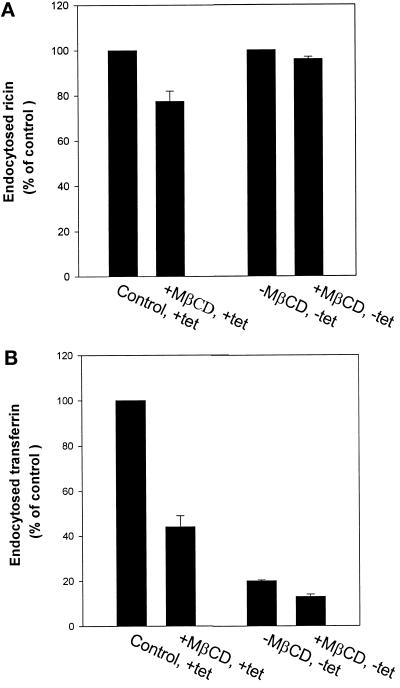
The data described above suggest that removal of cholesterol has a much stronger effect on clathrin-dependent than on clathrin-independent endocytosis. To further investigate this question we studied the effect of MβCD on transferrin and ricin endocytosis in HeLa K44A cells. These cells express mutant dynamin on removal of tetracycline, and clathrin-dependent endocytosis will then become inhibited (Damke et al., 1994). HeLa K44A cells grown with and without tetracycline were incubated with and without MβCD for 15 min at 37°C, and then 125I-transferrin or 125I-ricin was added. As shown in Figure 5A, ricin endocytosis was essentially unaffected by MβCD in the absence of tetracycline (mutant dynamin is expressed); however, MβCD decreased the endocytosis of ricin by ∼20% in the presence of tetracycline (both clathrin-dependent and clathrin-independent endocytosis are functioning) (Figure 5A). As shown in Figure 5B, endocytosis of transferrin is inhibited by MβCD to more than half in the presence of tetracycline, whereas transferrin endocytosis is blocked (>80%), as expected, when mutant dynamin is expressed and inhibits the clathrin-dependent pathway.
Figure 5.
Effect of MβCD on endocytosis of ricin (A) and transferrin (B) in HeLa cells stably transfected with the cDNA for mutant dynamin (dynK44A). The cells were washed briefly in HEPES-buffered medium and incubated with and without MβCD (10 mM) for 15 min at 37°C. 125I-labeled transferrin or 125I-labeled ricin was then added to the cells, and the amounts of endocytosed transferrin or ricin were measured as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
The Effect of MβCD on Endocytosis Is Related to Removal of Cholesterol
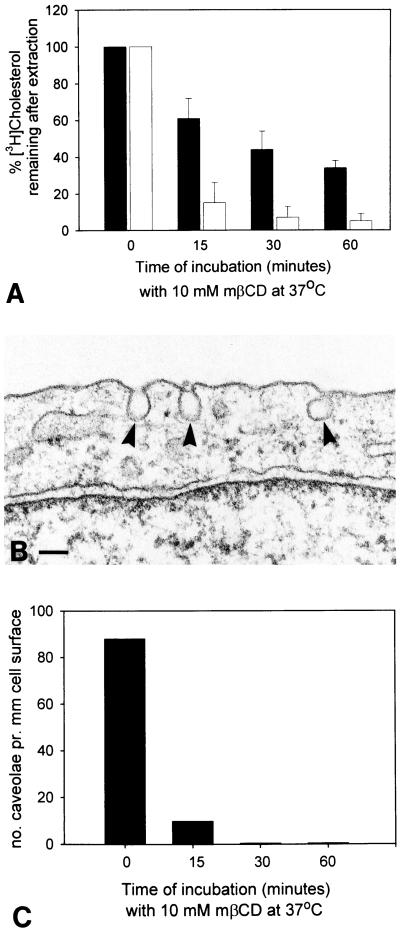
In agreement with previous studies (Neufeld et al., 1996; Keller and Simons, 1998) we found that MβCD efficiently extracts cholesterol from HEp-2 cells (Figure 6A). Caveolae with the characteristic deeply invaginated shape were frequently observed in control HEp-2 cells. As an alternative approach to evaluating the effect of MβCD on plasma membrane cholesterol, we used the observation that typically invaginated caveolae (Figure 6B) can only be maintained in the presence of cholesterol (Rothberg et al., 1990; Chang et al., 1998; Hailstones et al., 1998). Figure 6C shows that MβCD extraction of cholesterol has a pronounced effect on the number of invaginated caveolae.
Figure 6.
Effect of MβCD on cellular cholesterol and invaginated caveolae. A shows the percentage of [3H]cholesterol remaining in HEp-2 cells after extraction with MβCD as indicated. Black bars represent cells incubated with [3H]cholesterol for 20 h, whereas open bars are cells incubated for 15 min. Data from three independent experiments with samples performed in quadruplicate. Mean ± SD; n = 3. B shows characteristic invaginated caveolae (arrowheads) as observed in HEp-2 cells (without incubation with MβCD). Bar, 100 nm. In C, the frequency of such caveolae has been quantified after cholesterol extraction with MβCD as indicated; pooled data from two of the three experiments shown in A.
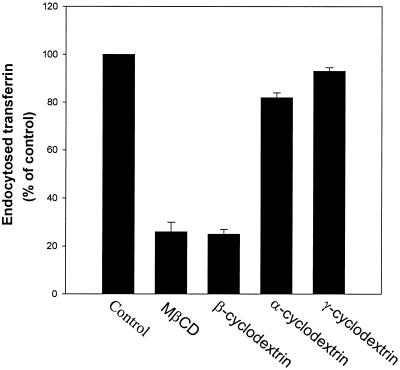
Although previous studies have shown that MβCD specifically removes cholesterol from the plasma membrane (Ohtani et al., 1989; Kilsdonk et al., 1995; Klein et al., 1995; Neufeld et al., 1996; Keller and Simons, 1998), we investigated whether the decreased transferrin endocytosis in the presence of MβCD was due to loss of cholesterol from the plasma membrane. Two other cyclodextrins, α-cyclodextrin and γ-cyclodextrin, do not bind cholesterol in the same way as MβCD (Ohtani et al., 1989), and we therefore tested whether they would affect transferrin endocytosis. In contrast, β-cyclodextrin, like MβCD, will extract cholesterol from the plasma membrane (Ohtani et al., 1989; Klein et al., 1995). HEp-2 cells were preincubated with 10 mM α-, β-, methyl-β-, or γ-cyclodextrins for 15 min at 37°C, before the addition of 125I-transferrin. Five minutes later, the amount of endocytosed transferrin was measured. As shown in Figure 7, both MβCD and β-cyclodextrin inhibited endocytosis of transferrin (>50%), whereas neither α- nor γ-cyclodextrin had any significant effect. Furthermore, when transferrin endocytosis was measured after preincubation of the cells with a MβCD-complex that was already saturated with cholesterol before it was added to the cells, there was no reduction in the endocytosis of transferrin (our unpublished results).
Figure 7.
Effect of α-, β-, methyl-β-, and γ-cyclodextrins on transferrin endocytosis. HEp-2 cells were washed briefly in HEPES-buffered medium and incubated with and without α-, β-, methyl-β-, or γ-cyclodextrins (10 mM) for 15 min at 37°C. 125I-labeled transferrin was then added to the cells, and the amounts of endocytosed transferrin were measured as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
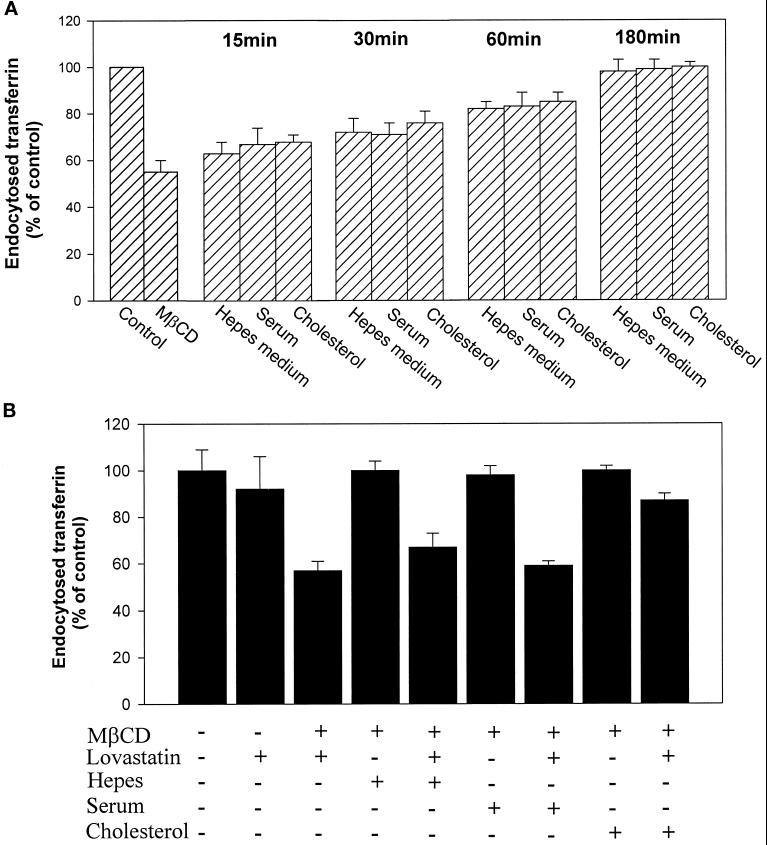
To further investigate the dependence of endocytosis on cholesterol, we studied whether the inhibition of transferrin endocytosis by MβCD was reversible. First we tried to compensate for the loss of plasma membrane cholesterol by adding cholesterol to the cells. The cells were preincubated with MβCD for 15 min at 37°C, and then HEPES-buffered medium with or without 5% fetal calf serum or 400 μg/ml water-soluble cholesterol was added before endocytosis of 125I-transferrin was measured. As shown in Figure 8A, there was a time-dependent recovery of the transferrin endocytosis. The uptake of transferrin was still inhibited by ∼20% after a 1-h incubation even when serum or cholesterol was added; however, after 3-h incubation, the inhibition by MβCD was totally reversed. The finding that transferrin endocytosis recovered even in the absence of added serum or cholesterol suggested that the recovery could be due to cholesterol synthesis in the cells. This hypothesis was strengthened by the finding that lovastatin, an inhibitor of cholesterol synthesis, did inhibit the recovery of transferrin endocytosis unless the water soluble form of cholesterol was added (Figure 8B). Unless the cells were pretreated with MβCD, lovastatin only decreased transferrin endocytosis by ∼10%, suggesting that there is only a small decrease in the level of plasma membrane cholesterol during 3 h in the absence of cholesterol synthesis. Thus, our results clearly indicate that the effect of MβCD observed is due to extraction of cholesterol.
Figure 8.
Reversal of MβCD-induced inhibition of transferrin endocytosis in the absence (A) or presence (B) of lovastatin. In both cases MDCK II cells were washed briefly in HEPES-buffered medium and incubated with and without MβCD (10 mM) for 15 min at 37°C. The cells were then incubated in HEPES-buffered medium with and without 5% FCS or water-soluble cholesterol (400 μg/ml) in the presence or absence of lovastatin (5 μg/ml) from 15 min to 3 h (A) or for 3 h (B) before 125I-labeled transferrin was added. The amounts of endocytosed transferrin were measured as described in MATERIALS AND METHODS. The error bars show deviations between duplicates.
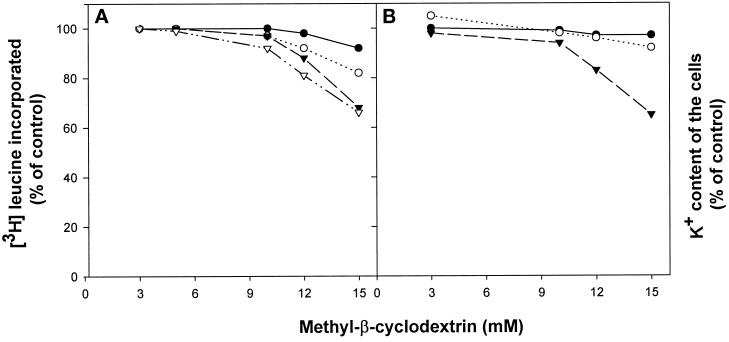
It was recently reported that removal of cholesterol with MβCD did not induce release of lactate dehydrogenase, suggesting that there are no large changes in the permeability of the plasma membrane (Keller and Simons, 1998). This is in agreement with our finding that up to 10 mM MβCD did not reduce protein synthesis in our cells to any significant extent (Figure 9A). Also, as shown in Figure 9B, 10 mM MβCD did not cause any significant membrane leakage of potassium. Furthermore, in MDCK II cells there was no increase in the cell-associated amount of 45Ca2+ even when the isotope was added to cells in a Ca2+-free buffer (our unpublished results). In HEp-2 cells there was a small increase (20%) under the same conditions, but there was no significant change when the isotope was added to a buffer containing normal amounts of CaCl2 (2 mM) (our unpublished results). Thus, in agreement with the data published by Keller and Simons (1998), our data indicate that MβCD strongly decreases clathrin-dependent endocytosis without causing any large permeability changes.
Figure 9.
Effect of MβCD on protein synthesis (A) and on the intracellular potassium content (B). (A) HEp-2 (○), NIH/3T3 (▿), MDCK II (●), and A431 (▾) cells were washed briefly in HEPES-buffered medium and incubated with and without MβCD (10 mM) for 15 min at 37°C. [3H]leucine was then added to the cells, and after 15 min further incubation the amounts of incorporated [3H]leucine were measured as described in MATERIALS AND METHODS. (B) HEp-2 (●), MDCK II (○), and A431 (▾) cells were washed briefly in HEPES-buffered medium and incubated with and without MβCD (10 mM) for 15 min at 37°C. The cells were then washed with MgCl2, and the amounts of intracellular potassium were measured as described in MATERIALS AND METHODS.
Effect of MβCD on Clathrin-coated Pits and TfR Distribution
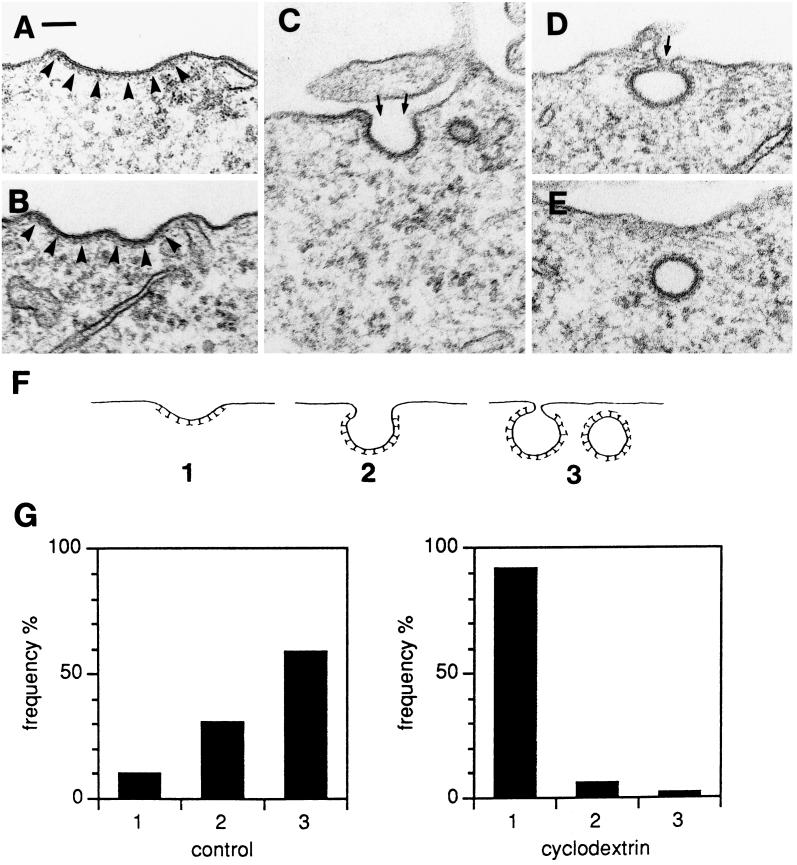
The inhibition of endocytosis of transferrin (and EGF) by MβCD could reflect that removal of cholesterol prevented clathrin-coated pit formation, i.e., assembly of clathrin coats on the cytoplasmic face of the membrane; however, clathrin-coated pits were readily observed by electron microscopy in MβCD-treated cells, although they clearly appeared more flattened than in control cells. To investigate how MβCD affected the morphological appearance of clathrin-coated pits quantitatively, we classified the pits as follows: shallow pits, which are flattened or only slightly invaginated (Figure 10, A and B); invaginated pits, that is, when the pits are clearly invaginated but the necks connecting them with the exterior are still open wide (slightly wider or narrower than the diameter of the pit itself) (Figure 10C); and pits that are almost or completely pinched off, where the neck is very narrow (Figure 10D), or absent in the particular plane of sectioning (which could very well mean that the pit is surface-connected in another plane of sectioning; see Petersen and van Deurs [1983]) (Figure 10E). These three categories of clathrin-coated pits are shown schematically in Figure 10F and will be referred to below as type 1, 2, or 3. When control HEp-2 cells and cells treated with 10 mM MβCD for 15 min at 37°C were compared with respect to the relative frequency of the three types of clathrin-coated pits (Figure 10G), the effect of MβCD was striking: type 2 and in particular type 3 dominated in control cells, whereas MβCD caused a clear predominance of type 1 pits.
Figure 10.
Examples of clathrin-coated pits at various degrees of invagination (A–D); E shows an apparently free, clathrin-coated vesicular profile that, however, may be surface-connected in another plane of sectioning. The clathrin coat is indicated by arrowheads in A and B. The small arrows in C and D indicate the necks connecting the pits to the exterior. Bar, 100 nm. (F) For quantification, the coated pits and apparently free vesicles (A–E) were subdivided into three types. (G) Coated pits in control HEp-2 cells and cells treated with 10 mM MβCD for 15 min (approximately 200 coated pits in each experiment) were scored at random and classified as 1, 2, or 3 as defined in F. The relative frequency of the different types of coated pits is shown. Bar, 100 nm.
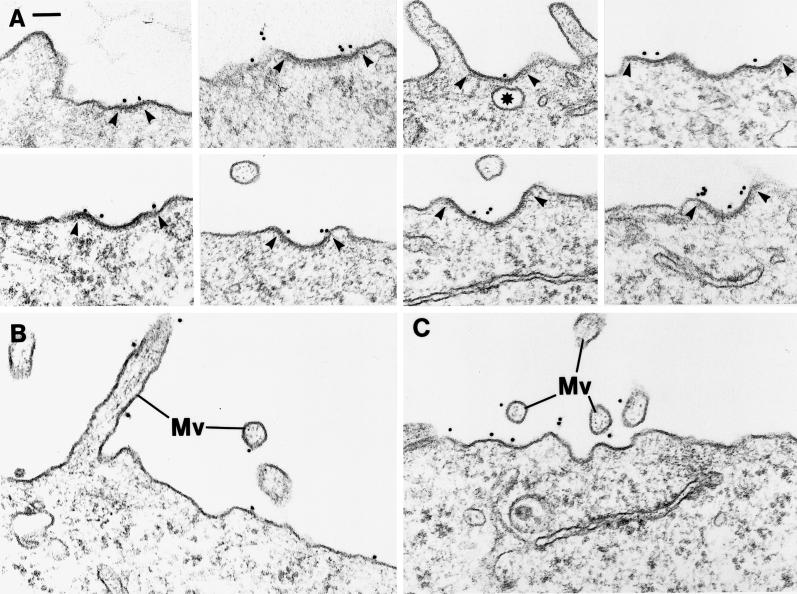
Having shown that MβCD does not affect endocytosis in general or does prevent assembly of clathrin-coated pits at the cell surface but interferes with the ability of flattened pits to invaginate, it was important to determine whether TfRs could still accumulate in the pits of MβCD-treated cells. We therefore used an immunogold-labeling protocol to detect TfRs on the surface of HEp-2 cells (Hansen et al., 1992). As shown in Figure 11A, TfR gold labeling was distinct in the shallow, clathrin-coated pits mostly found in the MβCD-treated cells. Indeed, gold labeling was also seen on the cell surface outside clathrin-coated pits and on microvilli (Figure 11, B and C). In control HEp-2 cells, 8.7% of the TfRs as detected by immunogold labeling (using 2 μg/ml of the B3/25 anti-TfR antibody on prefixed cells) are localized to coated pits that occupy 1.1–1.4% of the total surface area of these cells (Hansen et al., 1992). Accordingly, TfRs are concentrated approximately sevenfold in the coated pits (Table 1). After incubation with 10 mM MβCD, we found that 11.5% of the TfR gold labeling was localized to coated pits (Table 1); however, because the surface area now occupied by coated pits had increased by 25% (presumably reflecting the fact that efficient invagination and pinching off are perturbed), the amount of golds in coated pits still corresponds to a sevenfold TfR concentration (Table 1). It should also be noted that we found a 60% increase in the total TfR cell-surface labeling in MβCD-treated cells as compared with control cells (Table 1), in agreement with the biochemical data reported above. Thus MβCD treatment does not interfere with the ability of TfRs to become concentrated efficiently in coated pits. Taken together, our findings therefore indicate that rather than blocking all types of endocytosis or preventing TfRs from entering clathrin-coated pits, MβCD perturbs invagination of the coated pits.
Figure 11.
Effect of MβCD on clathrin-coated pits and TfR distribution. (A) Examples of shallow coated pits characteristic of MβCD-treated cells and of immunogold labeling for TfRs in the coated pits. Arrowheads mark the extension of the pits. Asterisk marks for comparison a clearly noncoated vesicular profile. (B and C), Examples of TfR immunogold labeling of portions of the cell surface without coated pits and of microvilli (Mv). HEp-2 cells were incubated for 15 or 30 min at 37°C with 10 mM MβCD, fixed, incubated with B3/25 anti-TfR antibody and then with goat anti-mouse antibody conjugated to 10 nm gold, and processed for EM. Bar, 100 nm.
Table 1.
Concentration efficiency of TfR in clathrin-coated pits in HEp-2 cells as determined by immunogold labelinga
| % of surface area occupied by clathrin-coated pits | % of TfR-gold in clathrin-coated pits | Concentration of TfR-gold in clathrin-coated pits; fold | |
|---|---|---|---|
| Control cells | 1.1–1.4 | 8.7 | ∼7× |
| Cells treated for 30 min with 10 mM MβCD | 1.4–1.8 | 11.5 | ∼7× |
HEp-2 cells were fixed before incubation with 2 μg/ml B3/25 anti-TfR antibody. Forty large cell profiles (with a total of 2990 and 4812 golds, respectively) were included in the quantification from each experiment.
Data and calculations based on Hansen et al., 1992.
DISCUSSION
In the present study we have investigated the role of cholesterol in endocytosis by using MβCD, which specifically removes cholesterol from the plasma membrane (Ohtani et al., 1989; Kilsdonk et al., 1995; Klein et al., 1995; Neufeld et al., 1996). Our results show that endocytosis of transferrin and EGF was strongly reduced by addition of MβCD. This reduction could be due 1) to a general inhibition of all endocytic activity, 2) to a perturbation of the ability of clathrin-coated membrane areas to form, invaginate, or pinch off to form free, coated vesicles, or 3) to an exclusion of the receptors from clathrin-coated pits. The first possibility can be excluded because the general membrane marker ricin is still internalized. Furthermore, MβCD clearly reduced ricin endocytosis to a much larger extent when both clathrin-dependent and clathrin-independent endocytosis were operating than when only clathrin-independent endocytosis was functioning. The results indicate that MβCD strongly reduces clathrin-dependent endocytosis, whereas there is a clathrin-independent endocytic pathway that is less affected. Because invagination of caveolae also is affected by MβCD, this clathrin-independent endocytosis seems to be caveolae-independent. It has previously been found that a downregulation of clathrin-dependent endocytosis can be associated with an upregulation of clathrin-independent endocytosis (Damke et al., 1995), and there is a possibility that this might occur also on treatment with MβCD. Second, EM revealed that coated pits were indeed formed in MβCD-treated cells, but a marked flattening of these structures had taken place. Third, the quantitative immunogold data clearly show that the TfRs are not prevented from being concentrated in (the shallow) coated pits after MβCD treatment. A concentration of TfRs in clathrin-coated pits of approximately sevenfold both in MβCD-treated cells and in control cells is in agreement with previous data (Hansen et al., 1992).
TfRs undergo endocytosis and recycling, and the number of TfRs on the surface is consequently dependent on both the amount of receptors which is internalized, and the amount that is recycled. The finding that binding of transferrin to the cell surface was increased nearly twofold by MβCD indicates that MβCD inhibits endocytosis without reducing the recycling of the TfRs to the same extent. There seems to be a somewhat stronger effect of MβCD on the morphology of the clathrin-coated pits than on the uptake of transferrin. This might be due to the formation of some invaginated coated pits that rapidly pinch off. Our results strongly suggest that removal of cholesterol perturbs clathrin-coated pit invagination and, therefore, coated vesicle formation.
It has previously been reported that the membrane in coated pits is not disrupted by the sterol-binding drugs filipin or digitonin, although the membrane adjacent to these specialized domains readily binds these agents (Montesano et al., 1979). On the other hand, endosomal membrane becomes susceptible to filipin disruption as soon as the clathrin coat is released (McGookey et al., 1983), suggesting that the clathrin coat directly or indirectly binds cholesterol and prevents access to filipin. In agreement with those results, nystatin was reported not to affect transferrin endocytosis (Deckert et al., 1996), and our own unpublished data showed that filipin has no effect on transferrin endocytosis in HEp-2 cells. Also, it is not clear that complexes between cholesterol and sterol-binding drugs would affect endocytosis in the same way as removal of cholesterol by MβCD.
Sterol-binding drugs such as filipin, nystatin, and digitonin have been found to inhibit endocytosis from invaginated caveolae and caveolae-like domains (Schnitzer et al., 1994; Deckert et al., 1996; Orlandi and Fishman, 1998). Invaginated caveolae disappear in cells that are depleted of cholesterol or exposed to sterol-binding agents (Rothberg et al., 1990, 1992; Schnitzer et al., 1994). In agreement with those data, our results clearly show that MβCD leads to disappearance of invaginated caveolae in HEp-2 cells. That MβCD treatment leads to loss of invaginated caveolae is in agreement with the recent results of Hailstones et al. (1998) showing that different cholesterol depletion treatments, including β-trimethyl cyclodextrin treatment, remove morphologically recognizable invaginated caveolae. Interestingly, they found that invaginated caveolae only form when the cholesterol level is >50% of control values. Also, Orlandi and Fishman (1998) found that the activity of cholera toxin was completely inhibited by β-cyclodextrin, and they concluded that productive entry of cholera toxin occurs from caveolae. Furthermore, they reported that diphtheria toxin retained 80% of its toxic activity in the presence of β-cyclodextrin, and they therefore assumed that entry from clathrin-coated pits was essentially normal. This apparently contradicts our results; however, no direct measurement on uptake from clathrin-coated pits after β-cyclodextrin treatment was performed by Orlandi and Fishman (1998), and the apparent discrepancy can be explained if removal of cholesterol both reduces uptake from coated pits and at the same time facilitates membrane translocation of the toxin. In fact, filipin, which does not affect uptake from clathrin-coated pits, increased the toxicity of diphtheria toxin (Orlandi and Fishman, 1998).
Heiniger et al. (1976) reported that L cells, in which hydroxymethylglutaryl-CoA reductase activity and cholesterol synthesis were inhibited by addition of 25-hydroxycholesterol, had a reduced rate of endocytosis of the fluid-phase marker HRP. The uptake of HRP was restored by the addition of cholesterol (Heiniger et al., 1976). HRP is endocytosed, however, by both clathrin-dependent and clathrin-independent endocytosis (Oliver, 1982), and the fact that cholesterol depletions of L cells seem to have a stronger effect on total endocytosis in these cells (50%) than the effect we observed on HRP endocytosis in the cell types we have tested could be due to a larger proportion of uptake by clathrin-dependent endocytosis in L cells. Interestingly, Chang et al. (1992) reported that removal of cholesterol drastically increased the number of low-density lipoprotein receptors in MA104 cells. The authors reported a slightly reduced internalization index, but because the calculation of such an index only takes into account the amount of surface-bound ligand versus internalized ligand, in this case at the end of a long incubation, the data might actually be in agreement with a reduced rate of endocytosis of the receptor from clathrin-coated pits. Two other cyclodextrins (Ohtani et al., 1989)—α-cyclodextrin, which has been reported to remove phospholipids from the plasma membrane, and γ-cyclodextrin, which is also not specific for cholesterol—had much less effect on transferrin endocytosis than MβCD. This supports the notion that it is the removal of cholesterol per se that is essential for the inhibitory effect on clathrin-dependent endocytosis in the MβCD-treated cells. On removal of MβCD, the transferrin endocytosis was fully restored by continued incubation of the cells, even in serum-free medium. The recovery was inhibited by addition of lovastatin, suggesting that the cholesterol level in the plasma membrane was restored by newly synthesized cholesterol. This idea is supported by the finding that endocytosis did recover when a water-soluble form of cholesterol was added together with lovastatin. When lovastatin is used to inhibit cholesterol synthesis, fatty acid modification of intracellular proteins also is inhibited (Alberts et al., 1980; Brown and Goldstein, 1980), and it is therefore essential for the conclusion, that cholesterol is required for clathrin-dependent endocytosis, that addition of cholesterol can counteract the effect of treatment with lovastatin.
It was reported recently that extraction of cholesterol with MβCD did not affect the amounts of lactate dehydrogenase in baby hamster kidney cells (Keller and Simons, 1998). In agreement with those findings, our results show that treatment with 10 mM MβCD had no effect on the intracellular potassium content nor on the protein synthesis, indicating that the plasma membrane permeability was not strongly affected. Furthermore, the lack of change in transepithelial resistance in MβCD-treated MDCK I cells (our unpublished results) and MDCK II cells (Keller and Simons, 1998), together with our finding that binding of ricin and Shiga-toxin to the cell surface was unaffected by MβCD (our unpublished results), indicates that there are no large changes in the bilayer structure in cells treated with MβCD compared with the control cells. All of these data indicate that the flattening of the clathrin-coated pits is directly correlated to the removal of cholesterol.
It has been suggested that annexin II, which seems to be involved in both the regulated exocytic pathway (Creutz, 1992) and the endocytic pathway (Gruenberg and Emans, 1993), interacts directly with cholesterol-rich domains of the bilayer, and perhaps with cholesterol itself (Harder et al., 1997). Such a direct protein–cholesterol interaction was recently revealed for the membrane protein VIP21-caveolin (Murata et al., 1995). The cholesterol-binding drug filipin induces dissociation of annexin II and actin-binding proteins from endosomes (Harder et al., 1997). In contrast, there is no evidence that cholesterol is important for binding of clathrin. As shown here, the clathrin coat stays at the plasma membrane after treatment of cells with MβCD. It is only the curvature that is affected. Actin was recently reported to be involved in clathrin-dependent endocytosis, because endocytosis was inhibited by perturbation of the actin cytoskeleton (Parton et al., 1994; Schnitzer and Oh, 1994; Deckert et al., 1996). Nevertheless, actin does not seem to be required for the formation of the coated-pit invagination (Lamaze et al., 1997). Thus, the effect of MβCD described here is probably not due to an effect on the actin cytoskeleton; however, cholesterol could be important for the function of a protein involved in inducing curvature of clathrin-coated pits, such as affecting the activity of GTP-binding proteins that regulate coated-pit function (Schmid, 1997). Also, removal of cholesterol from the membrane might affect association of other molecules with the clathrin-coated pits, it might affect the interaction of membrane-associated proteins with each other, or it might somehow directly affect the ability of the other lipids in the clathrin-coated membrane domains to form an invaginated structure. As mentioned in the INTRODUCTION, cholesterol is important for the structure and function of membrane proteins (Muller and Shinitzky, 1979; Criado et al., 1982; Bloch, 1991; Kilsdonk et al., 1995), and furthermore, a change in the cholesterol content will affect the membrane viscosity, the exposure of membrane proteins to the surroundings, and therefore also the interaction between membrane proteins and cytosolic proteins (Shinitzky and Inbar, 1974; Shinitzky and Rivnay, 1977). Such changes might be responsible for the inhibition of formation of clathrin-coated pits observed here.
In conclusion, our results clearly indicate that the concentration of cholesterol in the plasma membrane of mammalian cells is essential for clathrin-dependent endocytosis, and it will be important for the understanding of this pathway to investigate why this is the case.
ACKNOWLEDGMENTS
We are grateful to Anne-Grethe Myrann, Ulla Hjortenberg, Mette Ohlsen, Kirsten Pedersen, and Keld Ottosen for their excellent technical assistance. This work was supported by the Norwegian Research Council for Science and the Humanities, The Norwegian Cancer Society, The Danish Cancer Society, The Danish Medical Research Council, the Novo-Nordisk Foundation, Blix legacy, Torsteds legacy, the Jahre foundation, the Nordic Cancer Union, a NATO Collaborative Research Grant (CRG 900517), a Human Frontier Science Program grant (RG404/96 M), and Jeanette and Søren Bothners legacy.
Abbreviations used:
- MβCD
methyl-β-cyclodextrin
- TfR
transferrin receptor
REFERENCES
- Alberts AW, et al. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-CoA reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K. Cholesterol: evolution of structure and function. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membrane. Amsterdam: Elsevier Science Publishers; 1991. pp. 363–381. [Google Scholar]
- Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. [PubMed] [Google Scholar]
- Chang JY, Chavis JA, Liu LZ, Drew PD. Cholesterol oxides induce programmed cell death in microglial cells. Biochem Biophys Res Commun. 1998;249:817–821. doi: 10.1006/bbrc.1998.9237. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Rothberg KG, Kamen BA, Anderson RGW. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Schwartz AL, Dautry-Varsat A, Lodish HF. Kinetics of internalization and recycling of transferrin and transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- Creutz CE. The annexins and exocytosis. Science. 1992;258:924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Criado M, Eibl H, Barrantes FJ. Effects of lipids on acetylcholine receptor. Essential need of cholesterol for maintenance of agonist-induced state transitions in lipid vesicles. Biochemistry. 1982;21:3622–3629. doi: 10.1021/bi00258a015. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debry P, Nash EA, Neklason DW, Metherall JE. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J Biol Chem. 1997;272:1026–1031. doi: 10.1074/jbc.272.2.1026. [DOI] [PubMed] [Google Scholar]
- Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deirdre PO, McIntosh P, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eker P, Holm PK, van Deurs B, Sandvig K. Selective regulation of apical endocytosis in polarized Madin–Darby canine kidney cells by mastoparan and cAMP. J Biol Chem. 1994;269:18607–18615. [PubMed] [Google Scholar]
- Fraker PJ, Speck JC., Jr Protein and cell membrane iodinations with a sparingly soluble chloramide, 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Emans N. Annexins in membrane transport. Trends Cell Biol. 1993;3:224–227. doi: 10.1016/0962-8924(93)90116-i. [DOI] [PubMed] [Google Scholar]
- Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res. 1998;39:369–379. [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Internalization efficiency of the transferrin receptor. Exp Cell Res. 1992;199:19–28. doi: 10.1016/0014-4827(92)90457-j. [DOI] [PubMed] [Google Scholar]
- Harder T, Kellner R, Parton RG, Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol Biol Cell. 1997;8:533–545. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiniger HJ, Kandutsch AA, Chen HW. Depletion of L-cell sterol depresses endocytosis. Nature. 1976;263:515–517. doi: 10.1038/263515a0. [DOI] [PubMed] [Google Scholar]
- Henley JR, Krueger WA, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T, Fukunaga K, Pitha JJ. Hydroxypropylcyclodextrins in parenteral use. I: Lipid dissolution and effects on lipid transfers in vitro. J Pharmacol Sci. 1992;81:521–523. doi: 10.1002/jps.2600810609. [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with β-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34:13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- Lange Y. Disposition of intracellular cholesterol in human fibroblasts. J Lipid Res. 1991;32:329–339. [PubMed] [Google Scholar]
- Larkin JM, Brown MS, Goldstein JL, Anderson RGW. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- McGookey DJ, Fagerberg K, Anderson RGW. Filipin-cholesterol complexes form in uncoated vesicle membrane derived from coated vesicles during receptor-mediated endocytosis of low density lipoprotein. J Cell Biol. 1983;96:1273–1278. doi: 10.1083/jcb.96.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Perrelet A, Vassalli P, Orci L. Absence of filipin-sterol complexes from large coated pits on the surface of culture cells. Proc Natl Acad Sci USA. 1979;76:6391–6400. doi: 10.1073/pnas.76.12.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Hematol. 1979;42:355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of α-, β- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem. 1989;186:17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- Oliver C. Endocytic pathways at the lateral and basal cell surfaces of exocrine acinar cells. J Cell Biol. 1982;95:154–161. doi: 10.1083/jcb.95.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, van Deurs B. Serial-section analysis of coated pits and vesicles involved in adsorptive pinocytosis in cultured fibroblasts. J Cell Biol. 1983;96:277–281. doi: 10.1083/jcb.96.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha J, Irie T, Sklar PB, Nye JS. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 1988;43:493–502. doi: 10.1016/0024-3205(88)90150-6. [DOI] [PubMed] [Google Scholar]
- Reinhart MP, Billheimer JT, Faust JR, Gaylor JL. Subcellular localization of the enzymes of cholesterol biosynthesis and metabolism in rat liver. J Biol Chem. 1987;262:9649–9655. [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S. Effect of temperature on the uptake, excretion and degradation of abrin and ricin by HeLa cells. Exp Cell Res. 1979;121:15–25. doi: 10.1016/0014-4827(79)90439-7. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Endocytosis without clathrin. Trends Cell Biol. 1994;4:275–277. doi: 10.1016/0962-8924(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Endocytosis, intracellular transport, and cytotoxic action of shiga toxin and ricin. Am Physiol Soc. 1996;76:949–965. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;6:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994;269:6072–6082. [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M, Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974;85:603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M, Rivnay B. Degree of exposure of membrane proteins determined by fluorescence quenching. Biochemistry. 1977;16:982–986. doi: 10.1021/bi00624a027. [DOI] [PubMed] [Google Scholar]
- Smaby JM, Kulkarni VS, Momsen M, Brown RE. The interfacial elastic packing interactions of galactosylceramides, sphingomyelins, and phosphatidylcholines. Biophys J. 1996;70:868–877. doi: 10.1016/S0006-3495(96)79629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Hémar A, Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994;107:3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Petersen OW, Olsnes S, Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]
- Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of caveolae in Caco-2 and MDCK II cells. J Cell Sci. 1998;111:825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]