Abstract
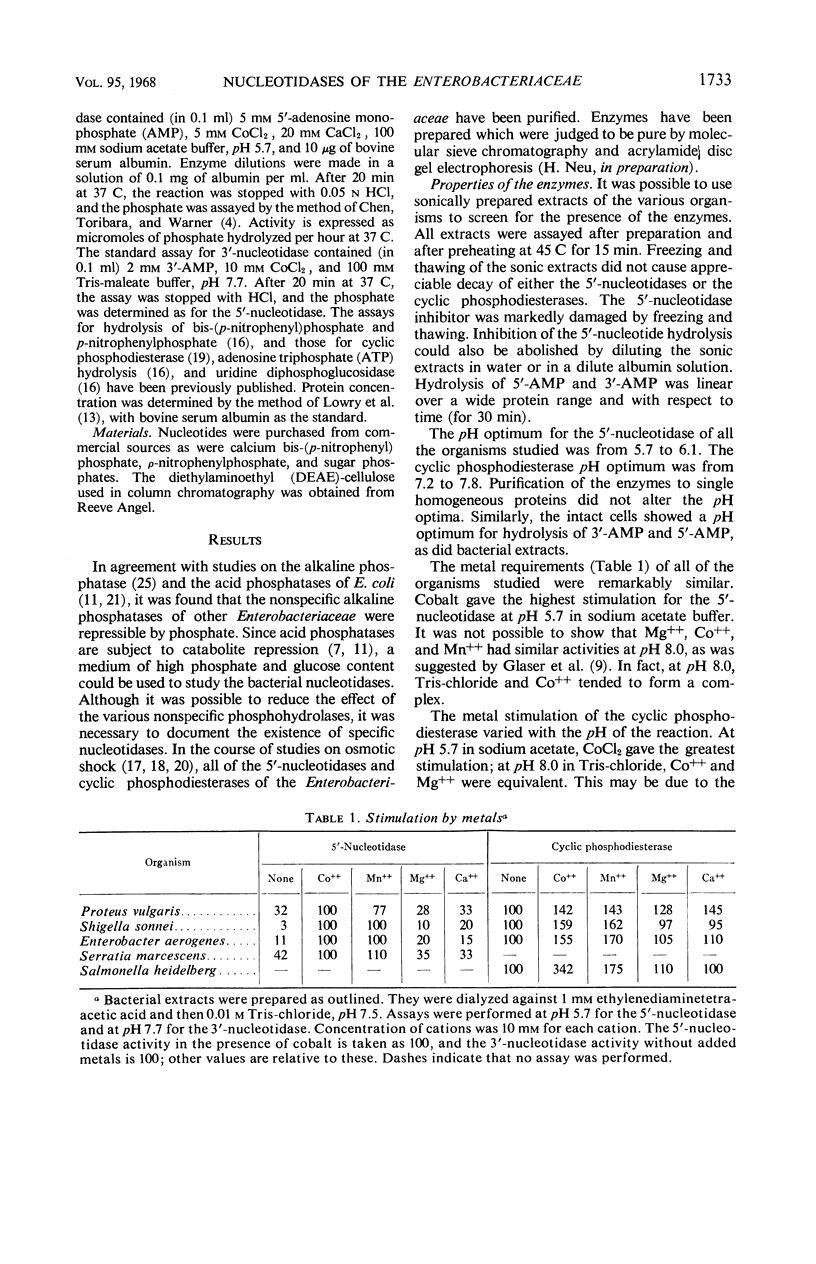
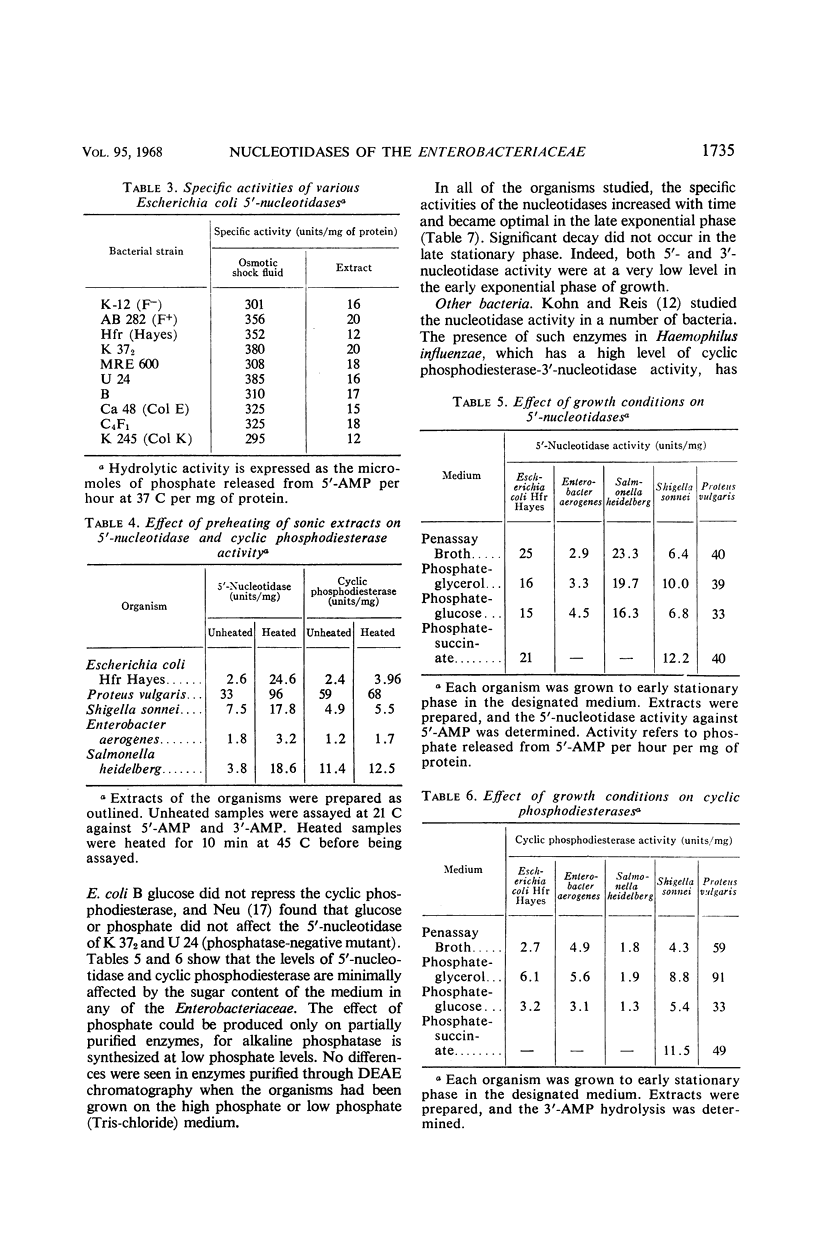
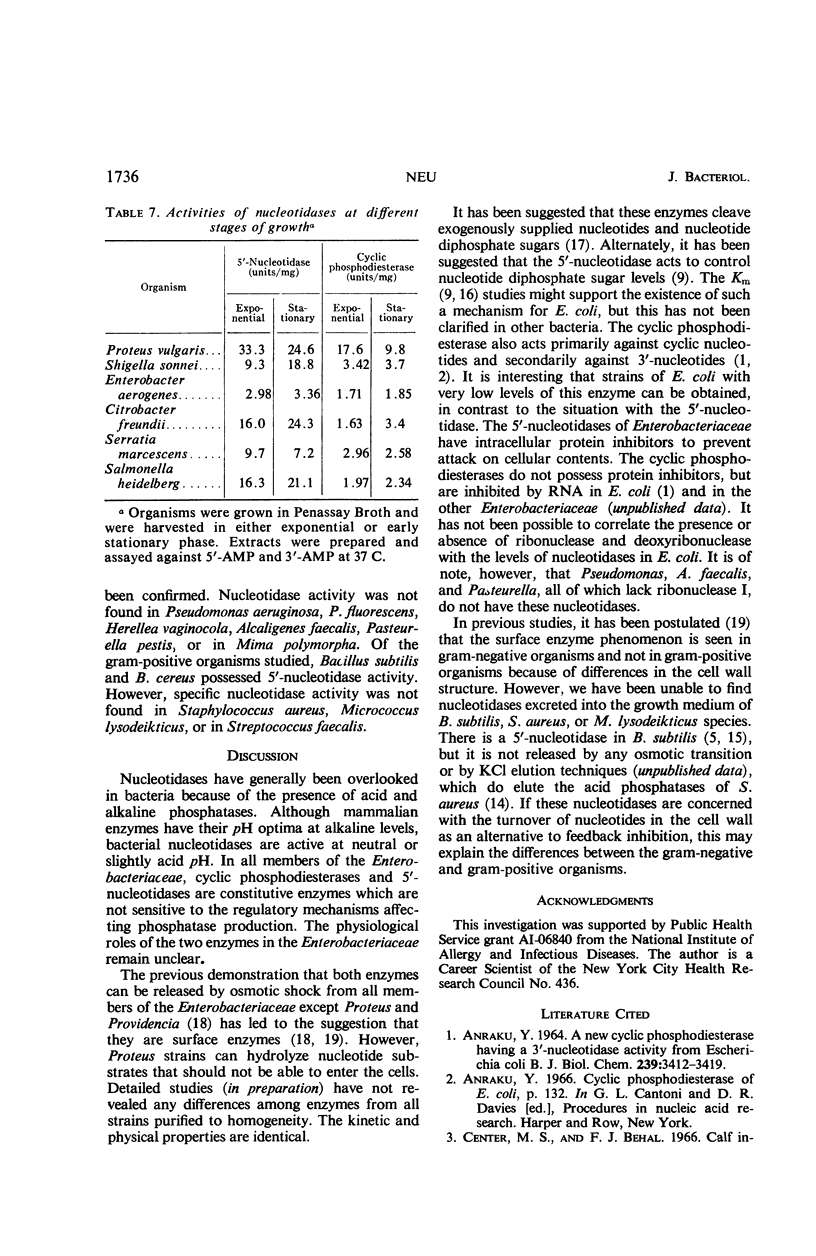
All members of the Enterobacteriaceae possess distinct 5′-nucleotidases and cyclic phosphodiesterases (3′-nucleotidases) that can be differentiated from the acid and alkaline phosphatases and the acid sugar hydrolases. The nucleotidases and cyclic phosphodiesterases of the various Enterobacteriaceae are remarkably similar in properties. All of the 5′-nucleotidases hydrolyze 5′-nucleotides, adenosine triphosphate, and uridine diphosphoglucose. Their pH optimum is from 5.7 to 6.1. The cyclic phosphodiesterases hydrolyze 3′-nucleotides, cyclic phosphonucleotides, bis-(p-nitrophenyl)phosphate, and p-nitrophenylphosphate. Their pH optimum is from 7.2 to 7.8. For both enzymes, cobalt showed optimal metal stimulation. An intracellular protein inhibitor for the 5′-nucleotidase is present in all of the Enterobacteriaceae. No inhibitor of cyclic phosphodiesterase activity exists, although hydrolysis of both cyclic phosphonucleotides and 3′-nucleotides is inhibited by ribonucleic acid. Neither of the enzymes is subject to control by phosphate level or by catabolite repression. Of the other bacteria studied, only Haemophilus and Bacillus subtilis contained significant 3′- or 5′-nucleotidase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Oct;239:3412–3419. [PubMed] [Google Scholar]
- Demain A. L., Hendlin D. Phosphohydrolases of a Bacillus subtilis mutant accumulating inosine and hypoxanthine. J Bacteriol. 1967 Jul;94(1):66–74. doi: 10.1128/jb.94.1.66-74.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Anraku Y., Heppel L. A. The occurrence of a protein inhibitor for 5'-nucleotidase in extracts of Escherichia coli. Biochem Biophys Res Commun. 1966 Sep 8;24(5):628–632. doi: 10.1016/0006-291x(66)90369-x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Brockman R. W., Heppel L. A. Purification and properties of two acid phosphatase fractions isolated from osmotic shock fluid of Escherichia coli. Biochemistry. 1967 Jun;6(6):1743–1751. doi: 10.1021/bi00858a024. [DOI] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- KOHN J., REIS J. L. BACTERIAL NUCLEOTIDASES. J Bacteriol. 1963 Oct;86:713–716. doi: 10.1128/jb.86.4.713-716.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malveaux F. J., San Clemente C. L. Elution of loosely bound acid phosphatase from Staphylococcus aureus. Appl Microbiol. 1967 Jul;15(4):738–743. doi: 10.1128/am.15.4.738-743.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. II. Surface localization and purification of the Escherichia coli 5'-nucleotidase inhibitor. J Biol Chem. 1967 Sep 10;242(17):3905–3911. [PubMed] [Google Scholar]
- ROGERS D., REITHEL F. J. Acid phosphatases of Escherichia coli. Arch Biochem Biophys. 1960 Jul;89:97–104. doi: 10.1016/0003-9861(60)90018-7. [DOI] [PubMed] [Google Scholar]
- SHUSTER L., KAPLAN N. O. A specific b nucleotidase. J Biol Chem. 1953 Apr;201(2):535–546. [PubMed] [Google Scholar]
- SULKOWSKI E., BJORK W., LASKOWSKI M., Sr A specific and nonspecific alkaline monophosphatase in the venom of Bothrops atrox and their occurrence in the purified venom phosphodiesterase. J Biol Chem. 1963 Jul;238:2477–2486. [PubMed] [Google Scholar]
- SWARTZ M. N., KAPLAN N. O., FRECH M. E. Significance of heat-activated enzymes. Science. 1956 Jan 13;123(3185):50–53. doi: 10.1126/science.123.3185.50. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- von HOFSTEN Acid phosphatase and the growth of Escherichia coli. Biochim Biophys Acta. 1961 Mar 18;48:171–181. doi: 10.1016/0006-3002(61)90529-7. [DOI] [PubMed] [Google Scholar]


