Abstract
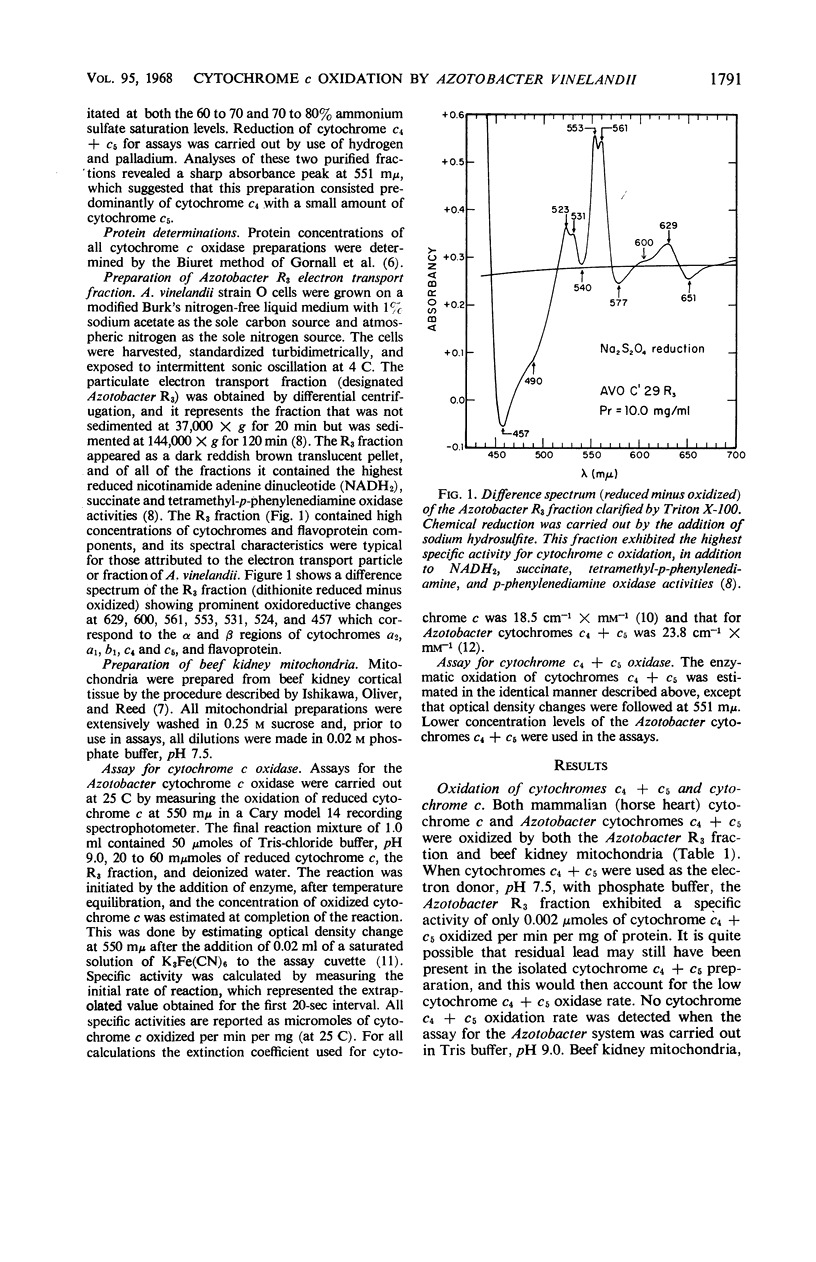
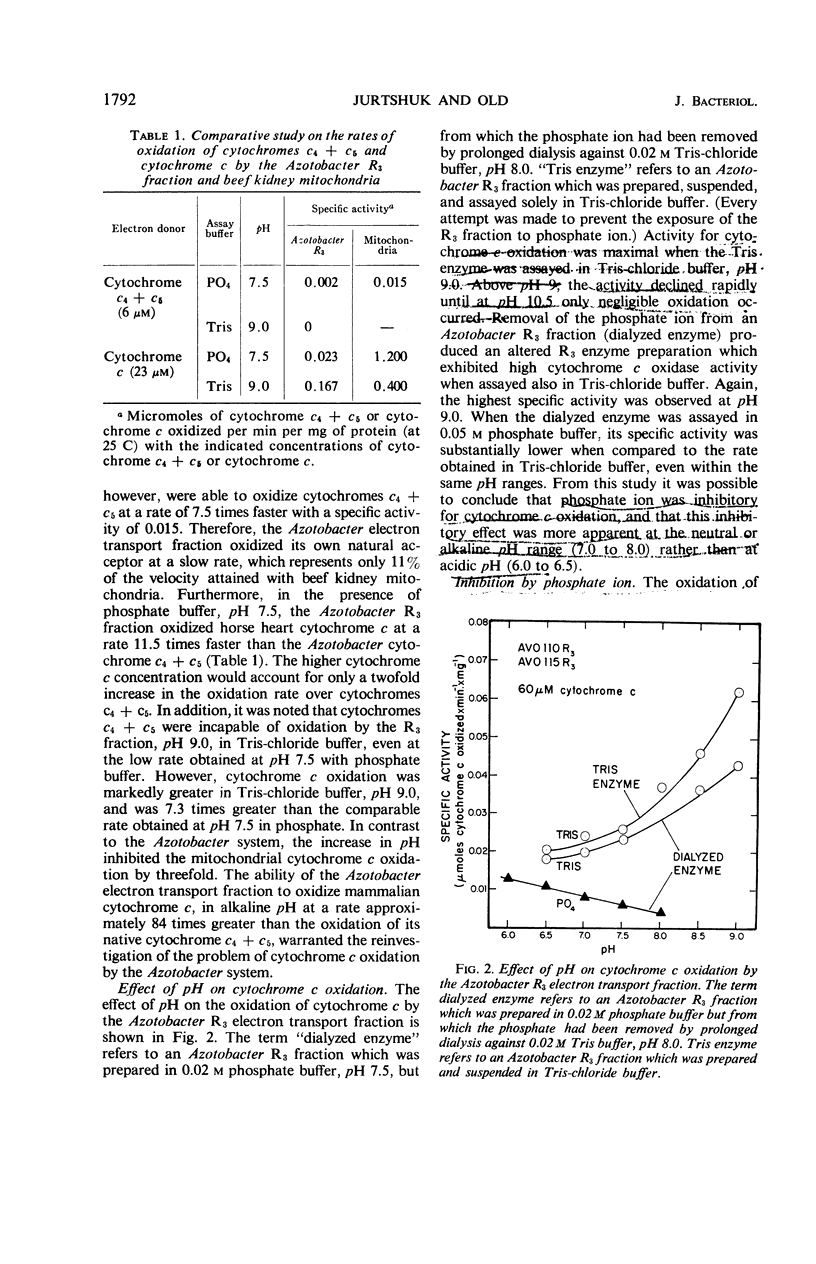
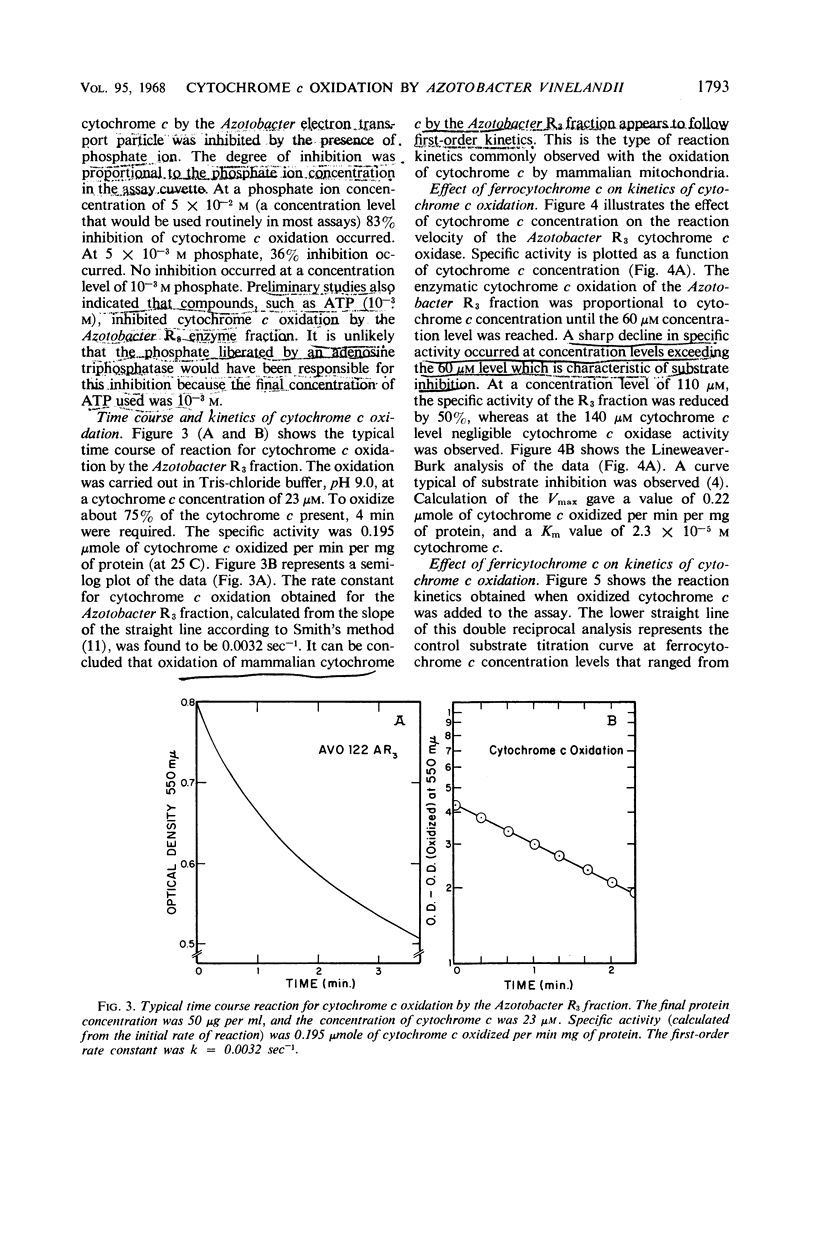
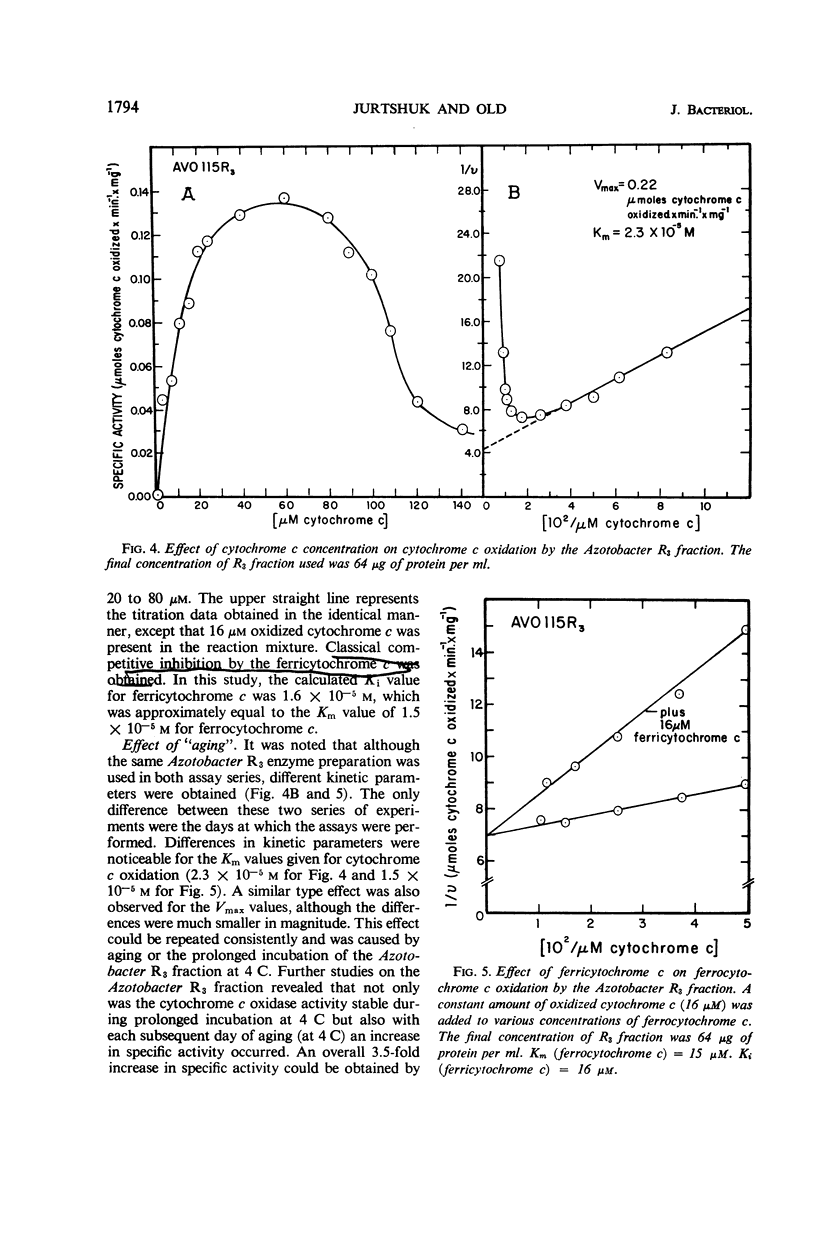
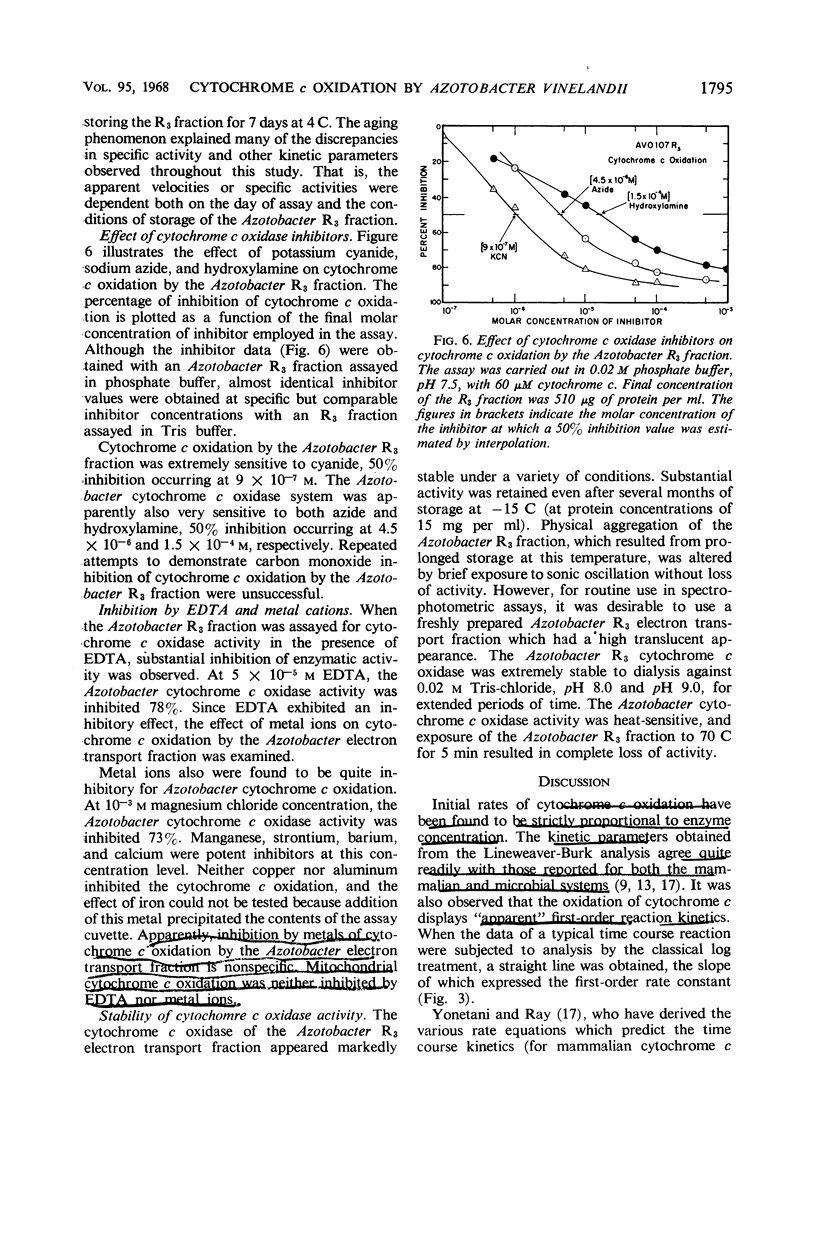
The spectrophotometric oxidation of horse heart ferrocytochrome c was examined by use of the particulate electron transport fraction (R3) of Azotobacter vinelandii strain O. Unlike cytochrome c, purified preparations of native Azotobacter cytochromes c4 + c5 were oxidized only slowly by the electron transport fraction. The oxidation of mammalian cytochrome c proceeded at an appreciable rate and displayed “apparent” first-order kinetics at a pH optimum of 9.0 with tris(hydroxymethyl)aminomethane-chloride buffer. The calculated Vmax value was 0.22 μmole of cytochrome c oxidized per min per mg of protein (25 C) and a Km value for cytochrome c of 2.3 × 10−5m was obtained. Ferricytochrome c was a “strict” competitive inhibitor for this oxidation. Cytochrome c oxidation by the Azotobacter electron transport system was markedly sensitive to cyanide, azide, and hydroxylamine, although carbon monoxide inhibition could not be demonstrated. It was sensitive also to high concentrations of phosphate, ethylenediaminetetraacetate, and some metal cations. “Aging” or prolonged storage of the Azotobacter R3 fraction, at 4 C for 10 days, resulted in a threefold increase in specific activity. The cytochrome c peroxidase type of reaction did not occur with the R3 electron transport fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azoulay E., Couchoud-Beaumont P. Etude de la cytochrome-oxidase de Pseudomonas aeruginosa. Biochim Biophys Acta. 1965 Nov 22;110(2):301–311. [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Oliver R. M., Reed L. J. Alpha-Keto acid dehydrogenase complexes, V. Macromolecular organization of pyruvate and alpha-ketoglutarate dehydrogenase complexes isolated from beef kidney mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):534–541. doi: 10.1073/pnas.56.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Aston P. R., Old L. Enzymatic oxidation of tetramethyl-p-phenylenediamine and p-phenylenediamine by the electron transport particulate fraction of Azotobacter vinelandii. J Bacteriol. 1967 Mar;93(3):1069–1078. doi: 10.1128/jb.93.3.1069-1078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAYNE E. C., NASON A. Cytochrome c oxidase from Azotobacter vinelandii. J Biol Chem. 1958 Apr;231(2):889–898. [PubMed] [Google Scholar]
- MARGOLIASH E. The use of ion exchangers in the preparation and purification of cytochrome c. Biochem J. 1954 Apr;56(4):529–535. doi: 10.1042/bj0560529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A. Purification, some properties and the specific biological activity of cytochromes c4 and c5 from Azotobacter vinelandii. Biochem J. 1956 Nov;64(3):582–589. doi: 10.1042/bj0640582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHARTON D. C., GRIFFITHS D. E. Studies on the electron transport system. XXXIX. Assay of cytochrome oxidase. Effect of phospholipids and other factors. Arch Biochem Biophys. 1962 Jan;96:103–114. doi: 10.1016/0003-9861(62)90458-7. [DOI] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. 3. Kinetics of the peroxidatic oxidation of ferrocytochrome c catalyzed by cytochrome c peroxidase. J Biol Chem. 1966 Feb 10;241(3):700–706. [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. I. Purification and some properties. J Biol Chem. 1965 Nov;240(11):4503–4508. [PubMed] [Google Scholar]


