Abstract
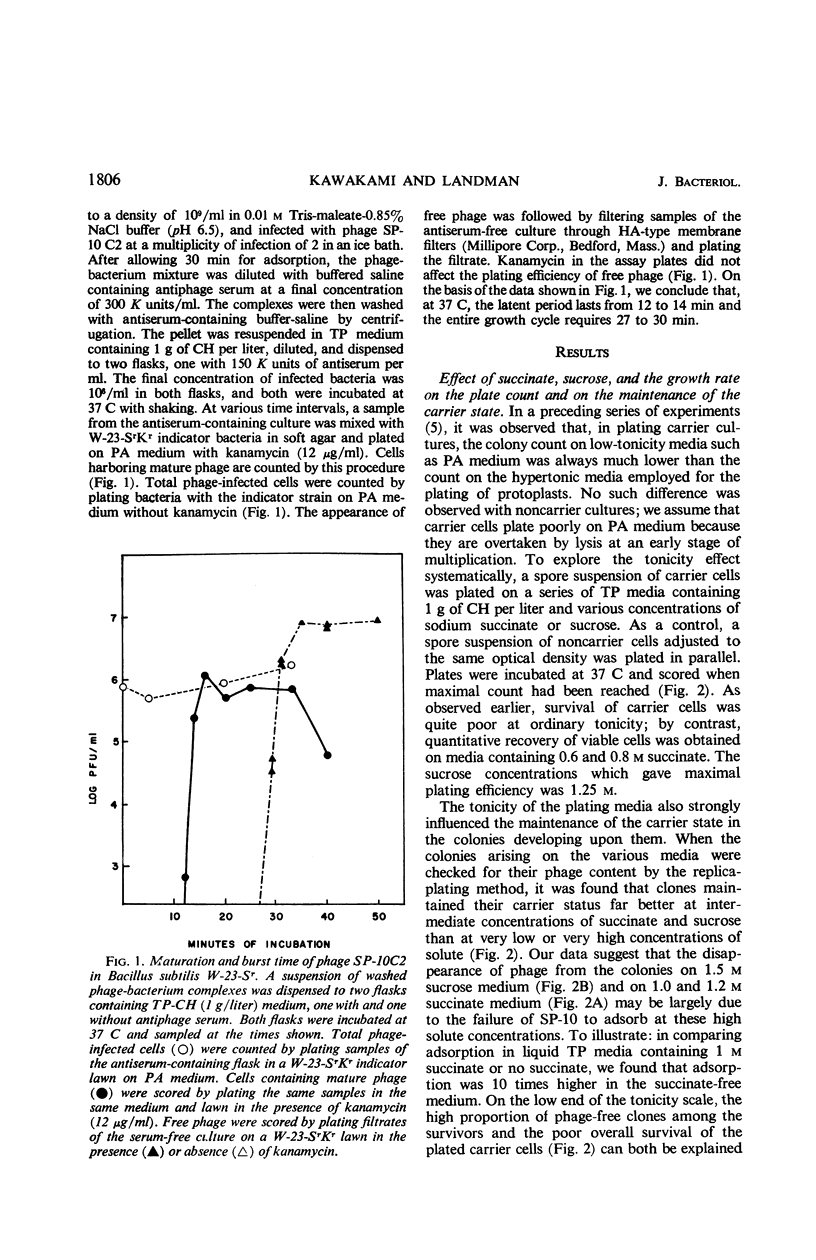
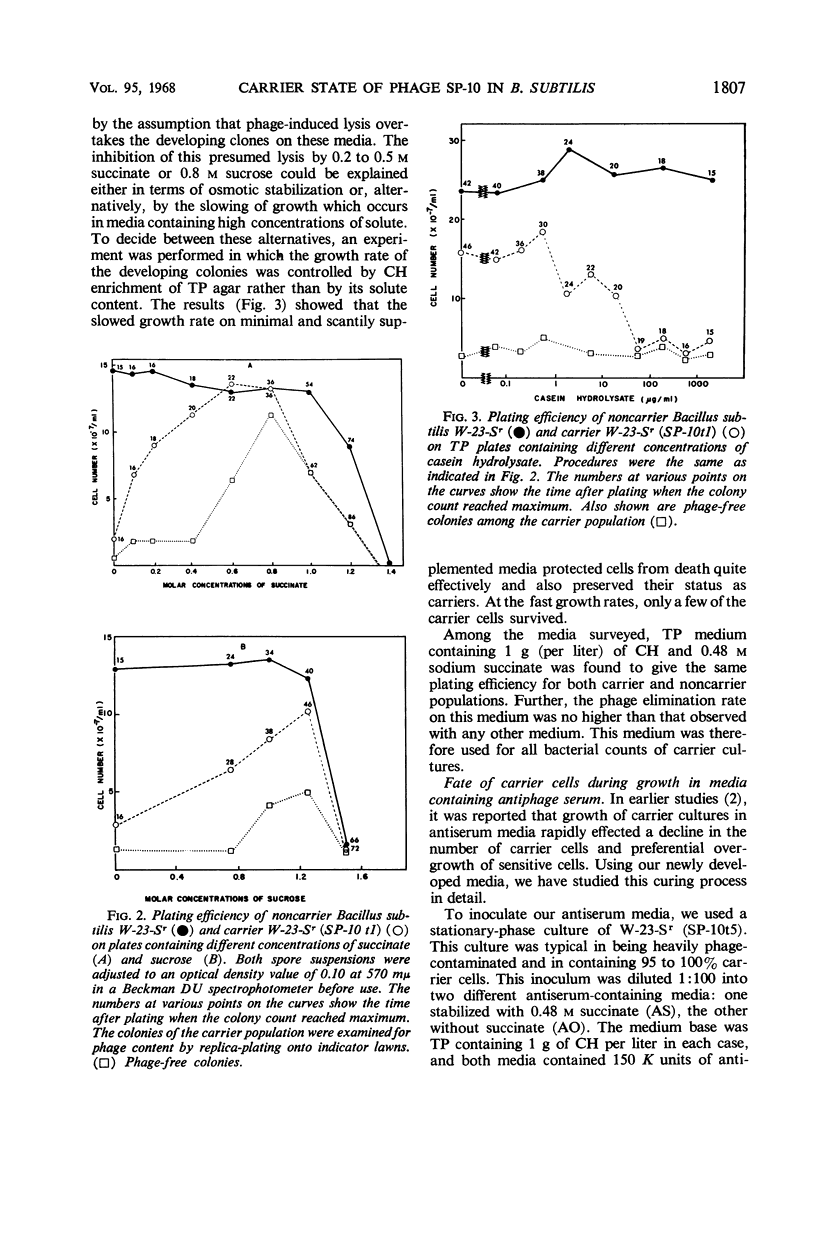
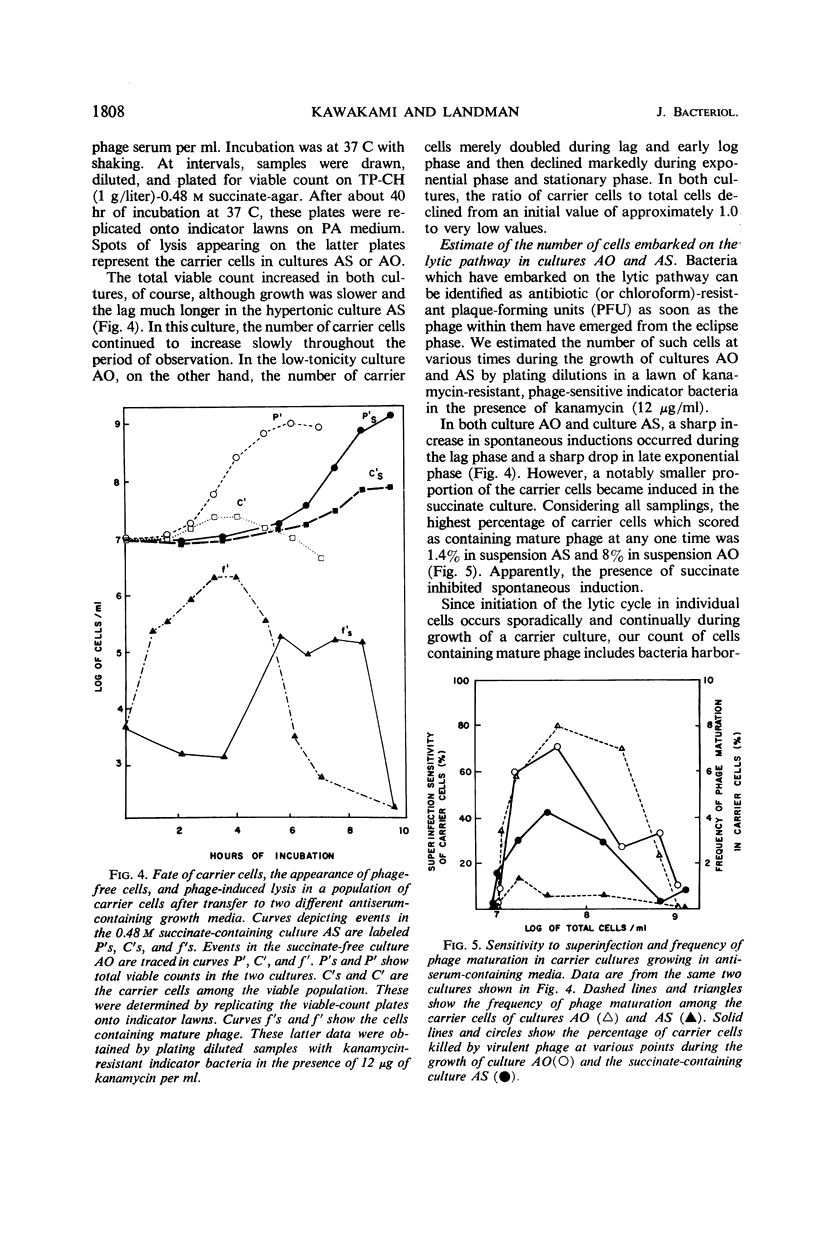
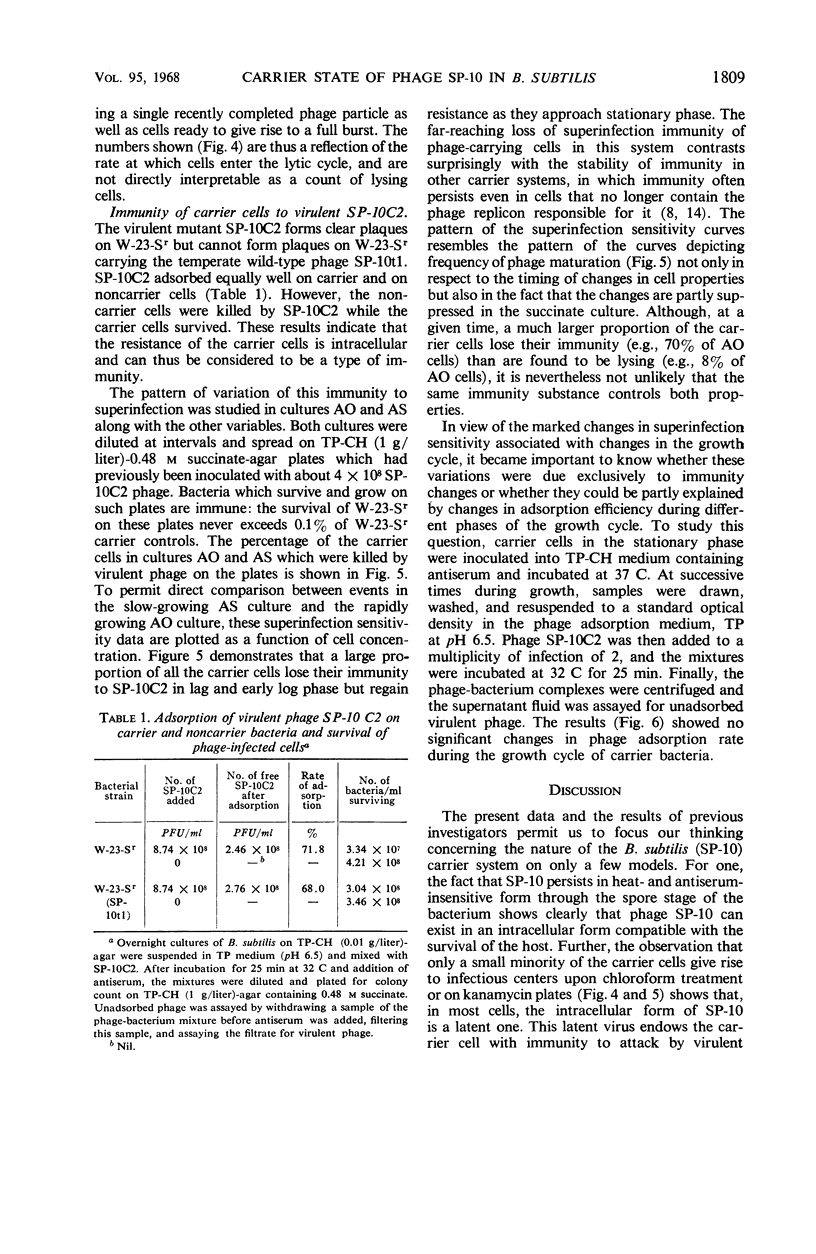
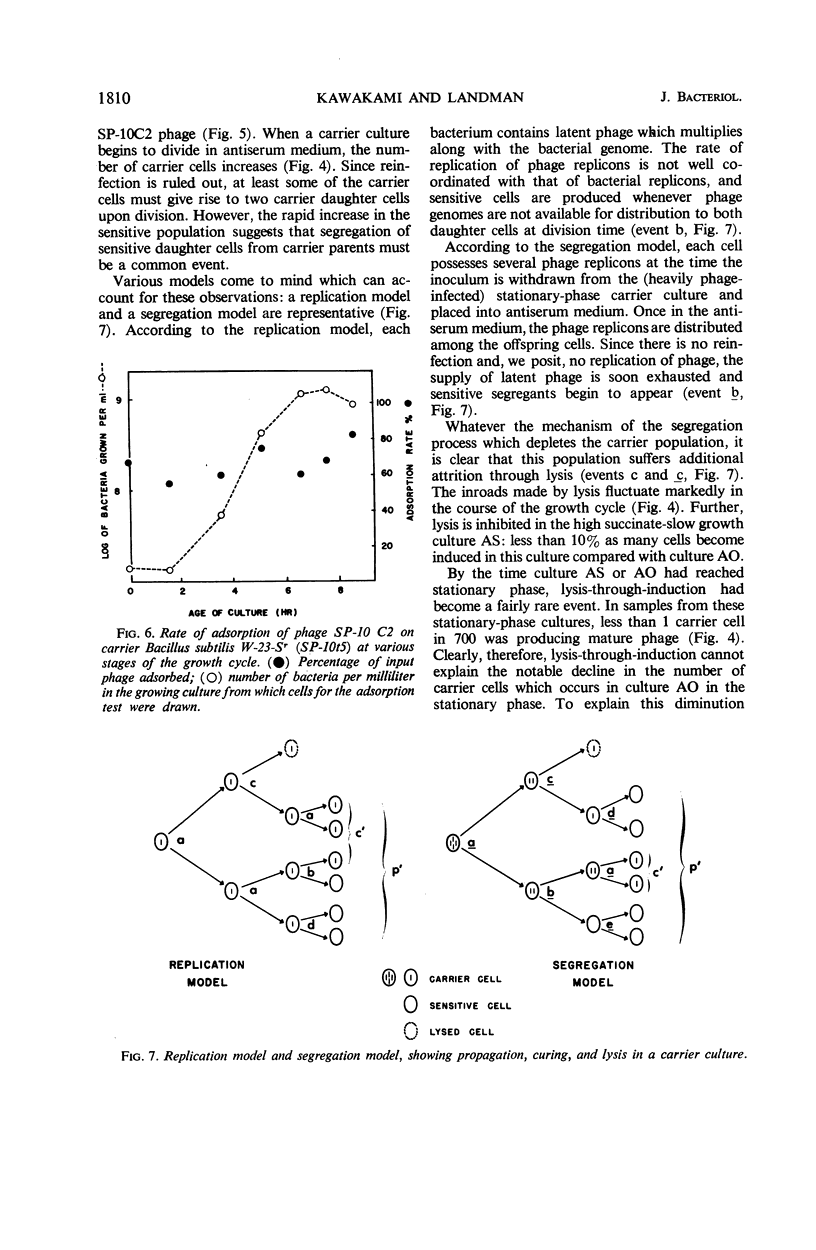
Although the association of phage SP-10 with Bacillus subtilis W-23-Sr persists in heat- and antiserum-resistant form through the spore stage, it is unstable in vegetative cells and frequently terminates in loss of the carried phage or in lysis. On low-tonicity media, the plating efficiency of carrier cells is low. However, high concentrations of succinate or sucrose or a slowed growth rate preserve viability: on 0.48 m succinate-agar, the viable count per optical density unit is the same as that of a noncarrier control culture. Carrier clones retain phage on 0.48 m succinate-agar. At higher succinate levels, many colonies emerge free of phage; at 1 m succinate, all are cured, probably because high succinate inhibits reinfection. Growth of carrier cells in liquid medium with antiphage serum results in rapid curing; events in such cultures with and without succinate were studied quantitatively by tracing the emergence of sensitive cells, the multiplication and induction of carrier cells, and the sensitivity of carrier cells to superinfection with virulent phage. During log phase, 40 to 70% of the carrier cells became sensitive to virulent phage, although the same cells were insensitive during lag and stationary phase. Apparently, fluctuations in repressor levels are responsible. Spontaneous induction of carrier cells followed a qualitatively similar pattern, perhaps in response to changes in level of the same repressor. Production of sensitive segregants by carrier followed a different course, presumably because the repressor does not affect segregation. Many sensitive cells were found two to three divisions after inoculation in antiserum medium. This suggests that each inoculum cell contained one or only a few phage replicons. The data are compatible with the idea that the carrier state in media without antisera is maintained entirely by reinfection and without replication of phage in the latent state. Alternative models which involve replication of latent phage are not ruled out, however.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- HOFFMANN BERLING H., MAZE R. RELEASE OF MALE-SPECIFIC BACTERIOPHAGES FROM SURVIVING HOST BACTERIA. Virology. 1964 Mar;22:305–313. doi: 10.1016/0042-6822(64)90021-2. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Landman O. E. Retention of episomes during protoplasting and during propagation in the L state. J Bacteriol. 1966 Aug;92(2):398–404. doi: 10.1128/jb.92.2.398-404.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI K., BARKSDALE L., GARMISE L. Phenotypic alterations associated with the bacteriophage carrier state of Shigella dysenteriae. J Gen Microbiol. 1961 Mar;24:355–367. doi: 10.1099/00221287-24-3-355. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., FRASER D. K., ADAMS J. N., BURROUS J. W. Lysogenization, transduction and genetic recombination in bacteria. Cold Spring Harb Symp Quant Biol. 1958;23:71–82. doi: 10.1101/sqb.1958.023.01.010. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- ROMIG W. R., BRODETSKY A. M. Isolation and preliminary characterization of bacteriophages for Bacillus subtilis. J Bacteriol. 1961 Jul;82:135–141. doi: 10.1128/jb.82.1.135-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. INCORPORATION OF BACTERIOPHAGE GENOME BY SPORES OF BACILLUS SUBTILIS. J Bacteriol. 1964 Jun;87:1499–1502. doi: 10.1128/jb.87.6.1499-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]


