Abstract
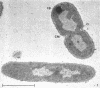
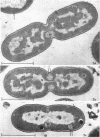
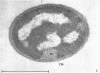
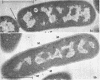
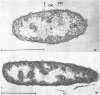
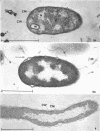
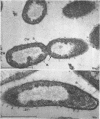
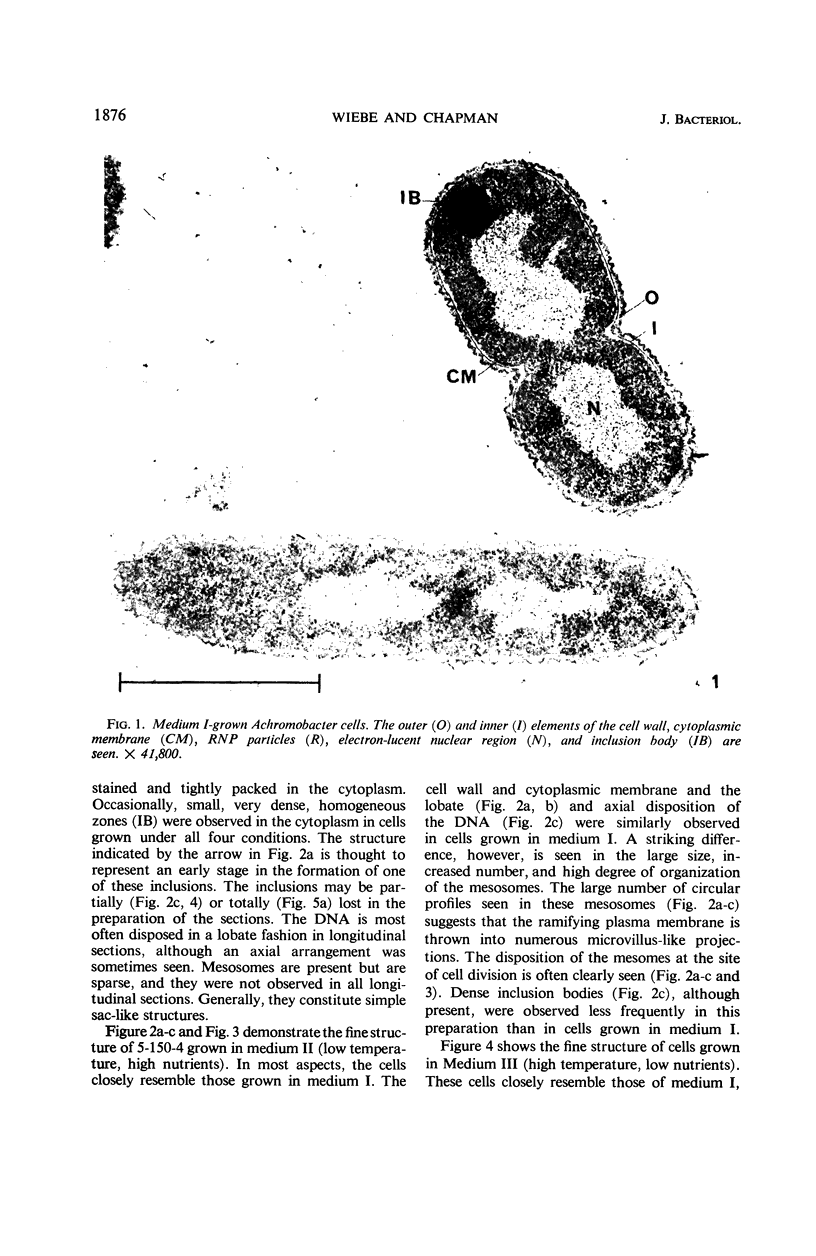
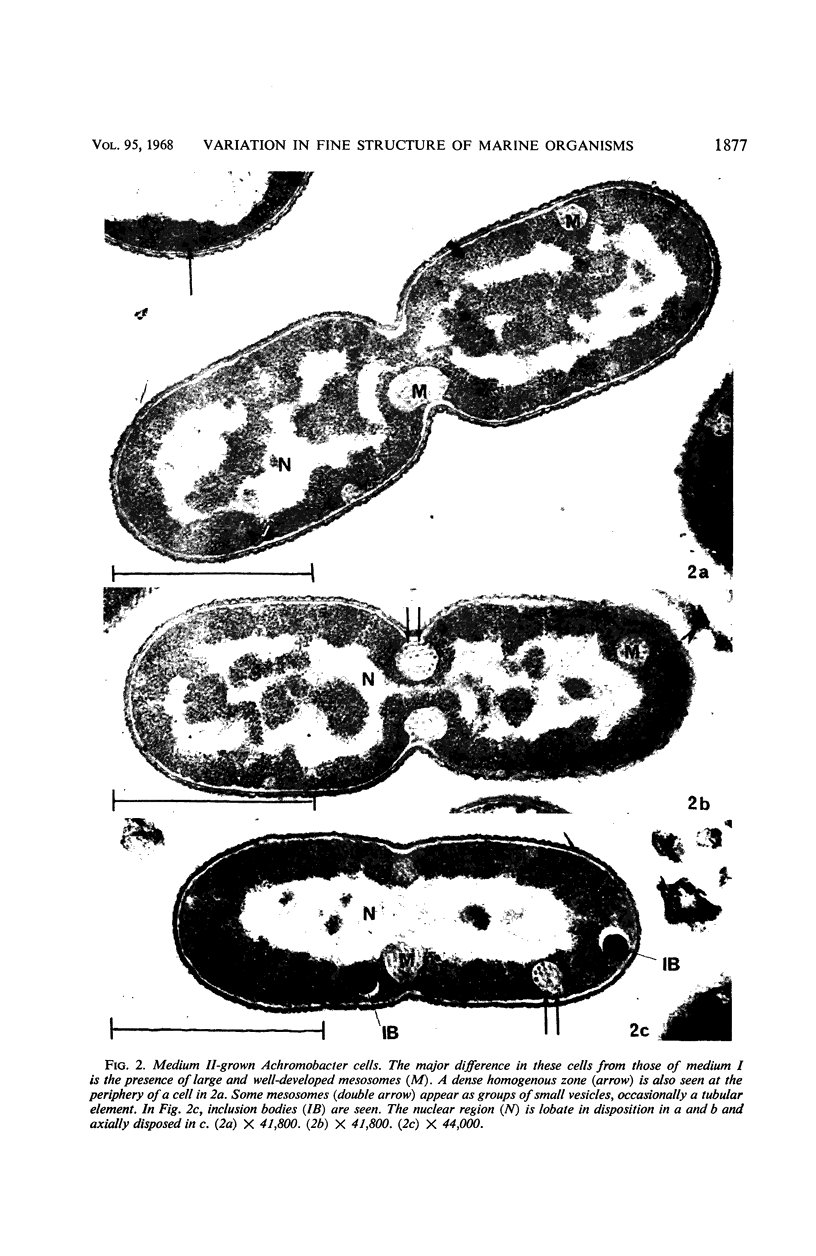
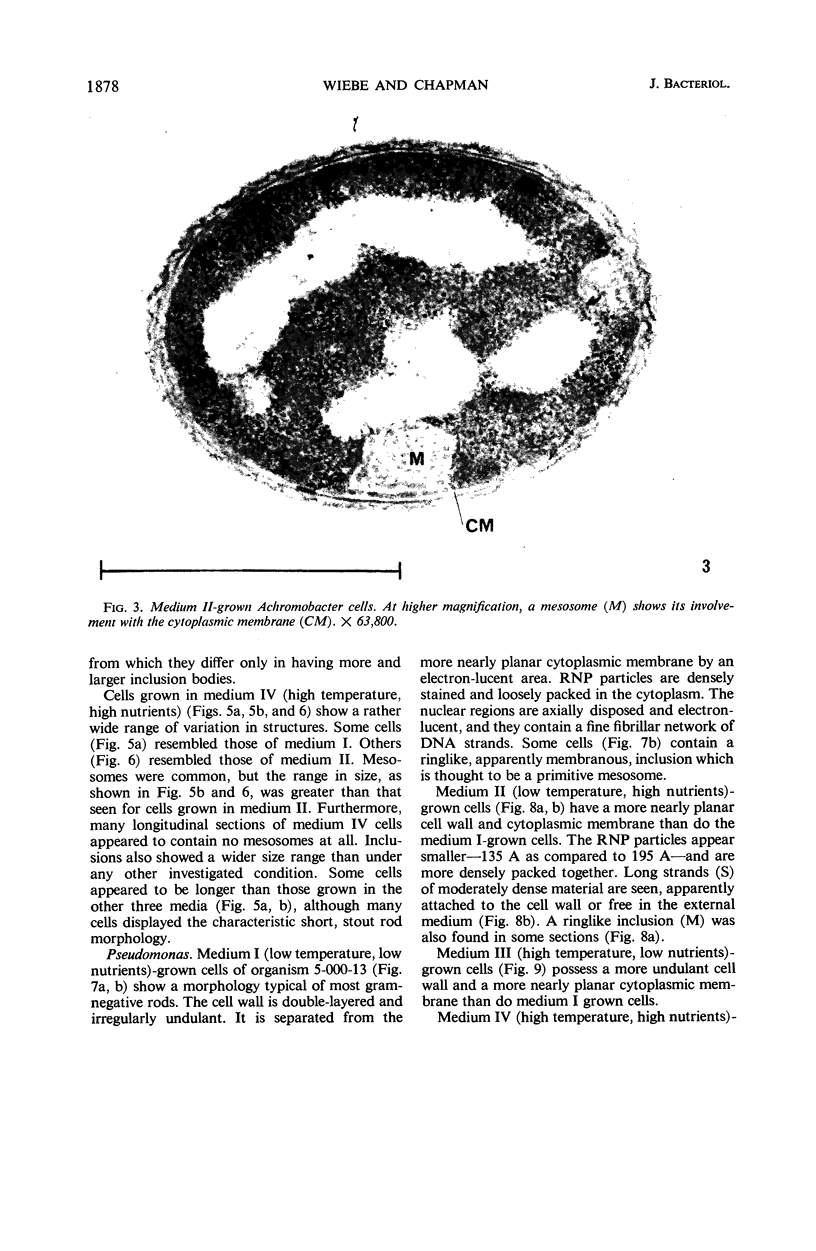
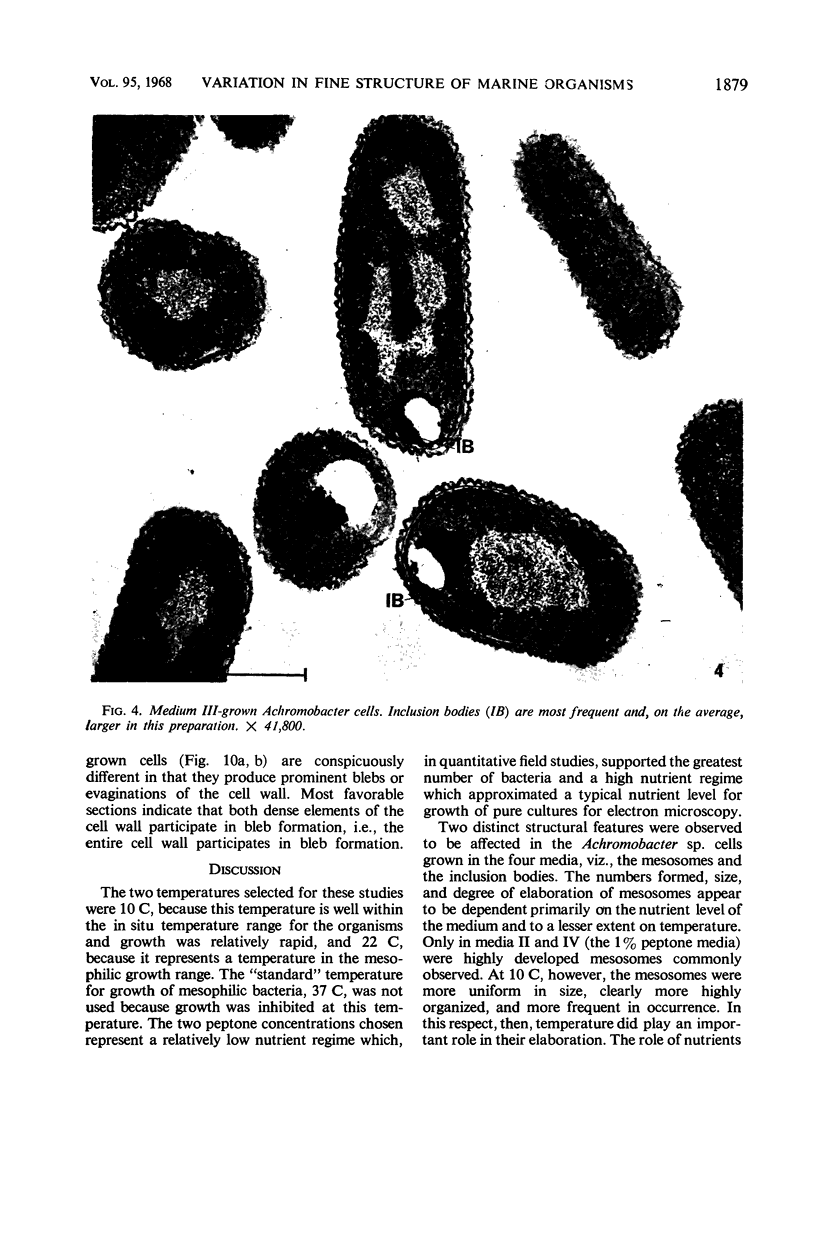
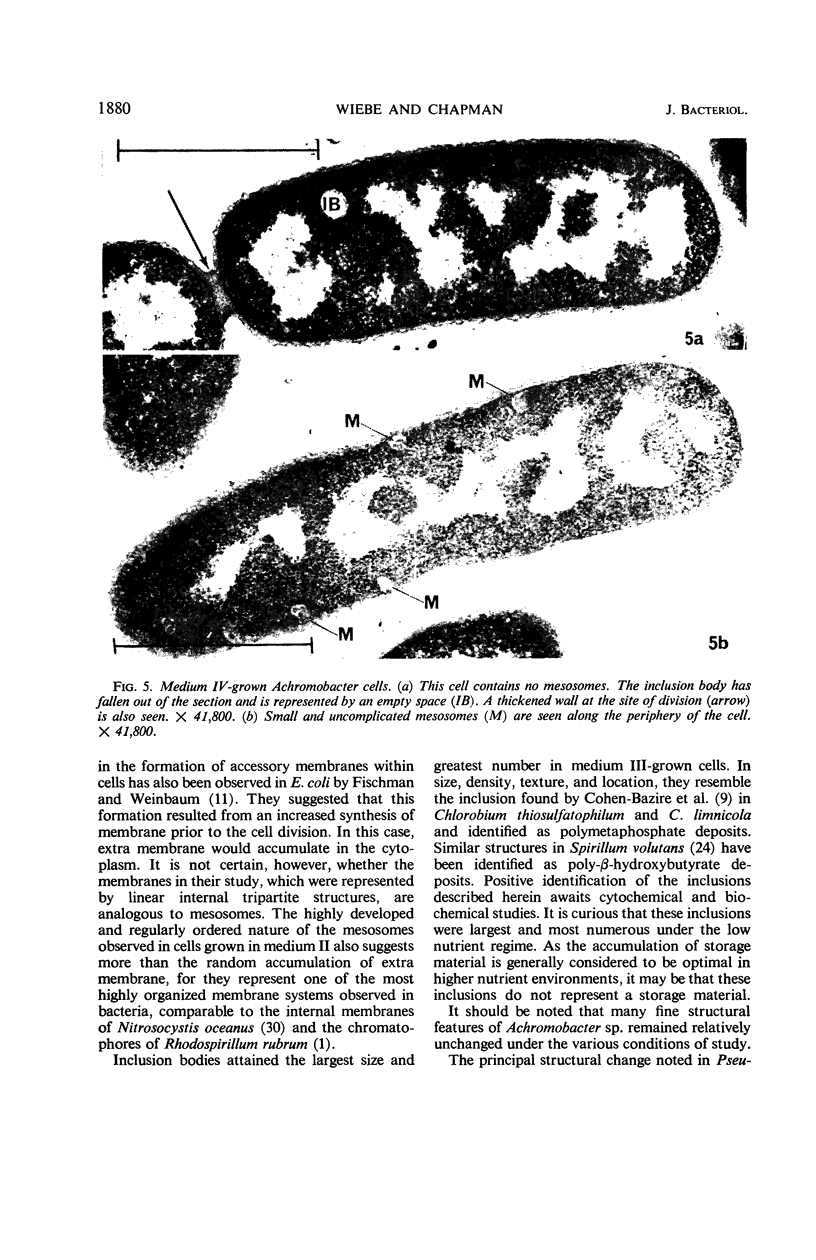
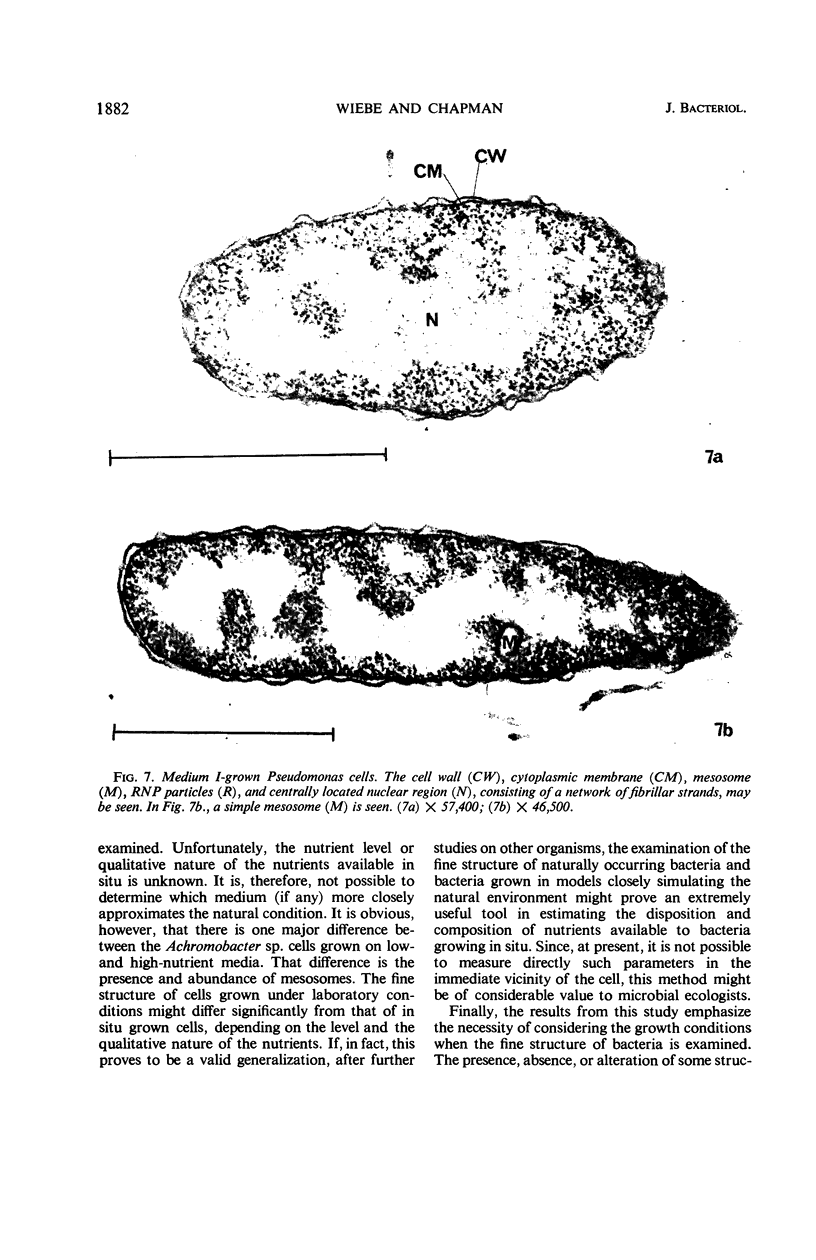
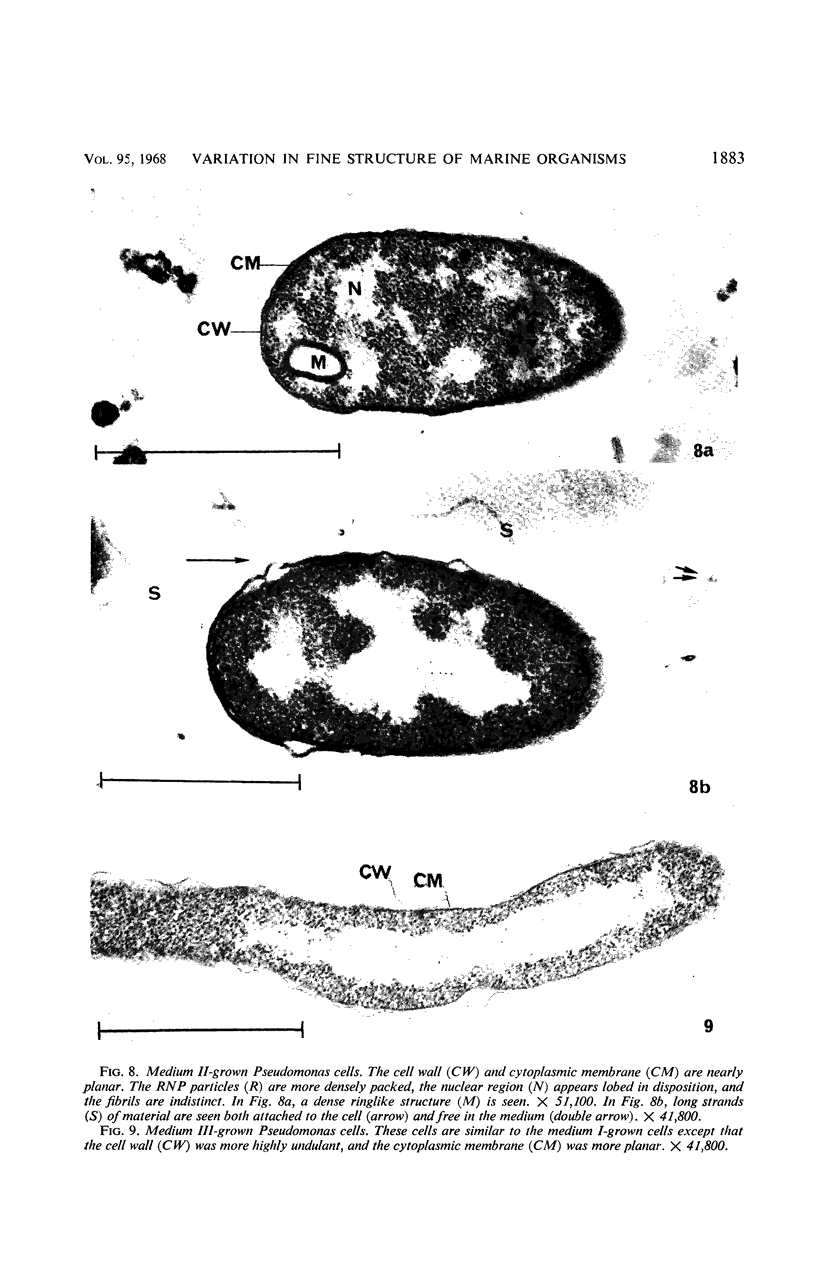
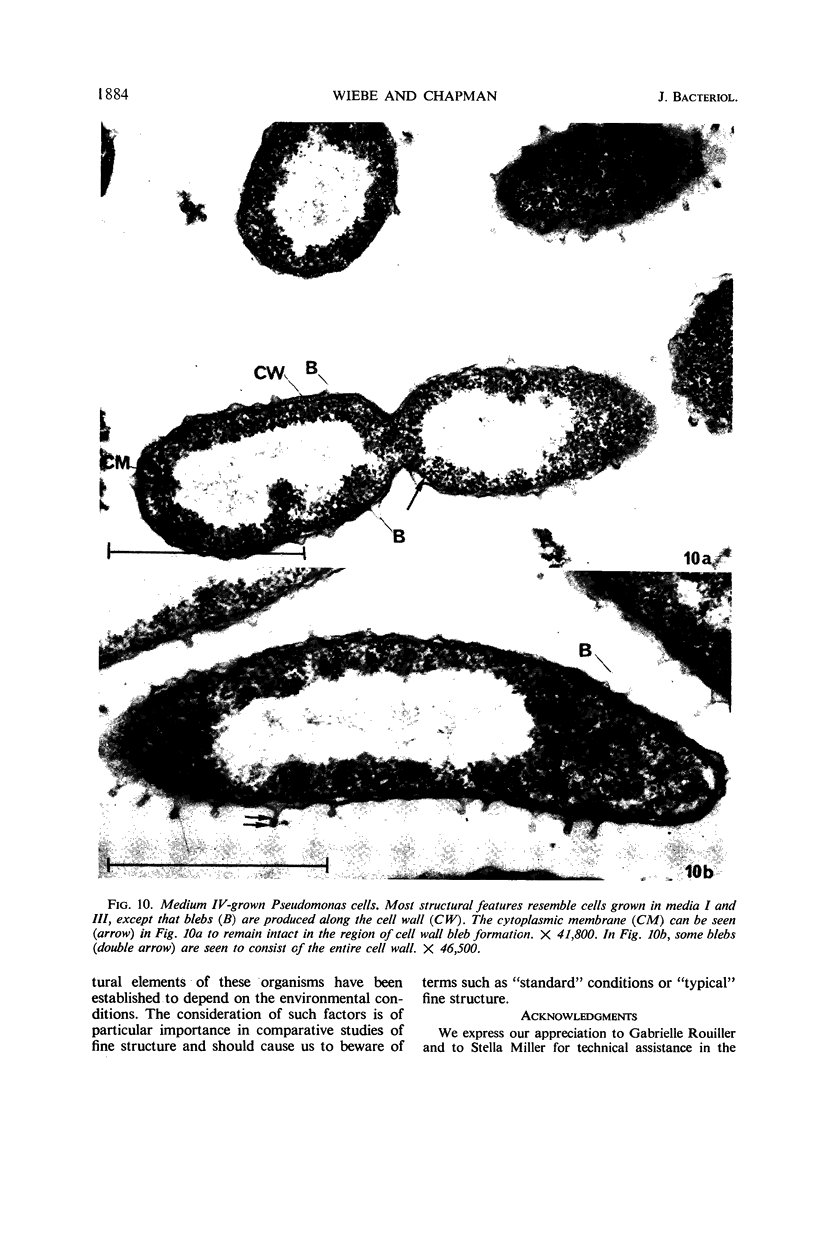
Certain features of the fine structure of a marine achromobacter and a marine pseudomonad were dependent upon the conditions of growth. Cells of achromobacter grown at 10 C in a low peptone-seawater (SW) medium displayed the characteristic morphology of the achromobacter: a regularly undulant outer element of the cell wall and a planar inner element, tightly packed ribonucleoprotein (RNP) particles in the cytoplasm, deoxyribonucleic acid (DNA) disposed in a lobate manner, and dense inclusion bodies. Few mesosomes, however, were seen. Cells of achromobacter grown at 10 C in a high peptone-SW medium had larger and more highly organized mesosomes. At 22 C, in a low peptone-SW medium, no mesosomes were seen, but the inclusions were more frequently seen and were larger in the achromobacter cells. At 22 C, in a high peptone-SW medium, these cells revealed the greatest variation in cellular morphology. They contained both small and large mesosomes, or no mesosomes, and both small and large inclusions, or no inclusions. Pseudomonad cells at 10 C in a low peptone-SW medium revealed a typical gram-negative morphology: double-layered, irregularly undulant cell wall; more nearly planar cytoplasmic membrane; densely stained, lightly packed RNP particles; finely fibrillar, axially disposed DNA; simple mesosomes. At 10 C, in a high peptone-SW medium, pseudomonad cells revealed associated strands of material and intracytoplasmic ringlike structures. At 22 C, in a low peptone-SW medium, pseudomonad cells had a more undulant cell-wall and a more nearly planar cytoplasmic membrane. At 22 C, in a high peptone-SW medium, these cells revealed prominent blebs of the cell wall.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOATMAN E. S., DOUGLAS H. C. Fine structure of the photosynthetic bacterium Rhodomicrobium vannielii. J Biophys Biochem Cytol. 1961 Nov;11:469–483. doi: 10.1083/jcb.11.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSH G. L., CHAPMAN G. B. Electron microscopy of symbiotic bacteria in developing oocytes of the American cockroach, Periplaneta americana. J Bacteriol. 1961 Feb;81:267–276. doi: 10.1128/jb.81.2.267-276.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. I. Cytological changes associated with the exposure of Escherichia coli to colistin sulfate. J Bacteriol. 1962 Jul;84:169–179. doi: 10.1002/path.1700840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscope observations on the behavior of the bacterial cytoplasmic membrane during cellular division. J Biophys Biochem Cytol. 1959 Oct;6:221–224. doi: 10.1083/jcb.6.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscopy of ultra-thin sections of bacteria. II. Sporulation of Bacillus megaterium and Bacillus cereus. J Bacteriol. 1956 Mar;71(3):348–355. doi: 10.1128/jb.71.3.348-355.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., PFENNIG N., KUNISAWA R. THE FINE STRUCTURE OF GREEN BACTERIA. J Cell Biol. 1964 Jul;22:207–225. doi: 10.1083/jcb.22.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota-Robles E. H. Internal membranes in cells of Escherichia coli. J Ultrastruct Res. 1966 Dec;16(5):626–639. doi: 10.1016/s0022-5320(66)80010-2. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman D. A., Weinbaum G. The formation of multiple layers of membrane-like structures in Escherichia coli B. J Cell Biol. 1967 Feb;32(2):524–528. doi: 10.1083/jcb.32.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA M. M., GRULA E. A. ACTION OF CYCLOSERINE ON A SPECIES OF ERWINIA WITH REFERENCE TO CELL DIVISION. Can J Microbiol. 1965 Jun;11:453–461. doi: 10.1139/m65-060. [DOI] [PubMed] [Google Scholar]
- Jacob F. Genetics of the bacterial cell. Science. 1966 Jun 10;152(3728):1470–1478. doi: 10.1126/science.152.3728.1470. [DOI] [PubMed] [Google Scholar]
- KAYE J. J., CHAPMAN G. B. CYTOLOGICAL ASPECTS OF ANTIMICROBIAL ANTIBIOSIS. III. CYTOLOGICALLY DISTINGUISHABLE STAGES IN ANTIBIOTIC ACTION OF COLISTIN SULFATE ON ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:536–543. doi: 10.1128/jb.86.3.536-543.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Contribution à l'étude du noyau bactérien. Schweiz Z Pathol Bakteriol. 1955;18(5):1122–1137. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. IV. Cytochemical changes. J Bacteriol. 1967 Jan;93(1):367–378. doi: 10.1128/jb.93.1.367-378.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G. Direct evidence for a cytoplasmic membrane in sectioned bacteria. Can J Microbiol. 1957 Apr;3(3):531–532. doi: 10.1139/m57-056. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., FRANCOMBE W. H., MAYALL B. H. The effect of penicillin on the structure of staphylococcal cell walls. Can J Microbiol. 1959 Dec;5:641–648. doi: 10.1139/m59-078. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., Morgan C., Rosenkranz H. S., Rose H. M. Effect of hydroxyurea on virus development. I. Electron microscopic study of the effect on the development of bacteriophage T4. J Bacteriol. 1966 Feb;91(2):823–833. doi: 10.1128/jb.91.2.823-833.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Carr H. S., Rose H. M. Electron microscopy of chloramphenicol-treated Escherichia coli. J Bacteriol. 1967 Jun;93(6):1987–2002. doi: 10.1128/jb.93.6.1987-2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- Rowley D. Phagocytosis and immunity. The central role of phagocytosis in immune reactions. Experientia. 1966 Jan 15;22(1):1–5. [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- Sedar A. W., Burde R. M. The demonstration of the succinic dehydrogenase system in Bacillus subtilis using tetranitro--blue tetrazolium combined with techniques of electron microscopy. J Cell Biol. 1965 Oct;27(1):53–66. doi: 10.1083/jcb.27.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine A. F., Chapman G. B. Fine structure and host-virus relationship of a marine bacterium and its bacteriophage. J Bacteriol. 1966 Nov;92(5):1535–1554. doi: 10.1128/jb.92.5.1535-1554.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. The composition and structure of bacterial spores. J Cell Biol. 1963 Mar;16:579–592. doi: 10.1083/jcb.16.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe W. J., Chapman G. B. Fine structure of selected marine pseudomonads and achromobacters. J Bacteriol. 1968 May;95(5):1862–1873. doi: 10.1128/jb.95.5.1862-1873.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]