Abstract
Using functional magnetic resonance imaging (fMRI), we investigated brain activity elicited by a computer-animated child’s actions that appeared consistent and inconsistent with a computer-animated adult’s instuctions. Participants observed a computer-animated adult verbally instructing a computer-animated child to touch one of two objects. The child performed correctly in half the trials and incorrectly in the other half. We observed significantly greater activity when the child performed incorrectly compared to correctly in regions of the dorsolateral prefrontal cortex (DLPFC) that have been implicated in maintaining our intentions in working memory and implementing cognitive control. However, no such effects were found in regions of the posterior superior temporal sulcus (posterior STS) that have been posited to interpret other people’s behavior. These findings extend the role of the DLPFC in cognitive control to evaluating the social outcomes of other people’s behavior and provide important new constraints for theories of how the posterior STS contributes to social cognition.
Keywords: fMRI, social cognition, dorsolateral prefrontal cortex, superior temporal sulcus, mirror neuron
Introduction
Planning appropriate behavior in social situations sometimes requires us to determine whether one person’s actions appear consistent with a second person’s intentions. For example, in order to appropriately plan his or her next behavior toward a suspect, a police officer may need to evaluate whether the suspect’s behavior appears consistent (e.g., putting his hands up) or inconsistent (e.g., running away) with a second officer’s stated intentions for the suspect (e.g., “put your hands up!”). Making accurate evaluations of other people’s behavior is thought to require a ‘theory of mind’ (Premack & Woodruff, 1978; Wimmer & Perner, 1983); that is, an understanding that other people have minds, knowledge, and intentions that differ from our own. When one’s ‘theory of mind’ is disrupted by abnormal brain functioning, as in autism (Hill & Frith, 2003), one’s understanding of other people can be selectively impaired. Therefore, developing neurobiological models of social phenomena is an important step toward understanding how we interpret and evaluate other people’s behavior.
Current models suggest that two regions of the human brain - the dorsolateral prefrontal cortex (DLPFC) and the posterior superior temporal sulcus (posterior STS) - may play especially important roles in evaluating other people’s behavior. The DLPFC is thought to support cognitive control processes that enable us to voluntarily control our actions (Baddeley, 1986; MacDonald, Cohen, Stenger, & Carter, 2000; Weissman, Warner, & Woldorff, 2004). Specifically, the DLPFC is thought to maintain representations of our current intentions, along with the rules for accomplishing those intentions, in working memory (Miller & Cohen, 2001). The DLPFC is also posited to create and maintain links between our actions and their eventual outcomes in working memory, so that our previous experiences can guide the selection of future behaviors (Genovesio, Brasted, & Wise, 2006; Petrides, 1995; Tsujimoto & Sawaguchi, 2004, 2005). Consistent with this view, our ability to use feedback about previous actions to guide future behavior can be severely impaired following damage to the DLPFC (Barcelo & Knight, 2002; Grafman, Jonas, & Salazar, 1990).
Given its role in maintaining our intentions in working memory and in using feedback to evaluate whether our actions are consistent with those intentions, we hypothesize that the DLPFC may make an important contribution to cognitive control in social situations. Specifically, it may maintain our understanding of one person’s intentions in working memory and evaluate whether a second person’s actions appear consistent with those intentions. In this way, the DLPFC could incorporate the social outcomes of an observed individual’s actions into the planning of our own future behavior.
Several recent studies in humans have localized brain activity that varies with whether an actor’s behavior appears consistent with his own intentions (Grezes, Frith, & Passingham, 2004; Pelphrey, Morris, & McCarthy, 2004; Pelphrey, Singerman, T., & McCarthy, 2003; Saxe, Xiao, Kovacs, Perret, & Kanwisher, 2004). In general, such activity has not been reported in the DLPFC. However, in none of these studies were the actors’ intentions explicitly specified. In the absence of a clear context within which to interpret a person’s outward behavior, we may be less likely, or even unable, to evaluate whether that person’s actions appear to fit with their intentions. Moreover, to our knowledge no prior study has directly investigated whether brain activity in the DLPFC varies with whether one actor’s behavior appears consistent with a second actor’s intentions.
Recent models in social cognitive neuroscience suggest that the posterior STS may also participate in evaluating the significance of other people’s actions. For quite some time, it has been known that brain activity in the posterior STS is especially responsive to faces (Kanwisher, McDermott, & Chun, 1997; McCarthy, Puce, Gore, & Allison, 1997) and to biological motion (Allison, Puce, & McCarthy, 2000; Bonda, Petrides, Ostry, & Evans, 1996; Decety & Grezes, 1999), suggesting a special role for this brain region in processing social stimuli. More recent findings (Grezes et al., 2004; Pelphrey et al., 2004; Pelphrey et al., 2003; Saxe et al., 2004) have led to the proposal that, through its analyses of biological motion, the posterior STS is involved in determining whether other people’s actions appear consistent with our understanding of their intentions (Morris, Pelphrey, & McCarthy, 2005).
Two crucial aspects of the role of the posterior STS in evaluating other people’s actions remain unclear, however. First, it is not yet known whether the posterior STS evaluates whether one person’s actions appear consistent with a second person’s intentions. Indeed, it is unclear whether numerous brain regions thought to be important for social cognition are involved in making such evaluations, including medial prefrontal regions that are posited to represent the mental states of other people (Amodio & Frith, 2006) and inferior frontal regions that have been linked to understanding other people’s actions (Rizzolatti & Craighero, 2004). Second, although the posterior STS appears to participate in evaluating other people’s behavior when their intentions are not made explicit and must therefore be inferred (Grezes et al., 2004; Pelphrey et al., 2004; Pelphrey et al., 2003; Saxe et al., 2004), it is not known whether the posterior STS contributes to making such evaluations when other people’s intentions are clearly stated. Frequently, we must infer other people’s intentions because those intentions are not made explicit (Malle, Moses, & Baldwin, 2001). However, as illustrated by our example involving the police officers, there are times when it is important to evaluate whether one person’s actions appear consistent with a second person’s intentions when those intentions are clearly stated.
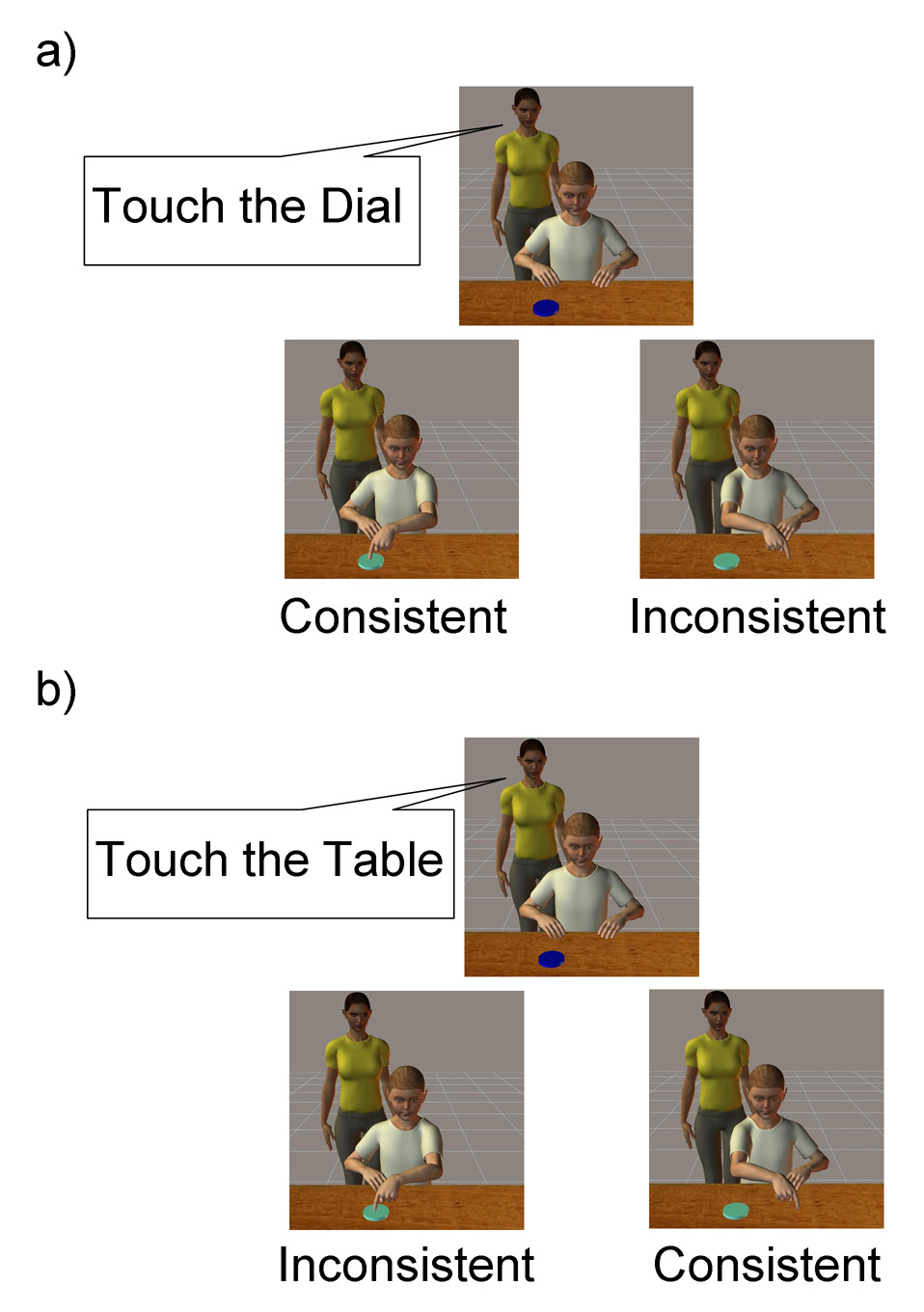
In the present study, we therefore used fMRI to investigate whether the DLPFC and the posterior STS contribute to cognitive control in social situations by evaluating whether one person’s actions appear consistent with a second person’s clearly-stated intentions. Study participants’ task was to view a series of 5-second computer animations. In half the animations, a computer-animated adult verbally instructed a computer-animated child to touch a blinking dial that was placed on a table (Fig. 1a); in the other half, the adult instructed the child to touch a different part of the table (Fig. 1b). Following each of these two possible instructions, the child performed the instructed action in half the trials and the opposite action in the other half. Thus, we manipulated whether the child’s actions matched his intentions (i.e., the adult’s instructions) independently of the actions themselves. We predicted that if the DLPFC and the posterior STS participate in evaluating whether one person’s actions appear consistent with a second person’s explicit intentions, then their activity should vary with whether the child follows the adult’s instructions.
Figure 1.
Experimental stimuli. Each animation began with a dial blinking on one end of a table (1 s). An adult standing behind a child then verbally instructed the child (1.5 s) to touch either (a) the blinking dial or to (b) a different part of the table upon which the dial was placed. The adult’s mouth movements were synchronized with a recording of an adult female voice to increase the realism of the animation. Following the adult’s instruction, the child touched moved one of his arms to touch either the dial or the table with equal probability and then moved his arm back to the initial starting position (2 s), after which the child remained in that position briefly (0.5 s) before the ITI began. The child followed instructions in 50% of trials and did not follow instructions in the other 50%.
Materials and methods
Participants
Eighteen healthy adults (10 male, age range: 18–29, 16 right-handed) participated in the study. All had normal or corrected-to-normal vision and no history of serious neurological trauma or disorders. Furthermore, none reported any problems with their hearing. Two participants were excluded due to excessive head motion during the experiment leaving sixteen participants (8 male, age range: 18–29 years, 15 right-handed). Participants gave informed consent prior to the experiment in accordance with the Duke Medical Center human subjects institutional review board. Before the MR session, each participant was shown examples of the different video clips. The experimenters highlighted the different types of instructions the adult could give and the different possible actions the child could perform, but did not describe the videos in terms of whether the child followed instructions. Participants were paid $20 per hour for their participation, which lasted approximately 2 hours.
Task
Participants viewed a series of 5-second computer animations created using Poser 6 software (Curious Labs Inc., Santa Cruz, California). Each animation began with a dial blinking on one end of a table (1 s). An adult standing behind a child then verbally instructed the child (1.5 s) to touch either the blinking dial (Fig. 1a) or a different part of the table upon which the dial was placed (Fig. 1b). The adult’s mouth movements were synchronized with a recording of an adult female voice to increase the realism of the animation. Following the adult’s instruction, the child touched the dial or touched the table with equal probability and then moved his arm back to the initial starting position (2 s), after which the child remained in that position briefly (0.5 s) before the inter-trialinterval (ITI) began.
Four animations were repeated twelve times each in every run. However, a total of eight different animations were presented across six runs. In three “dial-on-left” runs, the dial always appeared on the left side of the table from the child’s perspective (and thus the right side of the table from the participants’ perspective). The child touched the dial by moving his right arm to the left side of the table and touched the table by moving his left arm to the right side of the table. In three “dial-on-right” runs, the dial always appeared on the right side of the table from the child’s perspective (and thus the left side of the table from the participants’ perspective). The child touched the dial by moving his left arm to the right side of the table and touched the table by moving his right arm to the left side of the table. In total, the child performed correctly in 144 trials and incorrectly in the other 144 trials. Moreover, in half of each of these trial types (i.e., correct and incorrect), the child moved his right arm and in the other half the child moved his left arm. For each participant, the three “dial-on-left” runs were interleaved with the three “dial-on-right” runs. The nature of the first run (i.e., “dial-on-left” versus “dial-on-right”) was counterbalanced across participants.
The four computer animations in each run were presented in a first-order counterbalanced sequence such that each type of animation was preceded equally often by every type of animation in the run. The ITI ranged from 0 to 4 TRs following a roughly exponential distribution that favored short ITIs. Such jittering of the ITI increases the efficiency with which response estimates for distinct trial types are made in a multiple regression framework (Miezin, Maccotta, Ollinger, Petersen, & Buckner, 2000). During the ITI, a still image containing the child, the adult, and the dial in their initial states was presented. Thus, none of the trials began with a sudden onset or with a discontinuity in the positions of the characters or the dial relative to the prior trial.
Data Acquisition
A PC running CIGAL software (Voyvodic, 1999) presented the animations to participants through MR-compatible goggles and headphones. Structural images were collected using a T1-weighted spin echo sequence on a 4-Tesla GE whole-body scanner (TR=500 ms, TE=14 ms, flip angle=90°, 17 contiguous 7 mm-thick slices – in plane resolution =0.94 mm X 0.94 mm). Functional images, which measured the blood oxygenation level-dependent (BOLD) signal, were collected using a reverse spiral imaging sequence (TR=1.25 s, TE=40 ms, flip angle=90°, 17 contiguous 7-mm thick slices – in plane resolution, 3.75 mm × 3.75 mm). Each participant completed six runs of the task. During each run, 314 functional images were collected. The first six functional images contained no trials and were discarded.
Data Analysis
SPM2 (Friston et al., 1995) was used to correct the functional images for head motion, normalize the functional images to standard space, and spatially smooth the functional images with a three-dimensional Gaussian filter (FWHM = 8 mm). Next, the time series for each run was analyzed using customized software that implements a version of the general linear model, sometimes called the finite impulse response (FIR) model, which makes no assumptions about the shape of the BOLD response (Miezin et al., 2000; Ollinger, Corbetta, & Shulman, 2001; Ollinger, Shulman, & Corbetta, 2001). This model estimates the average stimulus-locked fMRI response for each trial type and has been validated in many prior studies (Miezin et al., 2000; Shulman et al., 1999; Weissman et al., 2004). Using this model, we estimated the average stimulus-locked fMRI response to each of the four types of 5-second-long animations across 18 TRs (i.e., 22.5 seconds). Because we jittered the ITI, each of these average responses reflected activity for a specific type of animation relative to our fixation baseline (Miezin et al., 2000; Shulman et al., 1999; Weissman et al., 2004). That is, despite the relatively short ITI (1.25 – 5 seconds), it did not reflect activity for the other types of animations in the study (Miezin et al., 2000). We then converted every time point of each average response to units of percent change from a session-specific, fixation baseline (i.e., the y-intercept term for that run). Next, for each participant and animation, we calculated the mean (across runs) of these percent change average responses, separately at every time point. This procedure yielded a participant-specific average fMRI response across time (i.e., 18 TRs) to each of the four animations. Finally, in each participant, we derived the average fMRI responses across time to animations depicting the child (1) performing an action that was consistent with the adult’s instructions, (2) performing an action that was inconsistent with the adult’s instructions, (3) moving his right arm, (4) moving his left arm, (5) touching the table, and (6) touching the dial. Voxelwise analyses of variance (ANOVAs) assessing interactions between Trial Type and Time Point (1–18) were restricted to particular areas (i.e., dorsolateral prefrontal regions, superior temporal regions, somatosensory cortex, and visual cortex) and were thresholded at F(17,255) = 2.52, p<0.001.
Region of Interest (ROI) Analyses
ROIs were defined using functional activations from two previous studies. ROIs in bilateral regions of the DLPFC were defined from one of our previous fMRI studies, which also assessed cognitive control mechanisms under conditions of audiovisual stimulation (Weissman et al., 2004). Each DLPFC ROI contained the exact voxels that were activated in our previous study. ROIs in the left MOG and the right posterior STS were defined from a prior study of social cognition that was nearly identical to the present study, the main exception being that no instructions preceded the computer-animated character’s actions (Pelphrey et al., 2004). In this prior study, these regions exhibited significantly greater peak activity when the animated character touched the table than when he touched the blinking dial. The authors interpreted this result as evidence that these regions evaluate whether other people’s actions appear consistent with their intentions. Each of these ROIs was a 27-voxel cube centered on the local maximum of activation in this prior study.
Stimulus-locked average fMRI responses to individual trial types were averaged across all voxels in each ROI. Planned contrasts were performed to assess whether peak activity differed for various trial types within each ROI. P-values less than 0.05 (one-tailed) were considered to be significant. All statistical tests in the present study were conducted using random effects analyses so that our conclusions would generalize to the population. Conversion from MNI to Talairach (Talairach & Tournoux, 1988) coordinates was implemented using two non-linear transformations (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
Results
Activity in the DLPFC distinguishes inconsistent from consistent actions
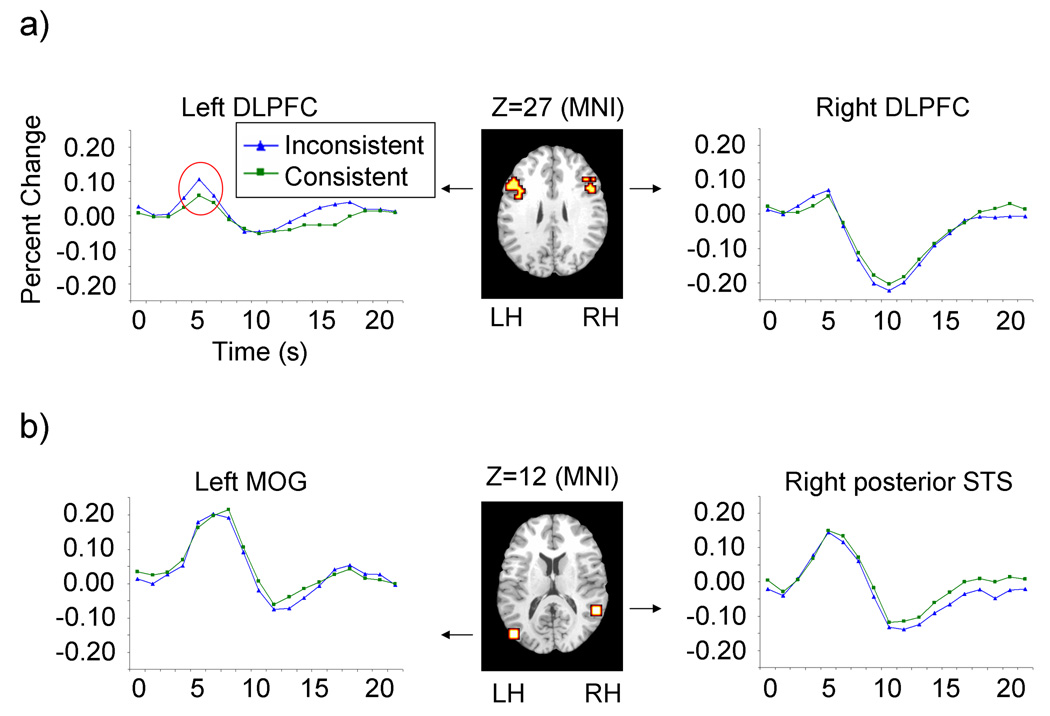
ROI analyses in the left DLPFC (Talairach x, y, and z coordinates: x = −47, y = 17, and z = 28 (−47, 17, 28); BA9; 30 voxels) and in the right DLPFC (47, 20, 26; BA 46; 16 voxels) revealed that peak activity in the left DLPFC was significantly greater, t(15)=1.77, p<0.05, when the child’s actions were inconsistent with the adult’s instructions than when they were consistent (Fig 2a, left) (this effect was not significant in the right DLPFC, p>0.25; Fig 2a, right). No additional differences in peak activity were found in the DLPFC for (1) touching the table versus touching the dial or (2) movements of the right versus the left arm (all p>0.20). Thus, activity in the left DLPFC specifically discriminated whether the child’s actions were inconsistent versus consistent with the adult’s instructions. A voxelwise ANOVA testing for interactions between Trial Type (consistent, inconsistent) and Time Point (1–18) in other dorsolateral prefrontal regions, and in medial prefrontal and inferior frontal regions that have been implicated in social cognition revealed no significant effects (all p-values greater than 0.001).
Figure 2.
Peak activity distinguishes whether or not the child follows the adult’s instructions in the dorsolateral prefrontal cortex (DLPFC), but not in the posterior superior temporal sulcus (posterior STS). (A) Regions of interest in the left and the right DLPFC overlaid on a normalized anatomical brain. Peak activity in the left DLPFC, highlighted with the red circle, was significantly greater when the child’s actions were inconsistent with the adult’s instructions than when they were consistent (this effect did not achieve significance in the right DLPFC). (B) Regions of interest in the right posterior STS and the left middle occipital gyrus (MOG) overlaid on a normalized anatomical brain. Peak activity in these regions did not differ depending on whether or not the child followed the adult’s instructions. Z coordinates for brain slices refer to MNI space. LH, left hemisphere; RH, right hemisphere.
Activity in the posterior STS does not distinguish inconsistent from consistent actions
ROI analyses in the left middle occipital gyrus (MOG) (−47, −77, 14; BA 19; 27 voxels) and in the right posterior STS (57, −47, 11; BA 22; 27 voxels) revealed no significant differences in peak activity for inconsistent versus consistent actions (both p>0.20; Fig 2b). A voxelwise ANOVA to test for interactions between Trial Type (consistent, inconsistent) and Time Point (1–18) in other superior temporal regions also revealed no significant effects (all p-values greater than 0.001). Thus, activity in the posterior STS did not discriminate whether the child’s actions were inconsistent versus consistent with the adult’s instructions.
Peak activity in the left MOG and in the right posterior STS ROIs also did not distinguish peak activity in most of the other conditions of our experiment. We found no differences in peak activity between touching the dial and touching the table (right posterior STS, p>0.27; left MOG, p>0.15) or between left and right arm movements in the right posterior STS (p>0.33). However, we did observe significantly greater peak activity for movements of the left versus the right arm in the left MOG (p<0.025). Assuming that participants fixated locations at or near the center of the display throughout each trial, the child’s right and left arms, respectively, would have been presented mostly in participants’ left and right visual fields. Thus, the greater activity for left than for right arm movements in the left MOG could have been driven by the contralateral organization of the visual system.
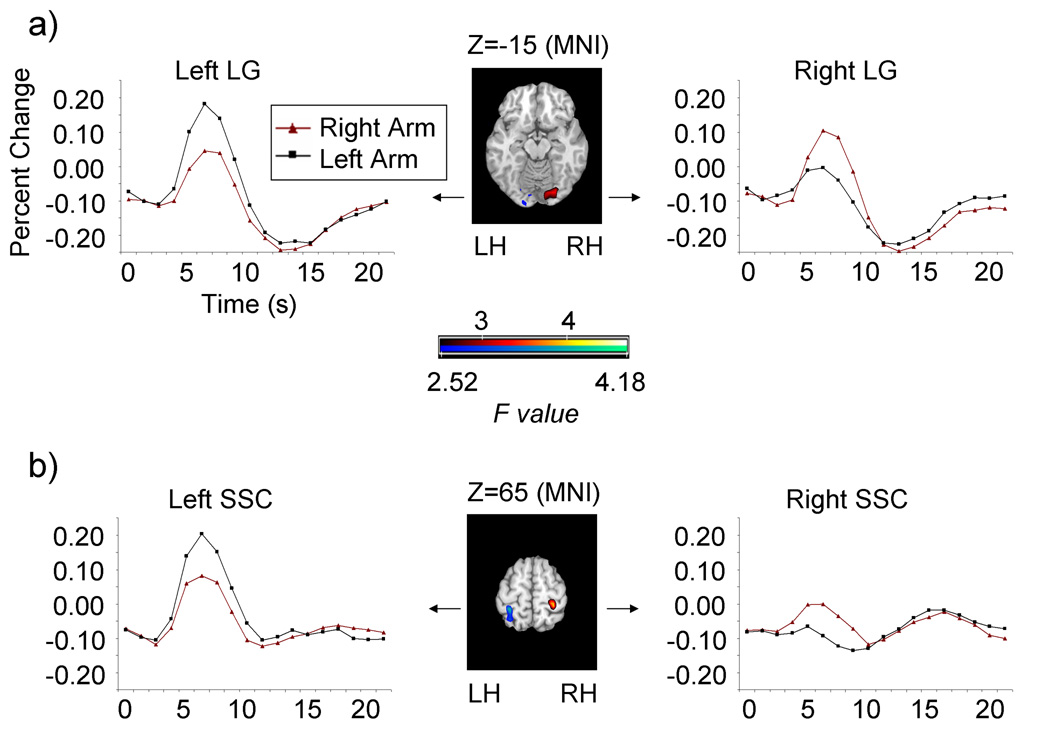
In line with this view, a voxelwise ANOVA revealed that right and left arm movements produced significantly different time courses of activity, F(17,255)=2.52, p<0.001, in both the left lingual gyrus (−18,−86,−8; BA 18; 14 voxels) and the right lingual gyrus (20, −84, −9; BA 18; 44 voxels) in the visual cortex (Fig. 3a). Follow-up ROI analyses revealed that peak activity in the left lingual gyrus was significantly greater for left versus right arm movements, t(15)=3.38, p<0.003 (one-tailed) while the opposite was true in the right lingual gyrus, t(15)=3.75, p<0.001 (one-tailed). Within the left and right lingual gyri, no additional differences in peak activity were observed for (1) inconsistent versus consistent actions or (2) touching the dial versus touching the table, (t(15)<1 in all cases).
Figure 3.
Contralateral activity associated with left and right arm movements in the lingual gyrus (LG) and the somatosensory cortex (SSC). (A). Activity in the left LG was significantly greater for left arm movements than for right arm movements, while the opposite was true for the right LG. (B). Activity in the left SSC was significantly greater when the child moved his left arm than he moved his right arm, while the opposite was true in the right SSC. In regions where right arm movements produced greater activity than left arm movements (color range, dark red to light yellow), F values are represented by the colors at the top of the color bar. In regions where left arm movements produced greater activity than right arm movements (color range, dark blue to light green), F values are represented by the colors at the bottom of the color bar. Z coordinates for brain slices refer to MNI space. LH, left hemisphere; RH, right hemisphere.
A mirror neuron system for touch in the human somatosensory cortex
Recent findings indicate that watching a person being touched on a particular part of the body results in the activation of corresponding regions within the perceiver’s somatosensory cortex (Blakemore, Bristow, Bird, Frith, & Ward, 2005). Moreover, when we directly face a person, as our study participants directly faced the child, we appear to simulate their experiences in body parts that mirror those of the individual (Pelphrey et al., 2004). Thus, when the computer-animated boy in the present study touched an object with his right hand, participants may have simulated touching an object with their left hands, leading to activation in the right somatosensory cortex, and vice-versa when the boy touched an object with his left hand.
In line with these predictions, a voxelwise ANOVA assessing Trial Type (right arm movement, left arm movement) by Time Point (1–18) interactions revealed significant differences, F(17, 255)=2.52, p<0.001, between the time courses of activity for right and left arm movements in both the left (−33, −45, 62; BA 5; 28 voxels) and the right (28, −38, 69; BA 5; 31 voxels) somatosensory cortex (Fig 3b). Follow-up ROI analyses confirmed that in the left somatosensory cortex left arm movements (watching the boy touch an object with his left hand) produced significantly greater peak activity than right arm movements (watching the boy touch an object with his right hand), t(15)=3.67, p<0.002 (one-tailed), while the opposite was true in the right somatosensory cortex, t(15)=3.40, p<0.003 (one-tailed). No additional differences in peak activity in these brain regions were observed for (1) inconsistent versus consistent arm movements or (2) touching the dial versus touching the table (t(15)<1 in all cases).
Adaptation effects
Given the highly repetitive nature of our task, it is possible that activation differences between inconsistent and consistent actions in brain regions underlying social cognition were most pronounced relatively early in the experiment. To investigate this possibility, we performed a voxelwise ANOVA (threshold: F(17, 255) = 2.99, p< 0.0001, 15 contiguous voxels) to test for interactions between Trial Type (consistent, inconsistent) and Time Point (1–18) in dorsolateral prefrontal, superior temporal, medial prefrontal, and inferior frontal regions using data from only the first two runs. The ANOVA indicated a lack of activation differences between inconsistent and consistent actions in dorsolateral prefrontal, superior temporal, and inferior prefrontal regions. Furthermore, ROI analyses in the dorsolateral prefrontal and superior temporal ROIs we identified earlier indicated that differences in peak activity between inconsistent and consistent actions were no greater when only the first two runs of data were included in the analysis than when all of the runs were included.
However, the voxelwise ANOVA did reveal highly robust, clearly interpretable activation differences between inconsistent and consistent actions in several medial prefrontal regions that are thought to be involved in social cognition. Activations in these medial prefrontal regions were centered in two regions of the right precuneus (4, −70, 49; BA 7; 53 voxels and 4, −65, 16; BA23; 23 voxels), the right anterior cingulate cortex (4, 24, 37; BA 32; 41 voxels), the left medial frontal gyrus (−8, 54, 9; BA 10; 23 voxels), and bilateral regions of the posterior cingulate cortex (BA 31; 17 voxels) that included peaks in both the left hemisphere (4, −27, 40) and the right hemisphere (−4, −27, 40). In every region, these activation differences reflected significantly greater peak deactivation for inconsistent than for consistent actions (all p < .02, two-tailed, except for the right posterior cingulate cortex for which p = 0.05, two tailed). We interpret these effects in greater detail in the Discussion section.
Discussion
Planning appropriate behavior in social settings often requires that we evaluate whether one person’s actions appear consistent with a second person’s intentions. For example, a parent may plan his or her next behavior toward a child (e.g., scolding or praising) by considering whether the child’s actions appear inconsistent (e.g., eating an entire ice cream cone) or consistent (e.g., sharing the ice cream cone with a sibling) with the other parent’s intentions for the child (e.g., sharing the ice cream cone). Evaluating whether one individual’s actions appear consistent with a second individual’s intentions can also be helpful for identifying the individuals’ relative social ranks which, in hierarchically-organized primate societies, strongly influences the selection of social behaviors (Bergman, Beehner, Cheney, & Seyfarth, 2003). Thus, understanding the psychological and neural mechanisms that enable us to evaluate the outcomes of observed social interactions is crucial for developing models of how we plan our behavior in social situations.
In the present study, we found that regions of the DLPFC, which have been implicated in cognitive control, were more active when a computer-animated boy’s actions appeared inconsistent with a computer-animated adult’s instructions than when they appeared consistent. However, this effect was absent in regions of the posterior STS that have been posited to play a crucial role in evaluating the appropriateness of other people’s actions within a particular situational context. These findings have major implications for neurobiological models of both cognitive control and social cognition.
The DLPFC is thought to contribute to cognitive control by maintaining our intentions in working memory (Miller & Cohen, 2001), including the rules for accomplishing those intentions. It is also posited to link our actions to their eventual outcomes so that previous experiences can be used to guide future behavior (Genovesio et al., 2006; Petrides, 1995; Tsujimoto & Sawaguchi, 2004, 2005). The present findings appear to extend the role of the DLPFC in cognitive control to maintaining our understanding of one person’s intentions in working memory and evaluating whether a second person’s actions appear consistent with those intentions. The DLPFC may thereby allow the social outcomes of other people’s actions to influence the planning of our future behavior. For example, the DLPFC may help us to learn new social behaviors by observing the trials and tribulations of other individuals performing them, an ability that chimpanzees also possess (Savage-Rumbaugh, McDonald, Sevcik, Hopkins, & Rubert, 1986). Given these considerations, our findings suggest that the DLPFC is a major site of interaction between the processes underlying cognitive control and those supporting social cognition.
The posterior STS has been posited to make a major contribution to social cognition by evaluating whether other people’s actions, conveyed through biological motion, appear consistent with their intentions (Morris et al., 2005). In the present study, we found no evidence to support a role for the posterior STS in evaluating whether one person’s actions are consistent with a second person’s intentions. This result appears to constrain the role of the STS in social cognition to evaluating whether an observed person’s actions appear consistent with his or her own intentions.
Alternatively, the posterior STS may be involved in determining whether one person’s actions are consistent with a second person’s intentions, but mainly when those intentions are not explicitly stated. Indeed, in many prior studies of the posterior STS and its role in social cognition, the actors’ intentions were not clearly specified (Grezes et al., 2004; Pelphrey et al., 2004; Pelphrey et al., 2003; Saxe et al., 2004) and therefore needed to be inferred by study participants. In these more ambiguous situations, the posterior STS might contribute to generating an inference about what an observed person is doing by performing highly elaborated perceptual processing of whatever biological motion information is available. When an observed person’s actions do not appear appropriate in a particular context, and an inference is therefore relatively difficult to make, the need for elaborated perceptual processing by the posterior STS might be especially high. Clearly, future studies will be needed to test this possibility. At present, we conclude that the posterior STS is not involved in evaluating whether one person’s actions appear consistent with a second person’s intentions, at least when those intentions are explicitly stated.
Since our animations portrayed relatively simple behaviors and were highly repetitive, we considered the possibility that some regions underlying social cognition might have been most involved in our task relatively early in the experiment. To test this hypothesis, we performed additional analyses using data from only the first two runs. These analyses revealed effects in dorsolateral prefrontal and superior temporal regions that were highly similar in magnitude to those we reported in our overall analysis. However, they also revealed several medial prefrontal regions, including the precuneus, bilateral regions of the posterior cingulate cortex, the anterior cingulate cortex, and the medial prefrontal cortex, in which inconsistent actions produced larger deactivations than consistent actions (i.e., regions in which consistent actions evoked greater activity than inconsistent actions). Many of these medial prefrontal regions have been posited to form mental representations of people’s psychological attributes, including their beliefs, intentions, and goals (Amodio & Frith, 2006). Therefore, one possible interpretation of our findings is that participants were better able to form mental representations of the child’s intentions when the child performed correctly (in which case the child’s intentions were clearly to follow the adult’s instructions) than when the child perform incorrectly (in which case the child’s intentions may have been less clear).
Our findings also provide further support for the existence of a mirror neuron system for touch (Blakemore et al., 2005). When we directly face an individual, as participants did in the present experiment, we often mirror that individual’s movements and sensations in a body part (e.g., the left hand) that mirrors the body part that is being manipulated by the observed individual (e.g., the right hand) (Pelphrey et al., 2004). In line with this view, activity in the right somatosensory cortex was significantly greater when participants observed the boy touch an object with his right hand (mirrored with the left hand) than when they observed the boy touch an object with his left hand (mirrored with the right hand), and the opposite pattern was observed in the left somatosensory cortex. Although we did not show that the same somatosensory regions were activated when participants observed the boy touch an object and when participants were actually touched (Keysers et al., 2004), the present findings appear to add to a growing body of data indicating a mirror neuron system for touch in the human somatosensory cortex.
In conclusion, our results have important implications for neurobiological theories of both cognitive control and social cognition. First, they suggest that the DLPFC is a crucial interface between the processes that underlie cognitive control and those that support social cognition. Second, they suggest that the contribution of the posterior STS to social cognition does not include evaluating whether the actions of one individual appear consistent with the intentions of a second individual, at least in situations where those intentions are explicitly stated. Future studies of the interplay between cognitive control and social cognition should investigate whether our findings generalize to interpreting more complex social interactions. More generally, they should further our understanding of the neural mechanisms that enable voluntary behavior in social environments.
Acknowledgements
This research was supported by NIH grants to D.H.W (1RO3DA021345-01) and and to M.G.W. (R01-MH60415, R01-NS051048, and 2-P01-NS41328, Proj.1). We thank James P. Morris for helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Citations
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Oxford: Clarendon Press; 1986. [Google Scholar]
- Barcelo F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–356. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Bergman T, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synethesia. Brain. 2005;128:1571–1583. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. The Journal of Neuroscience. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Grezes J. Neural mechanisms subserving the perception of human actions. Trends in Cognitive Science. 1999;3:172–178. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Genovesio A, Brasted PJ, Wise SP. Representation of Future and Previous Spatial Goals by Separate Neural Populations in Prefrontal Cortex. The Journal of Neuroscience. 2006;26:7305–7316. doi: 10.1523/JNEUROSCI.0699-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Jonas B, Salazar A. Wisconsin Card Sorting Test performance based on location and size of neuroanatomical lesion in Vietnam veterans with penetrating head injury. Perceptual and Motor Skills. 1990;71(3 Pt 2):1120–1122. doi: 10.2466/pms.1990.71.3f.1120. [DOI] [PubMed] [Google Scholar]
- Grezes J, Frith CD, Passingham RE. Inferring false beliefs from the actions of oneself and others: An fMRI study. NeuroImage. 2004;21:744–750. doi: 10.1016/S1053-8119(03)00665-7. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U. Understanding autism: insights from mind and brain. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Foqassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Malle BF, Moses LJ, Baldwin DA. Introduction: The significance of intentionality. In: Malle BF, Moses LJ, Baldwin DA, editors. Intentions and intentionality. Cambridge: MIT Press; 2001. pp. 1–26. [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation when approaching a virtual human on a virtual walk. Journal of Cognitive Neuroscience. 2005;17:1744–1752. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. I. The Method. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. II. Analysis. Neuroimage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the Intentions of Others: The Perceived Intentionality of an Action Influences Activity in the Superior Temporal Sulcus during Social Perception. Journal of Cognitive Neuroscience. 2004;16:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, T A, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003;41:156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Annals of the New York Academy of Sciences. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Chimpanzee problem-solving: a test for comprehension. Science. 1978;202:532–535. doi: 10.1126/science.705342. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh S, McDonald K, Sevcik RA, Hopkins WD, Rubert E. Spontaneous symbol acquisition and communicative use by pygmy chimpanzees (Pan paniscus) Journal of Experimental Psychology: General. 1986;115:211–235. doi: 10.1037//0096-3445.115.3.211. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perret DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, et al. Areas involved in encoding and applying directional expectations to moving objects. The Journal of Neuroscience. 1999;19(21):9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tsujimoto S, Sawaguchi T. Neuonal representation of response-outcome in the primate prefrontal cortex. Cerebral Cortex. 2004;14:47–55. doi: 10.1093/cercor/bhg090. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T. Context-dependent representation of response-outcome in monkey prefrontal neurons. Cerebral Cortex. 2005;15:888–898. doi: 10.1093/cercor/bhh188. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI integrating paradigm control, physiology, behavior, and on-line statistical analysis. NeuroImage. 1999;10:91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. The Journal of Neuroscience. 2004;24(48):10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]