Abstract
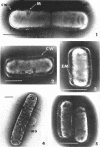
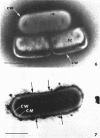
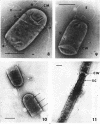
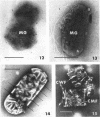
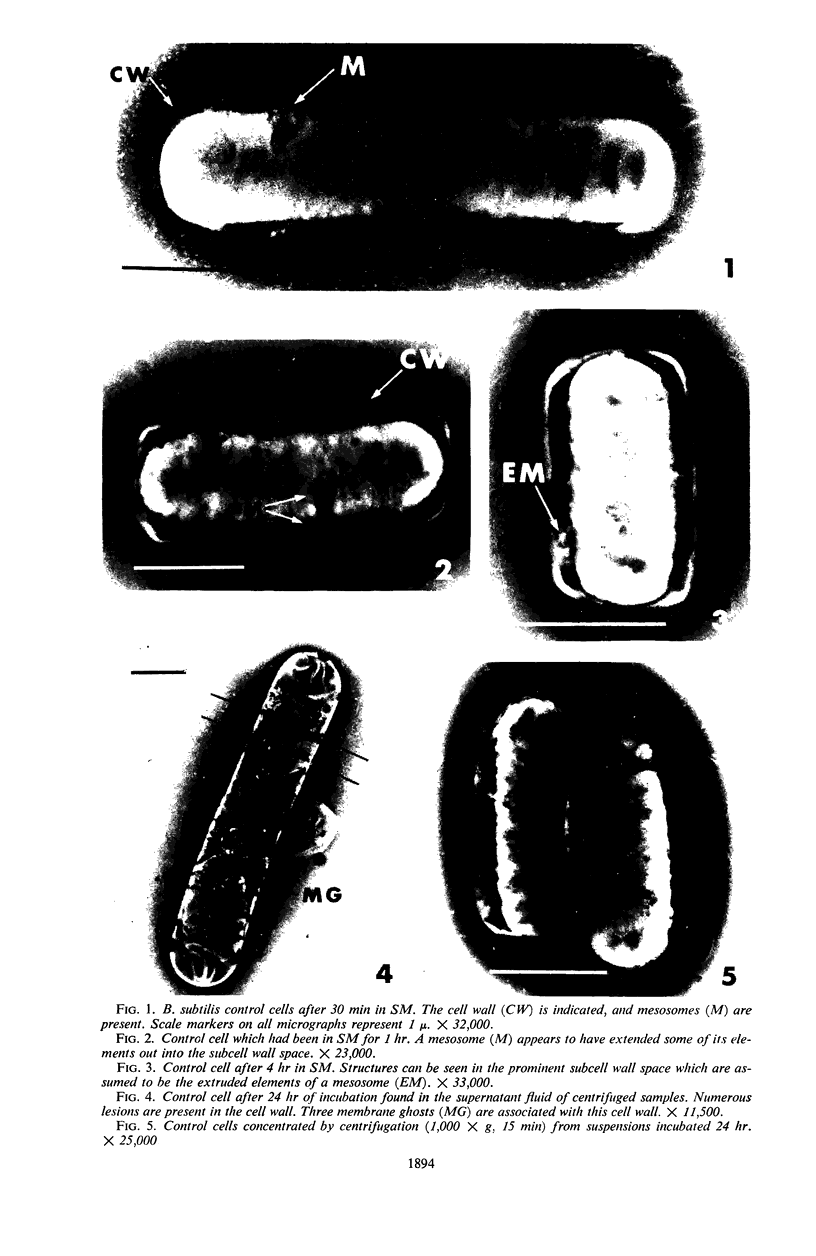
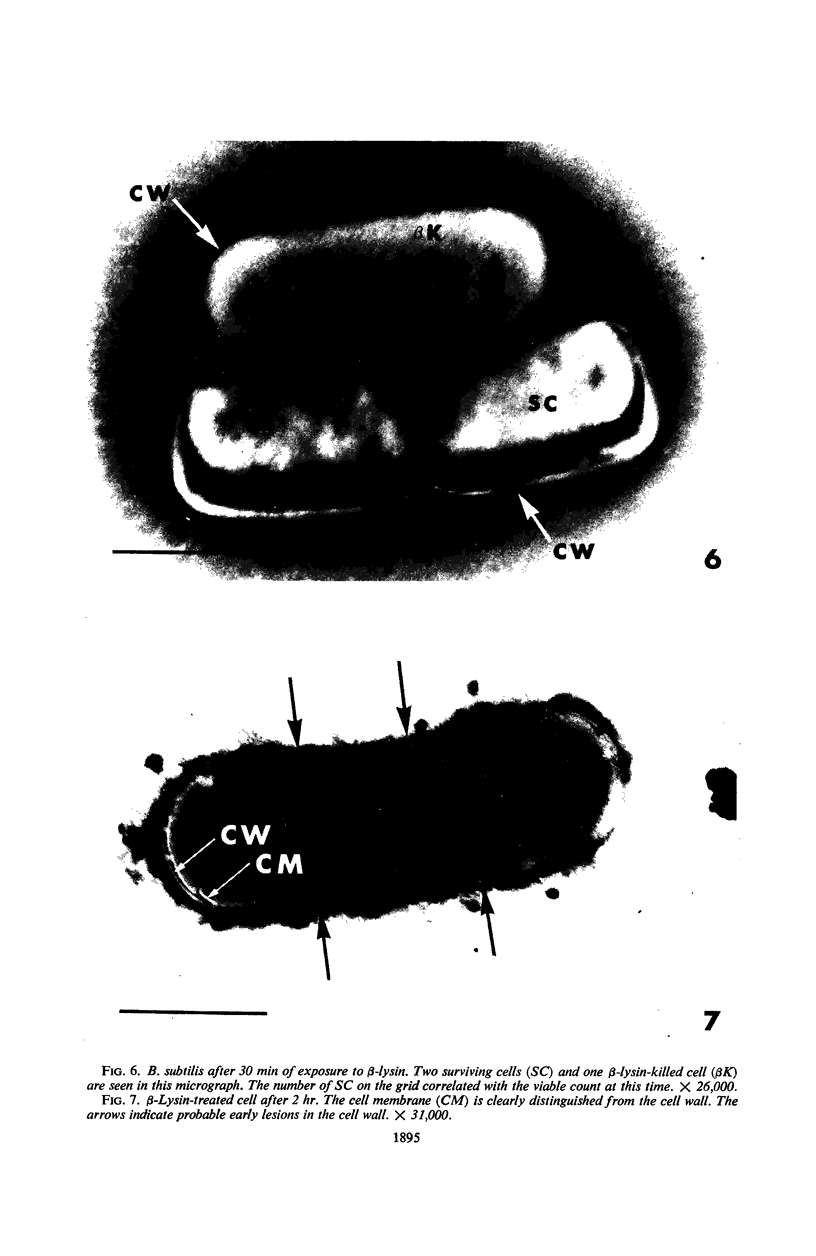
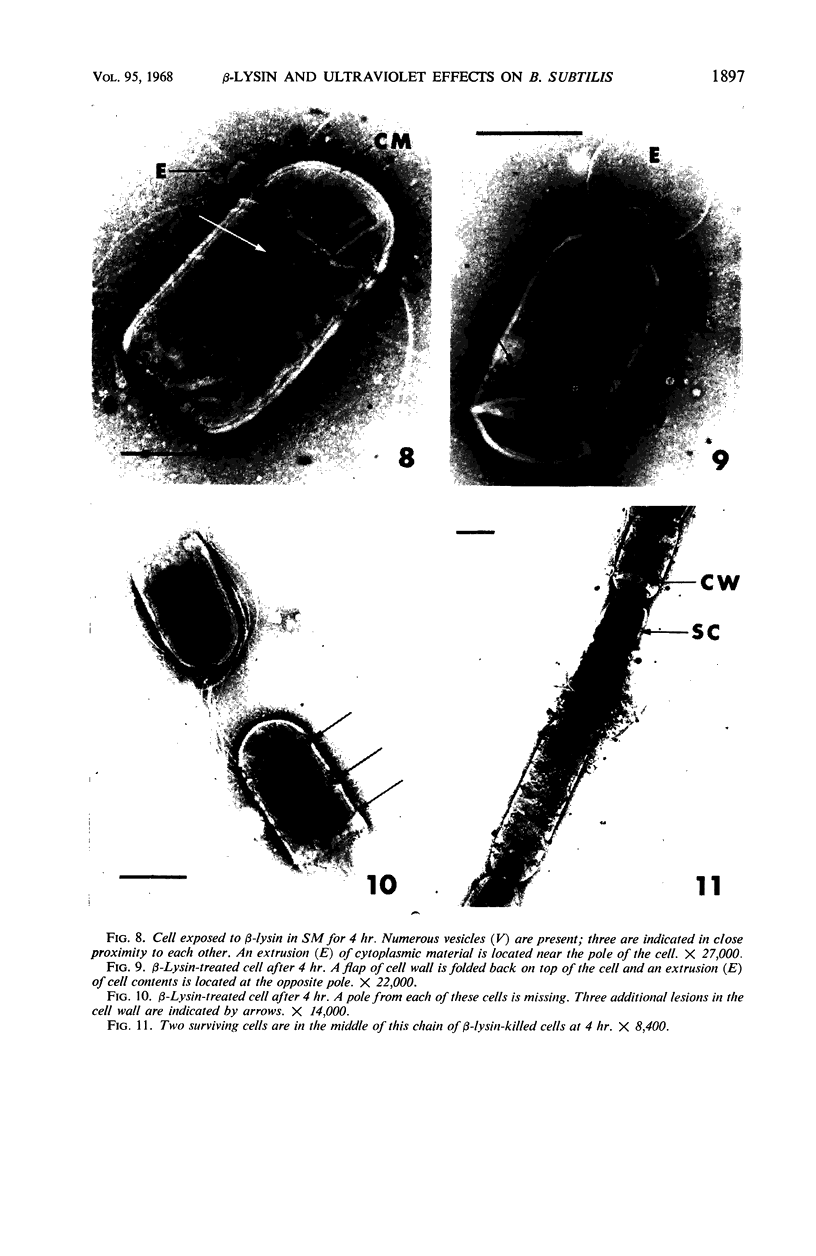
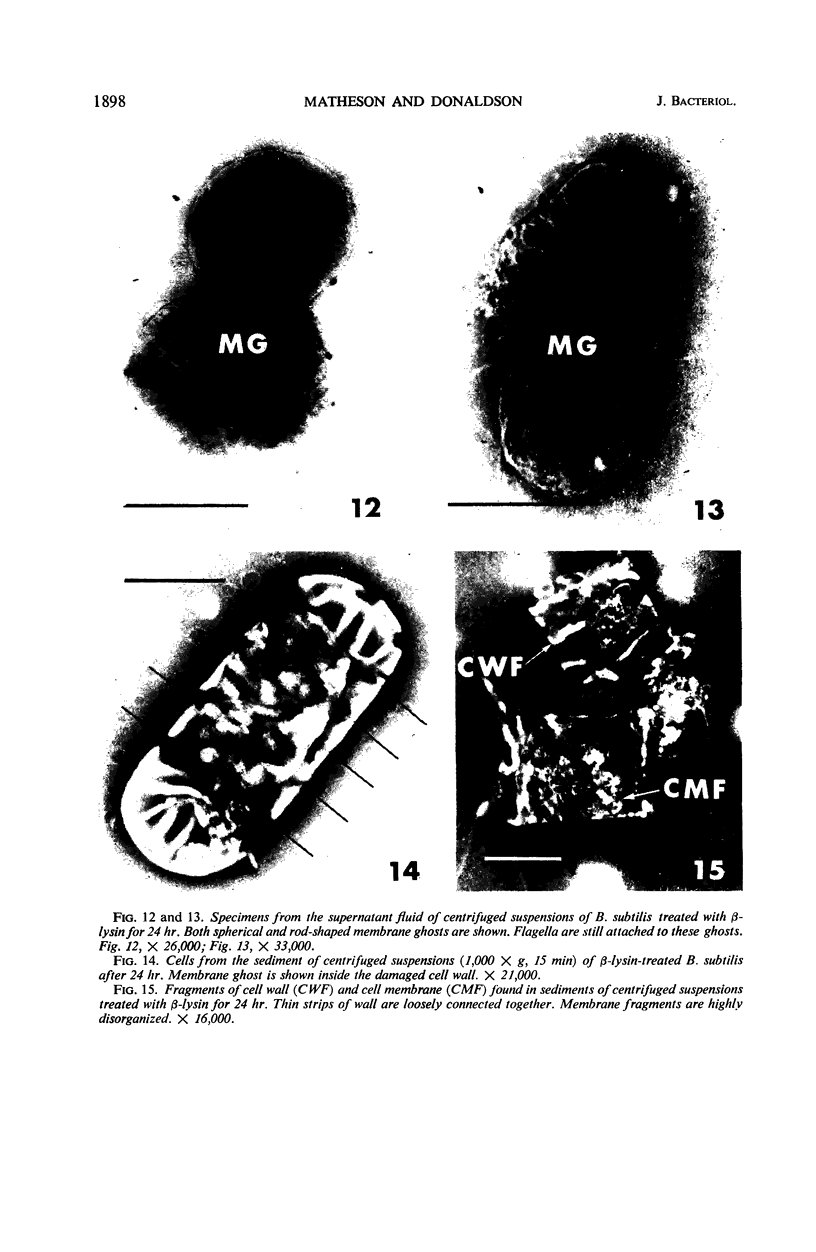
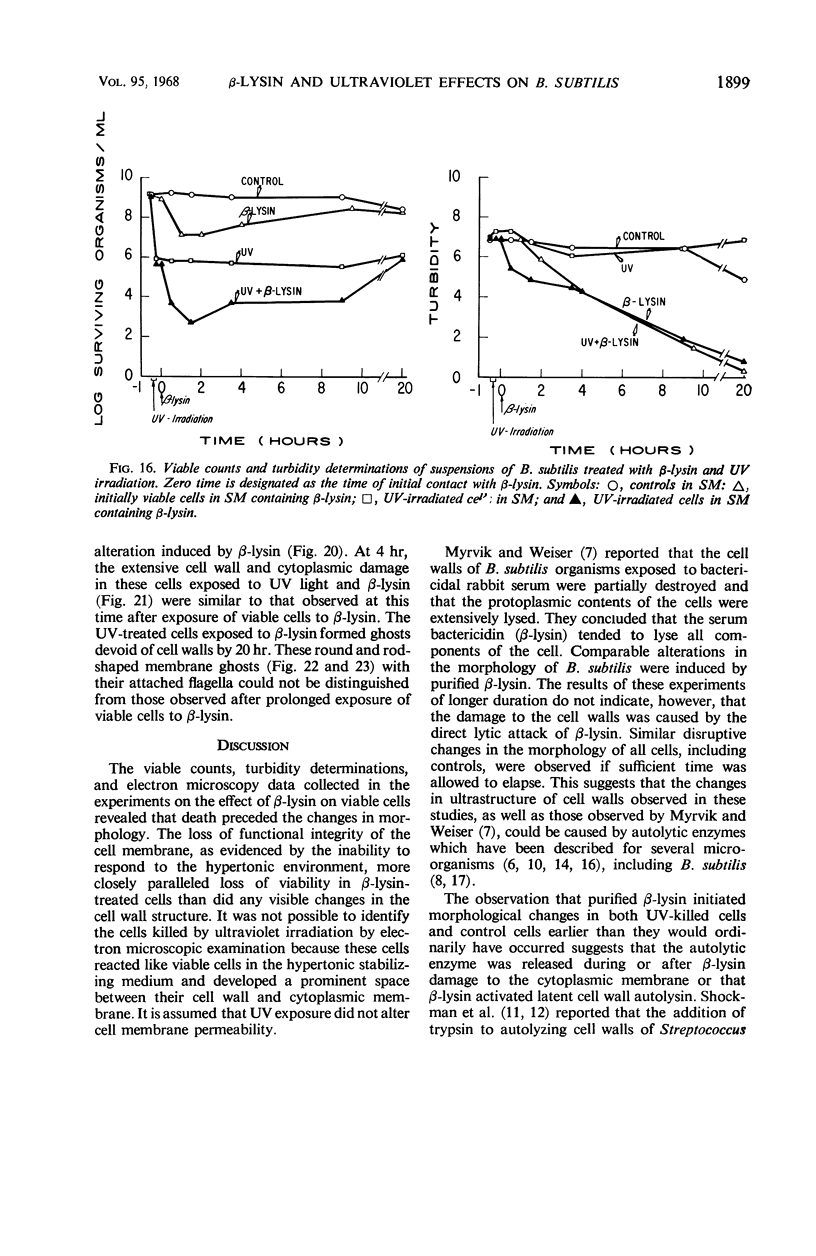
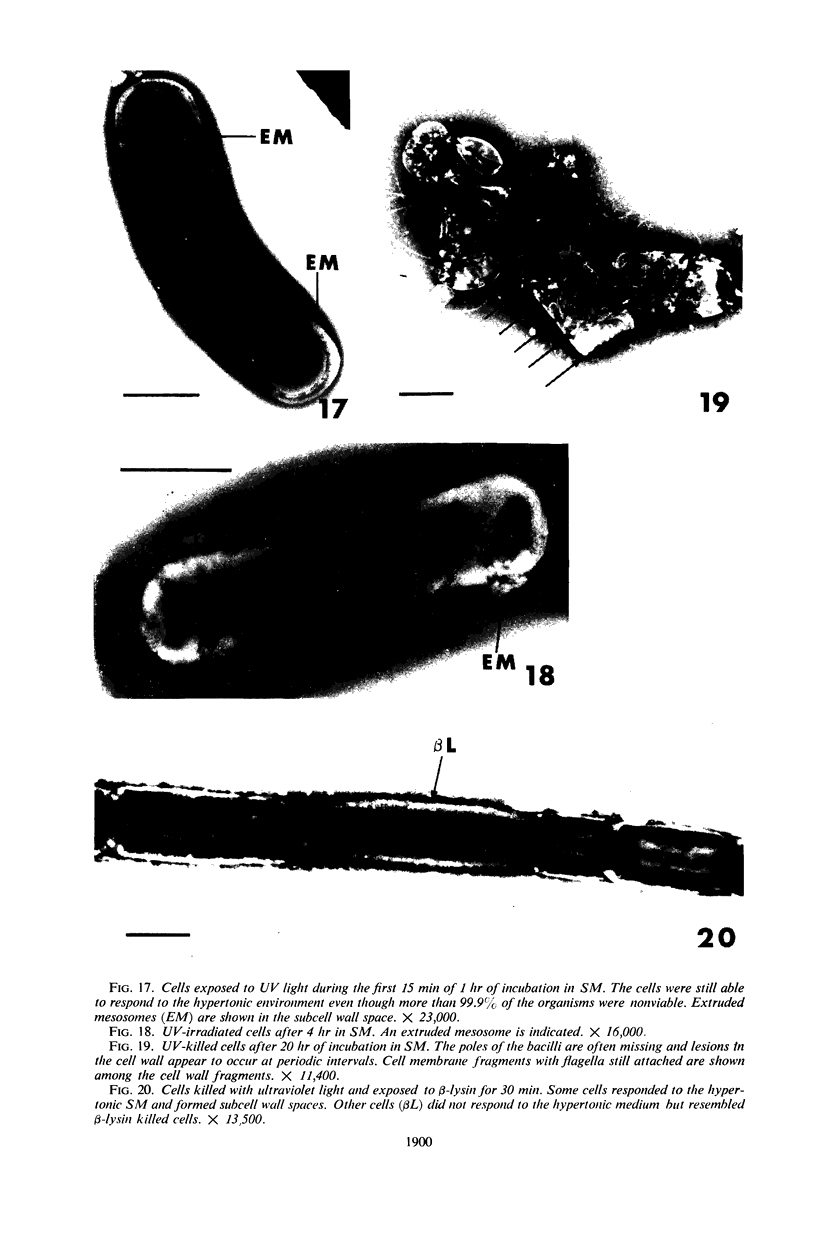
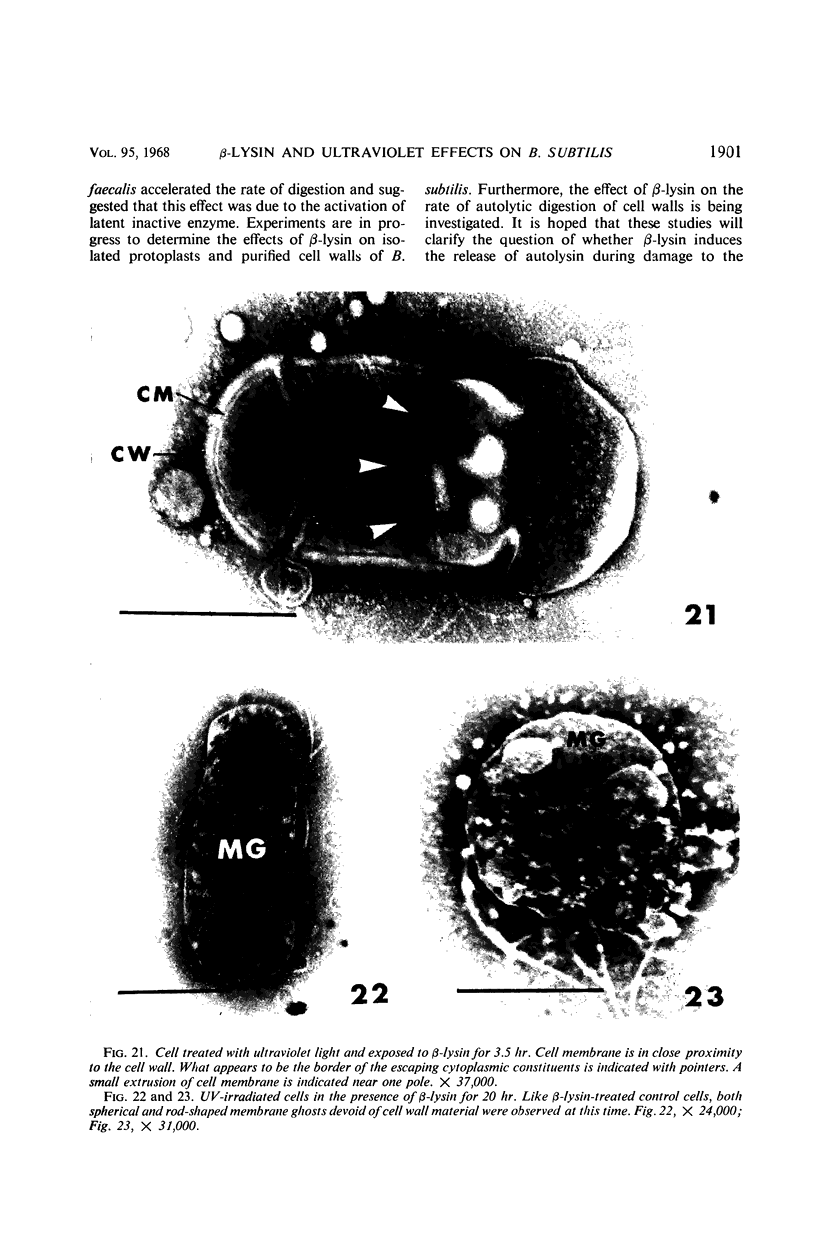
Viable counts, turbidities, and electron micrographs of Bacillus subtilis exposed to β-lysin and ultraviolet light (UV), singly or in combination, were compared in an attempt to relate death with changes in morphology. The decreases in survival of both the β-lysin- and UV-treated cells were rapid and preceded decreases in turbidity, as well as the changes in morphology. No significant differences were observed in turbidity reduction or morphological alterations of control cells from those of cells exposed to UV light. These cells developed prominent subcell wall spaces during incubation in the hypertonic stabilizing medium. No observable damage in either the cell wall or the cell membrane had taken place during 4 hr, but by 20 hr extensive damage of these two structures was apparent. The control and UV-treated cells exposed to β-lysin did not develop prominent subcell wall spaces. Within 2 hr, lesions were observable in their cell walls, and the cytoplasmic membranes were permeable to phosphotungstic acid. The damage to these structures became more extensive with time. Although the visible changes of control and UV-treated cells were evident much later than those induced by β-lysin, the morphological alterations in all cells were similar. It appeared that β-lysin caused an accelerated release of an autolytic enzyme which digested the cell walls.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DONALDSON D. M., ELLSWORTH B., MATHESON A. SEPARATION AND PURIFICATION OF BETA-LYSIN FROM NORMAL SERUM. J Immunol. 1964 Jun;92:896–901. [PubMed] [Google Scholar]
- DONALDSON D. M., JENSEN R. S., JENSEN B. M., MATHESON A. SEROLOGICAL RELATIONSHIPS AMONG BETA-LYSIN, PLAKIN, AND LEUKIN. J Bacteriol. 1964 Oct;88:1049–1055. doi: 10.1128/jb.88.4.1049-1055.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson D. M., Tew J. G. Differentiation of muramidase and beta-lysin. Proc Soc Exp Biol Med. 1966 May;122(1):46–49. doi: 10.3181/00379727-122-31046. [DOI] [PubMed] [Google Scholar]
- HOSODA J., NOMURA M. Nature of the primary action of the autolysin of Bacillus subtilis. J Bacteriol. 1956 Nov;72(5):573–581. doi: 10.1128/jb.72.5.573-581.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q. N., WEISER R. S. Studies on antibacterial factors in mammalian tissues and fluids. I. A serum bactericidin for Bacillus subtilis. J Immunol. 1955 Jan;74(1):9–16. [PubMed] [Google Scholar]
- Matheson A., Jensen R. S., Donaldson D. M. Serologic relationships between beta-lysins of different species. J Immunol. 1966 May;96(5):885–891. [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DES RELATIONS ENTRE M'ESOSOMES ET NOYAUX CHEZ BACILLUS SUBTILIS. C R Hebd Seances Acad Sci. 1963 Nov 13;257:3060–3063. [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. A cell-wall lytic enzyme associated with spores of Bacillus species. J Gen Microbiol. 1957 Feb;16(1):236–249. doi: 10.1099/00221287-16-1-236. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Electron microscopic aspects of membrane alterations during bacterial cell lysis. Exp Cell Res. 1967 May;46(2):245–251. doi: 10.1016/0014-4827(67)90062-6. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. PLASMOLYSIS IN BACILLUS MEGATERIUM. J Bacteriol. 1965 Apr;89:1151–1154. doi: 10.1128/jb.89.4.1151-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., LEUTGEB W. Autolytic enzymes as a source of error in the preparation and study of gram-negative cell walls. J Gen Microbiol. 1963 Jan;30:127–130. doi: 10.1099/00221287-30-1-127. [DOI] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]
- Young F. E. Fractionation and partial characterization of the products of autolysis of cell walls of Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):839–846. doi: 10.1128/jb.92.4.839-846.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]