Abstract
A major limitation in developing applications for the use of human embryonic stem cells (HESCs) is our lack of knowledge of their responses to specific cues that control self-renewal, differentiation, and lineage selection. HESCs are most commonly maintained on inactivated mouse embryonic fibroblast feeders in medium supplemented with FCS, or proprietary replacements such as knockout serum-replacement together with FGF-2. These undefined culture conditions hamper analysis of the mechanisms that control HESC behavior. We have now developed a defined serum-free medium, hESF9, for the culture of HESCs on a type I-collagen substrate without feeders. In contrast to other reported media for the culture of HESCs, this medium has a lower osmolarity (292 mosmol/liter), l-ascorbic acid-2-phosphate (0.1 μg/ml), and heparin. Insulin, transferrin, albumin conjugated with oleic acid, and FGF-2 (10 ng/ml) were the only protein components. Further, we found that HESCs would proliferate in the absence of exogenous FGF-2 if heparin was also present. However, their growth was enhanced by the addition of FGF-2 up to 10 ng/ml although higher concentrations were deleterious in the presence of heparin.
Keywords: defined serum-free culture, feeder-free
Human embryonic stem cells (HESCs) were originally derived from the inner cell masses of early embryos explanted onto inactivated mouse embryo fibroblast (MEF) feeder cells, in a medium comprising a 50:50 mix of DMEM and Ham's F12 (DMEM:F12), supplemented with fetal calf serum (FCS) (1, 2). Subsequently, a proprietary basal medium, KO-DMEM supplemented with proprietary ‘Knock-Out Serum Replacement’ (KSR) (Invitrogen), initially developed for murine ES cells (MESCs), has become widely used both for maintenance and derivation of HESCs. However, the precise formulation of KSR is not in the public domain and, although ‘serum-free’, it is likely to contain a variety of animal products (PCT/US98/00467; WO98/30679).
Culture without feeder cells in defined media has proved more problematic, although culture in the absence of feeders but with feeder-conditioned medium is effective (3). Addition of Leukemia Inhibitory Factor (LIF) to FCS-containing medium was sufficient to allow the culture of MESCs without feeders (4, 5). However, LIF does not prevent the differentiation of HESCs (1, 2), although it does activate the STAT3 pathway in these cells (6, 7). Indeed, together with a variety of other differences in their expression of surface antigen markers of the undifferentiated state (8) and capacity for differentiation (9), HESCs appear to differ markedly from MESCs in their responses to activation of signaling pathways associated with FGF and the TGFβ/BMP family of cytokines. For example, activin and Nodal inhibit differentiation of HESCs (10–12), whereas BMP induces their differentiation to trophoblasts (9) or extraembryonic endoderm cells (13). By contrast, BMP signaling inhibits the differentiation of MESCs (14). It is also evident that HESCs have a strong requirement of FGF-2 (15, 16), whereas MESCs do not appear to respond to this growth factor (17). However, pluripotent ‘Epiblast Stem Cells’ with similar growth factor requirements to HESCs have recently been derived from gastrula stage mouse and rat embryos (18, 19).
Several methods for the culture of HESCs in more defined media, and in the absence of feeders have been reported. Some require culture on Matrigel (Becton-Dickinson) but this contains a variety of extracellular matrix (ECM) components, most likely associated with an ill-defined mixture of growth factors (20–22). Others use fully chemically defined media together with specific ECM attachment factors (23). Nevertheless, there is no consensus as to the optimal formulation, or the nature of the cytokine requirements of HESCs to promote their self-renewal and inhibit their differentiation. One puzzle is the reported requirement for very high concentrations of FGF-2 (up to 100 ng/ml) (24); this suggests that either FGF-2 is operating through an unidentified receptor for which it has a low affinity, or that it is relatively unstable or inefficiently presented to the cells in the culture conditions used.
We have now investigated the culture of HESCs under fully defined culture conditions and have developed a medium that permits their prolonged culture in an undifferentiated state. Although this medium shares a number of features with those described by others, including culture on defined ECM attachment factors, it differs in a number of important respects. In particular, we have used a base medium, ESF (25) that we previously developed for use with MESCs, in contrast to DMEM:F12 commonly used in other formulations (25). This medium also excludes Hepes, but includes heparin, a cofactor for FGF-2, which is required for HESC maintenance.
Results
We first tested the ability of ESF7 medium [supporting information (SI) Table S1], which we had developed for use with MESCs (25), to support the growth of two HESC lines, HUES-1 (Fig. 1A) (26) and Shef1 (Fig. 1B) (8). These cells were harvested using collagenase, or trypsin/EDTA, respectively, as previously described, from cultures on MEFs, and transferred to type I collagen-coated flasks in ESF7 medium. However, the cells died after one day. On further study we found that Hepes is detrimental to even one day of HESC culture in the absence of serum, that LIF provided no advantage in 6-day culture, but that l-ascorbic acid-2-phosphate and FGF-2 enhanced HESC survival and growth over 3 passages (data not shown). From this initial study we developed a variant of the ESF7 formulation, designated hESF8 (Tables S1 and S2). In this medium, on type I collagen-coated flasks, both HUES-1 (Fig. S1A) and Shef1 cells (Fig. S1A) grew to form densely packed colonies consistent with the cells retaining an undifferentiated HESC morphology.
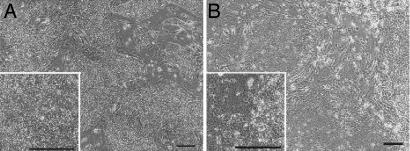
Fig. 1.
Phase contrast photomicrograph of HUES-1 (A) and Shef 1 (B) HESCs cultured on feeders in KSR-based medium. (Scale bar, 200 μm.)
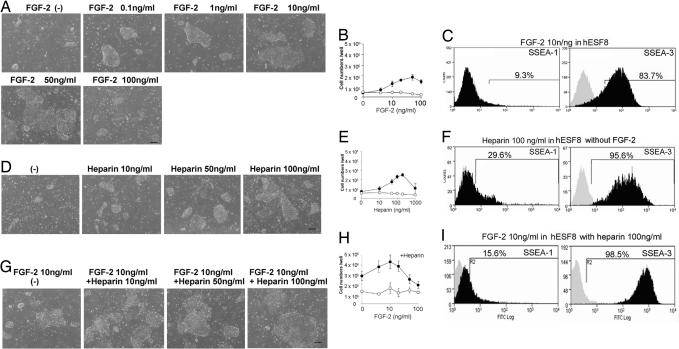
To determine the optimal concentration of FGF-2 for HESC growth, HUES-1 cells were seeded without feeders, on a type I-collagen substrate, in KO-DMEM/KSR or hESF8 medium containing varying concentrations of FGF-2. In addition, in some experiments, heparin, a cofactor known to stabilize FGF-2, was also added. When HUES-1 cells were cultured in KSR-based medium without feeders on collagen-coated plates, few cells survived and no undifferentiated, alkaline phosphatase-positive colonies were observed with or without FGF-2 in the presence or absence of heparin (data not shown). However, small undifferentiated colonies were observed in hESF8 medium lacking FGF-2, while increasing the dose of FGF-2 up to 50 ng/ml promoted the growth of larger colonies in this medium (Fig. 2A). High SSEA-3 expression and low SSEA-1 expression were seen in all cultures, irrespective of the FGF-2 concentration (Fig. 2B). However, at 100 ng/ml FGF-2, the colony sizes were again smaller, with apparently differentiated cells identified morphologically, also present.
Fig. 2.
Effect of FGF-2 and heparin on the HUES-1 cell growth. HUES-1 cells were cultured on type I collagen in hESF8 with varying concentrations of FGF-2 or heparin. (A) Phase contrast photomicrograph of HUES-1 cells with various concentration of FGF-2 without heparin. (Scale bar, 200 μm.) (B) Number cells harvested after 6 days of culture in KSR-based medium (open circles) or hESF8 (closed circles). Values are the mean ± SD for three measurements. The absence of error bars is due to the small SD in the results. (C) FACS profiles for SSEA-1 and SSEA-3 expression of cells cultured with 10 ng/ml FGF-2. Antigen histogram (black); control histogram (gray); the horizontal bar indicates the gating used to score the percentage of cells antigen positive. Similar profiles were obtained for cells from cultures grown with all concentrations of FGF-2 from 0 to 100 ng/ml (data not shown). (D) Phase contrast photomicrograph of HUES-1 cells cultured without FGF-2 but supplemented with various concentration of heparin. (Scale bar, 200 μm.) (E) Number cells harvested after 6 days of culture in KSR-based medium (open circles) or hESF8 (closed circles) with varying concentrations of heparin but no FGF-2. Values are the mean ± SD for three measurements. The absence of error bars is due to the small SD in the results. (F) FACS profiles for SSEA-1 and SSEA-3 expression of cells cultured with 100 ng/ml heparin. Antigen histogram (black); control histogram (gray); the horizontal bar indicates the gating used to score the percentage of cells antigen positive. Similar profiles were obtained for cells from cultures grown with all concentrations of heparin from 0 to 100 ng/ml (data not shown). (G) Phase contrast photomicrograph of HUES-1 cells cultured with various concentration of heparin in hESF8 containing 10 ng/ml FGF-2. (Scale bar, 200 μm.) (H) Number cells harvested after 6 days of culture in KSR-based medium (open circles) or hESF8 (closed circles) containing varying concentrations of FGF-2 and 100 ng/ml heparin. Values are the mean ± SD for three measurements. The absence of error bars is due to the small SD in the results. (I) FACS profiles for SSEA-1 and SSEA-3 expression of cells cultured hESF8 containing 100 ng/ml heparin and 10 ng/ml FGF-2 (hESF9). Antigen histogram (black); control histogram (gray); the horizontal bar indicates the gating used to score the percentage of antigen-positive cells. Similar profiles were obtained for cells from cultures grown with all concentrations of FGF-2 from 0 to 100 ng/ml (data not shown).
The addition of heparin to hESF8 medium, in the absence of FGF-2, also promoted HUES-1 cell proliferation in a dose-dependent manner; the greatest effect was seen at 100–200 ng/ml heparin, whereas 1000 ng/ml was markedly deleterious (Fig. 2 D and E). These cells also retained expression of SSEA-3 with low expression of SSEA-1 (Fig. 2F). Finally, we tested the combined effects of heparin and FGF-2. In hESF8 medium containing 100 ng/ml heparin, maximal cell densities were achieved with 10 ng/ml FGF-2, with high SSEA-3 and low SSEA-1 expression; >20 ng/ml FGF-2, maximal cell densities decreased (Fig. 2 G–I).
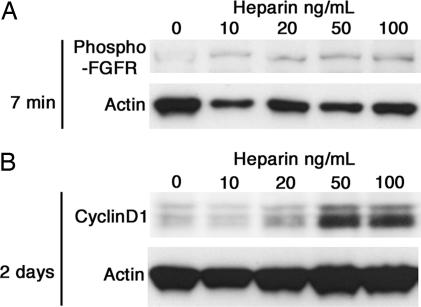
To confirm the effect of heparin on FGF signaling, we analyzed its effect on phosphorylation of the FGF receptor. Rapid phosphorylation of the FGF receptors was induced by heparin in a dose-dependent manner (Fig. 3A). Further, expression of cyclinD1 was also induced by heparin, confirming a potential effect on cell cycle regulation (Fig. 3B). Based on these observations we supplemented hESF8 medium with 10 ng/ml FGF-2 and 100 ng/ml heparin, and designated this, hESF9.
Fig. 3.
The effect of heparin on FGF signaling. HUES-1 cells were stimulated with different concentrations of heparin after 48-h starvation of FGF-2 and heparin. (A) Seven minutes after heparin addition, the cells were lysed and followed by western blot using an antibody detecting the phosphorylation of FGF receptors. (B) After 2 days of culture in the presence of heparin, the cells were lysed and subjected to western blot of cyclin D1. Actin is used as loading control.
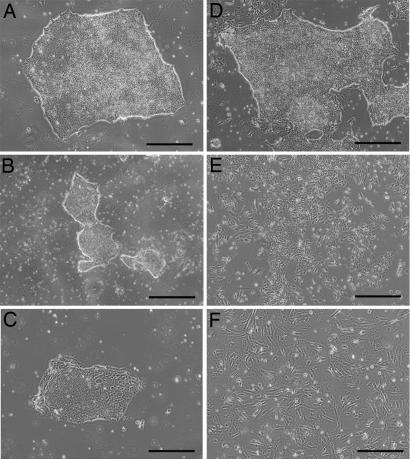
To determine whether different substrates might affect HESC growth, HUES-1 cells were cultured in hESF9 separately on type I collagen (10 μg/cm2), fibronectin (5 μg/cm2), laminin (5 μg/cm2), or gelatin (10 μg/cm2). In each case the cells produced typical undifferentiated colonies (Fig. 4 A–D) with similar profiles of SSEA-3, TRA-1–60, and SSEA-1 (data not shown). However, subjectively, we judged the colony morphologies more uniform on type I collagen, which we continued to use as the standard substrate in subsequent experiments with hESF9 medium. By contrast, when HUES-1 cells were cultured on Matrigel in hESF9 medium (Fig. 4E), or on these ECM components in KSR-based medium (Fig. 4F), extensive differentiation was observed.
Fig. 4.
Effect of ECM on HUES-1 culture. (A–E) Phase contrast photomicrographs of HUES-1 cells cultured in hESF9 medium, in flasks coated with collagen (A), gelatin (B), fibronectin (C), laminin (D), and Matrigel (E). (F) Cells were cultured in KSR-based medium on type I collagen. (Scale bar, 200 μm.)
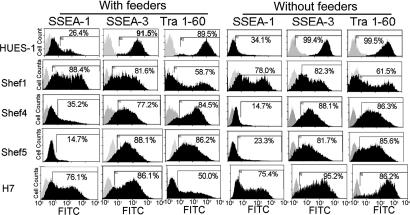
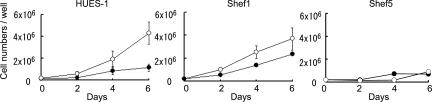
The hESF9 medium proved capable of supporting the culture of other HESC lines, Shef1, Shef4, Shef5, and H7, after plating on type I collagen. In each case, the expression of the marker antigens SSEA-1, SSEA-3, and TRA-1–60 was similar to cells grown in KSR-based medium on feeders (Fig. 5). Also, we measured the growth rates of Shef1 and Shef5 cells and, as for HUES-1 (Fig. 6). The growth rate and maximum cell densities reached by HUES-1 and Shef1 were higher when the cells were grown in hESF9. However, in the case of Shef5, although the final cell density was higher when grown in hESF9, the initial growth rate was lower.
Fig. 5.
FACS profiles for SSEA-1, SSEA-3, and TRA-1–60 expression by HUES-1, Shef1, Shef4, Shef5, and H7 HESC lines cultured on type I collagen in hESF9, in comparison with cells grown on feeders in KSR-based medium. Antigen histogram (black); control histogram (gray); the horizontal bar indicates the gating used to score the percentage of antigen-positive cells.
Fig. 6.
A comparison of the growth of different HESCs in hESF9 and KSR-based media. HUES-1, Shef1, and Shef5 cells were seeded on feeders in KSR-based medium (closed circles) or on type I collagen in hESF9 (open circles) at a cell density of 1 × 105 cells per well; mean and SD of three experiments. Cell numbers were counted every 2 days.
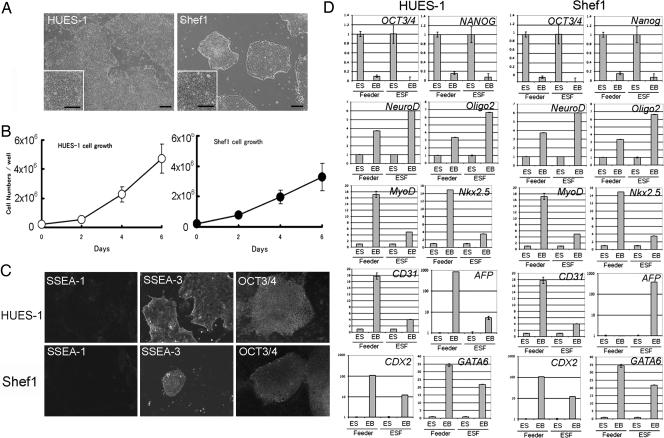
We subsequently tested long-term culture of HUES-1 to 25 passages and Shef1 to 15 passages on type I collagen in hESF9 medium without feeders. Although a few fibroblastic or neural cells appeared in the cultures at early passages, morphologies of undifferentiated HESC colonies were maintained as those cultured on feeders (Fig. 7A). The growth rates of these HESCs after five passages was similar to to those at passage 1 (Fig. 7B). The cells retained a normal karyotype (Fig. S2A). They also retained expression of SSEA-3, and OCT3/4, NANOG, SOX-,2 and REX-1 (Fig. 7C, Fig. S2 B and C). Further, the HUES-1 and Shef1 cells retained the capacity for extensive differentiation as indicated by analysis of ectoderm, mesoderm, and endoderm marker gene expression, NeuroD, Oligo 2, MyoD, Nkx2.5, CD31, AFP, Cdx2, and GATA6 following embryoid body formation (Fig. 7D).
Fig. 7.
Long-term culture of HESCs in the defined medium. HUES-1 and Shef1 cells were serially cultured on type I collagen in hESF9. The cells were split at 1:3 every week. (A) Phase contrast photomicrograph of HUES-1 at passage 21 and Shef1 cells at passage 14. (Scale bar, 200 μm.) (B) The growth of HUES-1 (passage 10) and Shef1 cells (passage 5) in the defined medium. (C) Immunohistochemical staining of HUES-1 (passage 24) and Shef1 (passage 14) for SSEA-1, SSEA-3, and OCT3/4. (D) Q-PCR analysis of gene expression in HUES-1 and Shef1 on feeder in KSR-based medium (feeder) and HUES-1 (passage 24) and Shef1 (passage 14) on collagen in hESF9 medium (ESF) those during in vitro differentiation (EB). The name of the gene of differentiation are noted in each bar graph. Expression levels were all normalized against GAPDH. The relative level of each gene in undifferentiated cells was defined as “1.”
Discussion
Our current results indicate that it is possible to culture HESCs in a defined medium, hESF9, in which insulin, transferrin, a low level of fatty acid-free albumin conjugated with oleic acid, and low levels of FGF-2 are the only protein components, together with a substrate composed only of type I collagen. In these studies we have grown two HESC lines, HUES-1 and Shef1, through multiple passages while they retained stable expression of undifferentiated markers of HESCs, and a capacity to differentiate. The medium we describe differs in several key respects from media already reported by others.
First, the base medium, ESF (25), which we developed for MESC culture, has a substantially different formulation from DMEM and DMEM:F12, which are widely used in other reports. Thus, the ESF basal medium includes lipoic acid, glutathione, p-aminobenzoic acid, which are absent from DMEM:F12. Also, the concentration of biotin, pyridoxine, tyrosine, and phosphate are higher than in DMEM:F12, and the osmolarity of ESF is lower (292 mosmol/liter). Further, we found that Hepes is relatively toxic for HESCs, and we have excluded it. In addition, as in an earlier serum-free medium that we designed for human EC cells (27), we found that inclusion of ascorbic acid was strongly beneficial. However, rather than ascorbic acid itself, which is relatively unstable, we used a long-acting vitamin C derivative, l-ascorbic acid-2-phosphate (28). Ascorbic acid is well known as a scavenger of free oxygen radicals, and its value may relate to these anti-oxidant properties. However, it is not required by MESCs, and another possibility is that the requirement reflects the inability of humans, unlike most other mammals, to synthesize this vitamin.
Second, some other reported media for HESCs have either included particularly high concentrations of FGF-2 (up to 100 ng/ml) (24), and/or members of the TGF-β family of growth factors (12, 23), and/or Wnt3A (20) and APRIL/BAFF (29). However, for hESF9 we have found that FGF-2 alone, at a concentration of 10 ng/ml, is sufficient. This requirement for low rather than high levels of FGF-2 may be due to our inclusion of heparin. Heparin, a soluble derivative of heparan sulfate, is a well known cofactor for FGF-2, but its use in defined HESC culture media has not been described. In many cases, a requirement for heparin might be satisfied by the production of heparan sulfate by the ES cells themselves, or by their differentiated derivatives. Several reports have strongly indicated a role for FGF signaling in the maintenance of HESCs, in contrast to MESCs, and have suggested that HESCs produce FGF-2 as an autocrine factor (30, 31). Our observation that the addition of heparin in the absence of exogenous FGF results in the phosphorylation of FGF receptors in HESCs is consistent with a role in stabilizing endogenously produced FGF. Likewise, our finding of substantially lower optimal concentrations of exogenous FGF than reported elsewhere might also reflect the stabilizing effect of heparin on this growth factor. Nevertheless, other mechanisms cannot be excluded. For example, heparin has been reported to enhance the activity of Wnt signaling and FGF signaling in HESCs (32).
In the absence of feeders, HESCs require attachment factors to promote their survival and proliferation (8). Matrigel, a basement membrane preparation from the Engelbreth-Holm-Swarm mouse tumor, is often used. However, it contains a complex and ill-defined mixture of fibronectin, laminin, type IV collagen, entactin, and heparan sulfate proteoglycans, and various growth factors such as FGF-2, EGF, PDGF, NGF, and TGF-β (16). Previous reports have described the use of N2/B27 with 20 ng/ml FGF-2 and Matrigel for feeder free culture of HESC growth (33). Some components such as heparin in Matrigel may support HESC growth with FGF-2. Some authors have replaced Matrigel with purified components, such as type IV collagen, fibronectin, laminin, and vitronectin, alone or in combination (8, 23, 34). In previous studies we found that laminin and fibronectin, but not type I collagen, tended to promote the differentiation of MESCs in defined medium (35). By contrast, in the present study we found that each of these factors was effective in supporting attachment and proliferation of HESCs, although type I collagen appeared the best.
The reasons for the apparent differences in effectiveness of the various formulations for defined HESC culture media are unclear. One possibility is that in some cases the substrate used for attachment of the HESCs, such as Matrigel, has been undefined and might contain extraneous growth factors that confound the analysis. Another possibility is that the lines used by different authors differ in their requirements, either because the undifferentiated stem cells themselves differ intrinsically in their response to, or their autocrine production of specific factors, or because they generate varying amounts of differentiated derivatives that produce factors acting back on the stem cells to promote or inhibit their proliferation. Until specific media formulations are tested by different laboratories on different HESC lines, these issues cannot be easily resolved. An ongoing program of the International Stem Cell Initiative (18) is currently addressing this problem by comparing a number of defined media on different HESC lines in different laboratories.
Materials and Methods
Cells.
Stock cultures of HESC lines HUES-1 and Shef1 were maintained in Knockout (KO)-DMEM (Invitrogen) supplemented with Knockout Serum Replacement (KSR, Invitrogen) on inactivated mouse embryo fibroblast feeder cells as described (8, 15, 26). In addition, a subline of H7, H7.s6 (1, 36), and two new HESC lines, Shef4 and Shef5, derived and maintained in our laboratory according to the same protocols as Shef1 were also used. For culture in defined media without feeders, cells were harvested with 0.5 mM EDTA.
Cell Culture Media.
FGF-2 was purchased from Peprotech Inc. (Rocky Hill, NZ). Type I collagen was from Nitta Gelatin, Co. (Osaka, Japan). The basal ESF medium was provided by the Cell Science & Technology Institute (Sendai, Japan), according to the formulation described (Table S2 and ref. 25). l-ascorbic acid-2-phosphate was obtained from Wako Pure Chemical Ltd. (Osaka, Japan). All other reagents were from Invitrogen and Sigma Aldrich.
Antigen Expression.
Cell surface antigen expression was determined by flow cytometry (37). For in situ immunohistochemisty, the cells were fixed with 4% paraformaldehyde and then incubated with first and second antibodies as described in ref. 37. The following monoclonal antibodies to surface marker antigens were prepared and used as described in ref. 37: MC480 (anti-SSEA1) (38), MC631 (anti-SSEA3) (39), and TRA-1–60 (40). Further antibodies to OCT3/4 (1 μg/ml; Santa Cruz) and NANOG (0.4 μg/ml, R&D Systems) were also used.
Reverse Transcription PCR (RT-PCR) and Quantitative RT-PCR.
Total RNA was extracted from HESCs using a kit (Qiagen) according to the kit instruction. RT-PCR was performed as described in ref. 41. Q-PCR was carried out using the SYBR Green JumpStartTM Kit on a Bio-Rad iCycler. Primer pairs used are listed in Table S3. Expression levels were all normalized against GAPDH. The relative level of each gene in undifferentiated cells was defined as “1.”
Western Blot Analyses.
Cells were lysed in SDS lysis buffer [50 mM Tris·HCl, pH 6.8, 2% SDS, 10% glycerol, with protease and phosphotase inhibitors Complete and phosSTOP (Roche)]. Antibodies used were anti-phospho-FGF Receptor (AF3285, R&D systems), mouse monoclonal anti-human cyclinD1 DCS-6 (DAKO), and mouse monoclonal anti-actin (Abcam).
Supplementary Material
Acknowledgments.
We thank Dr. Peter Tonge for scientific discussions and Katie Amps, Gregg Bingham, Gemma Bray, and Christine Pigott for excellent technical assistance. This work was supported by grants from the Medical Research Council, U.K. and the Juvenile Diabetes Research Foundation. M.K.F. was supported by a visiting research fellowship from Kanagawa Dental College. J. Na is supported by a Medical Research Council Stem Cell Career Development Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806136105/DCSupplemental.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 4.Smith AG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 5.Williams RL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 6.Daheron L, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey RK, et al. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52:2519–2525. doi: 10.2337/diabetes.52.10.2519. [DOI] [PubMed] [Google Scholar]
- 8.Draper JS, Moore HD, Ruban LN, Gokhale PJ, Andrews PW. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- 9.Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 10.Beattie GM, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 11.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development (Cambridge, UK) 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 12.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 13.Pera MF, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 14.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 15.Amit M, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 17.Esner M, Pachernik J, Hampl A, Dvorak P. Targeted disruption of fibroblast growth factor receptor-1 blocks maturation of visceral endoderm and cavitation in mouse embryoid bodies. Internat J of Dev Biol. 2002;46:817–825. [PubMed] [Google Scholar]
- 18.Adewumi O, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:806–813. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 19.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 21.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, et al. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem and Biophys Res Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig TE, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 24.Levenstein ME, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furue M, et al. Leukemia inhibitory factor as an anti-apoptotic mitogen for pluripotent mouse embryonic stem cells in a serum-free medium without feeder cells. In Vitro Cell Dev Biol. 2005;41:19–28. doi: 10.1290/0502010.1. [DOI] [PubMed] [Google Scholar]
- 26.Cowan CA, et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 27.Andrews PW, et al. Inhibition of proliferation and induction of differentiation of pluripotent human embryonal carcinoma cells by osteogenic protein-1 (or bone morphogenetic protein-7) Lab Invest. 1994;71:243–251. [PubMed] [Google Scholar]
- 28.Hata R, Senoo H. l-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J of Cell Physiol. 1989;138:8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato N, Sanjuan IM, Heke M, Uchida M, Naef F, Brivanlou AH. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260:404–413. doi: 10.1016/s0012-1606(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak P, et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells. 2005;23:1200–1211. doi: 10.1634/stemcells.2004-0303. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki N, et al. Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem. 2008;283:3594–3606. doi: 10.1074/jbc.M705621200. [DOI] [PubMed] [Google Scholar]
- 33.Yao S, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol of Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 36.Draper JS, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 37.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: Changes upon differentiation in culture. J Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solter D, Knowles B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Nat Acad Sci (USA) 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shevinsky L, Knowles B, Damjanov I, Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- 40.Andrews PW, Banting G, Damjanov I, Arnaud D, Avner P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984;3:347–361. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- 41.Matin MM, et al. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.