Abstract
Two transgenic mouse lines expressing an inducible form of the Cre recombinase (CreERTM) under the control of the human GFAP promoter have been generated and characterized. In adult mice, expression of the fusion protein is largely confined to astrocytes in all regions of the central nervous system. Minimal spontaneous Cre activity was detected and recombination was efficiently induced by intraperitoneal administration of tamoxifen in adult mice. The pattern of recombination closely mirrored that of transgene expression. The percentage of astrocytes undergoing recombination varied from region to region ranging from 35% to 70% while a much smaller portion (<1%) of oligodendrocytes and neural precursor cells showed evidence of Cre activity. These mouse lines will provide important tools to dissect gene function in glial cells and in gliomagenesis.
Keywords: Cre, Inducible recombination, Astrocyte, Mouse model
Introduction
Astrocytes are the most abundant glial cell type in the mammalian brain and play important roles in all aspects of the developing and mature organ (He and Sun 2007). This includes, but is not restricted to a housekeeping function in support of neurons, the establishment and maintenance of the blood-brain barrier and the regulation of synaptogenesis and synaptic plasticity. It is perhaps not surprising then, that astrocytes are proposed to be at the root of numerous pathological processes such as glial scarring following brain injuries, Alexander disease and the most common primary brain tumor, glioma (He and Sun 2007). The availability of the appropriate molecular tools to manipulate gene expression in vivo is therefore critical to further our understanding of astrocyte contributions to normal and disease states.
A large number of brain-specific bacteriophage P1 Cre recombinase-expressing mouse strains have been developed, including those using the glial fibrillary acidic protein (GFAP) promoter to drive astrocyte-specific expression of cre (Gaveriaux-Ruff and Kieffer 2007). The expression pattern directed by the GFAP promoter in transgenic mice has been extensively studied and mapped (Brenner and Messing 1996; Johnson et al. 1995). In adult mice its activity is restricted predominantly to astrocytes while embryonic expression targets neural precursor cells of the ventricular zone and spinal cord. In addition, a subset of Gfap-expressing glial cells located in the subventricular zone of the adult brain have been shown to function as adult neural stem cells capable of giving rise to the major cell types in the brain: mature astrocytes, oligodendrocytes and neurons (Doetsch 2003). Because Cre-mediated recombination is irreversible, embryonic expression in neural precursor cells directed by the GFAP promoter and/or aberrant expression resulting from transgene insertion effects have resulted in substantial Cre-mediated recombination in mature neurons as well as glial cells in all GFAP-cre mice characterized to date (Bajenaru et al. 2002; Casper and McCarthy 2006; Fraser et al. 2004; Kwon et al. 2001; Marino et al. 2000; McCarty et al. 2005; Zhuo et al. 2001). Therefore, the ability to restrict Cre activity during embryogenesis could greatly mitigate neuronal Cre-mediated recombination in GFAP-Cre mice and allow for the study of gene function in mature astrocytes without disruption of function in neurons.
Cre activity can be regulated in a temporal fashion by engineering a fusion protein with the ligand binding domain of a steroid hormone receptor, such as the estrogen receptor (Feil et al. 1996; Hayashi and McMahon 2002). Furthermore, a specific point mutation within the ligand-binding domain termed ERTM abolishes the binding to endogenous steroid hormones while retaining its interaction with synthetic estrogen analogs such as tamoxifen. The fusion protein, CreERTM, is inactive while sequestered in the cytoplasm by heat shock protein complexes. Binding of tamoxifen releases CreERTM from this complex allowing ligand-dependent translocation to the nucleus where the fusion protein is active and directs recombination between loxP sites (Hayashi and McMahon 2002). In this report, we describe the characterization of two mouse strains expressing the CreERTM fusion protein under the control of the human GFAP promoter.
Materials and methods
Transgenic and reporter mice
Standard subcloning techniques were used to construct the transgene placing the 2.2 Kb human GFAP promoter (a gift from D. Gutmann) (Brenner et al. 1994) upstream of the cDNA encoding the CreERTM fusion protein (provided by A. McMahon). This was followed by an internal ribosomal entry sequence (IRES) and the cDNA encoding bacterial β-galactosidase (β-gal) to facilitate detection of transgene expression. The construct was terminated by an SV40 intronic sequence and polyadenylation signal. Following pronuclear injection into fertilized FVB/NJ oocytes and implantation into foster mothers, the transgenic founder was identified by polymerase chain reaction (PCR) with primers recognizing the Cre sequence (5′ AGCGATCGCTGCCAGGAT 3′ and 5′ ACCAGCGTTTTCGTTCTGCC 3′). Integration of the transgene was confirmed by Southern blot using a probe recognizing the SV40 sequences. The mouse line was propagated by crossing with FVB/NJ wild-type mice. Cre activity was assessed by crossing into the Z/EG reporter mouse line (Jackson Laboratory, Bar Harbor, ME) (Novak et al. 2000). Reporter positive mice were identified by PCR using primers to the GFP gene (5′ CCGGGGTGGTGCCCATCCTGGT 3′ and 5′ GCTCCTGGACGTAGCCTTCGGGC 3′). All experiments involving mice were carried out in compliance with the Animal Care and Use Committee at St. Jude Children’s Research Hospital.
Induction of Cre activity
Tamoxifen (Sigma, St. Louis, MO) was dissolved in corn oil (Sigma) at a concentration of 20 mg/ml at 37°C, and then filter sterilized and stored for up to 7 days at 4°C in the dark. A 27 g needle tuberculin syringe (Becton Dickinson, Franklin Lakes, NJ) was used for intraperitoneal injections at the indicated doses. An equivalent volume of sterile filtered corn oil alone was used for vehicle injections. Multiple injections in the same mouse were separated by 24 h.
Histochemistry
Animals were perfused with 1× phosphate-buffered saline (PBS) followed by 2% paraformaldehyde (PFA) in PBS. Following dissection, tissues were post-fixed overnight in 2% PFA in PBS at 4°C, and then equilibrated to 25% sucrose in PBS for a further 24 h at 4°C. Tissues were subsequently embedded and cryosectioned at a thickness of 12 μm. Tissue slides were washed three times in PBS prior to staining.
For detection of β-gal activity, slides were washed in Rinse A (100 mM NaPO4, pH 7.3; 2 mM MgCl2; 5 mM EGTA) for 30 min followed by Rinse B (100 mM NaPO4 pH 7.3; 2 mM MgCl2; 0.01% sodium deoxycholate; 0.02% NP-40) for 5 min. Slides were then stained at 37°C for 3–4 days in Developing Buffer (1 mg/ml X-gal; 100 mM NaPO4; pH 7.3; 2 mM MgCl2; 0.01% sodium deoxycholate; 0.02% NP-40; 5 mM K3Fe(CN)6; 5 mM K4Fe(CN)6). After washing extensively in PBS, slides were counterstained with Nuclear Fast Red (Vector Labs, Burlingame, CA).
Immunohistochemistry and immunofluorescence
Cryosections were prepared as above. Primary antibodies used for immunodetection were against GFP (1:5000 for immunohistochemistry, 1:500 for immunofluorescence; Molecular Probes, Eugene, OR), S100β (1:200; Sigma), NeuN (1:500; Chemicon, Temecula, CA), CC-1 (1:50; Calbiochem, San Diego, CA), Gfap (1:200; Sigma) and S100 (1:500; Dako, Carpinteria, CA). Microwave antigen retrieval was performed for all antibodies except anti-GFP. For immunohistochemistry, biotinylated secondary antibodies were used in conjunction with horseradish peroxidase-conjugated streptavidin (Elite ABC, Vector Laboratories) treated with NovoRed or VIP (Fig. 5) substrate (Vector Laboratories) and counterstained with hematoxylin or methyl green (Vector Laboratories), respectively. For immunofluorescence, Alexa Fluor 488 (Molecular Probes) and cyanine 3 (Jackson Immunoresearch, West Grove, PA) conjugated secondary antibodies were employed along with Vectashield mounting media containing 4′,6-diamino-2-phenylindole (Vector Laboratories).
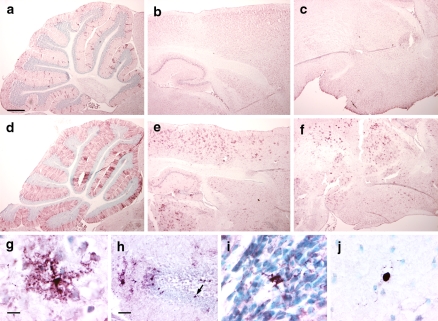
Fig. 5.
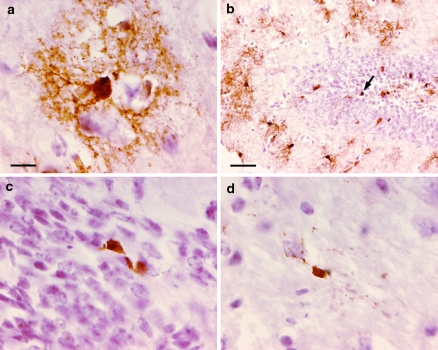
Cre-mediated recombination in adult GFAP-CreER TM B; Z/EG brains by immunohistochemistry for GFP. Mice were treated with tamoxifen as in Fig. 3. (a–c) GFAP-CreER TM B; Z/EG double transgenice mouse injected with vehicle daily for 5 days. (d–f) GFAP-CreER TM B; Z/EG double transgenic mouse injected with 9 mg/40 g tamoxifen daily for 5 days. Brain regions presented are sagittal sections through the cerebellum (panels a, d), cerebral cortex and hippocampus (panels b, e) and thalamus and hypothalamus (panels c, f). Scale bar in (a) is 0.5 mm and applies to panels (a–f). (g) High power image of GFP-positive cell in the cerebral cortex. (h) Dentate gyrus. The arrow indicates a GFP-stained cell located in the inner layer of the dentate gyrus. (i) Subventricular zone. (j) Corpus callosum. Scale bar in (g) is 10 μm and applies to panels (g, i, j). Scale bar in (h) is 50 μm
Quantitation
The percentage of astrocytes undergoing Cre-mediated recombination was determined in five GFAP-CreER TM A; Z/EG mice injected with 9 mg/40 g body weight tamoxifen daily for 5 days. Serial cryosections were stained with S100 and GFP and positively stained cells (identified by visibly stained nucleus) from equivalent surface areas in various regions of the brain were counted with the aid of the Bioquant system (Bioquant Image Analysis Corp., Nashville, TN). The average number of cells counted in each mouse for the cerebellum, cortex, hippocampus and thalamus/hypothalamus was 1260, 1430, 490, and 2500, respectively. For oligodendrocytes, CC-1 positive cells in the corpus callosum of cryosections from two brains were counted (3220 cells each) and compared to the number of double positive CC-1 and GFP staining cells in an adjacent section. The total number of subventricular zone nuclei in sections from four different animals was determined (an average of 2260 nuclei per mouse) and compared to the number of GFP positive cells in the same sections.
Results
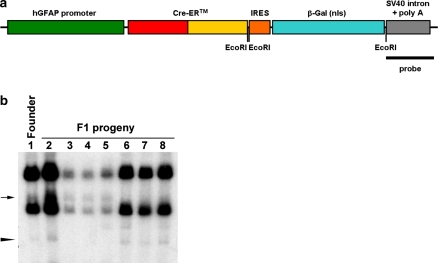
The cDNA encoding the CreERTM fusion protein was placed downstream of the 2.2 Kb human GFAP promoter (Fig. 1a) (Brenner et al. 1994). This was followed by an internal ribosomal entry site (IRES) and a LacZ cDNA encoding a nuclear-targeted version of E. coli β-galactosidase (β-gal). The construct was terminated by an SV40 large T intron and polyadenylation signal. After pronuclear injection of FVB/NJ eggs, one founder was obtained. Transgene integration in this line, hereafter termed GFAP-CreER TM, was verified by polymerase chain reaction (PCR) for Cre-specific sequences as well as by Southern blot hybridization (Fig. 1b). The latter result suggested the presence of two independent integration sites, a conclusion which was supported by a frequency of Cre-positive F1 progeny of 76% (63 Cre-positive out of 83 total pups). After several rounds of breeding to wild-type FVB mice, the two integration sites were separated into independent lines (GFAP-CreER TM A; Fig. 1b, lanes 6–8, and GFAP-CreER TM B; Fig. 1b, lanes 3–5). Furthermore, a single integration site in each line was verified by fluorescence in situ hybridization (FISH) for the transgene in MEF cells generated from each GFAP-CreER TM mouse line with chromosomal location of 9A5 for line A and 15E3 for line B (data not shown).
Fig. 1.
Generation of transgenic mouse lines. (a) Diagram of transgenic construct. The 2.2 Kb human GFAP promoter drives expression of cDNAs encoding the Cre-estrogen receptor ligand binding domain (CreERTM) fusion protein, an internal ribosome entry site (IRES) and E. coli β-galactosidase (β-gal). (b) Southern blot. Genomic DNAs from the transgenic founder mouse and a panel of F1 progeny were digested with EcoRI (location of restriction sites within the transgene are indicated in panel (a)) to detect fragments resulting from internal digest of the multicopy transgene as well as those containing the junction of the transgene with the insertion sites in genomic DNA. The fragments corresponding to the insertion site for GFAP-CreER TM A (arrowhead) and for GFAP-CreER TM B (arrow) are shown. The probe was directed against SV40 sequences as indicated in panel (a)
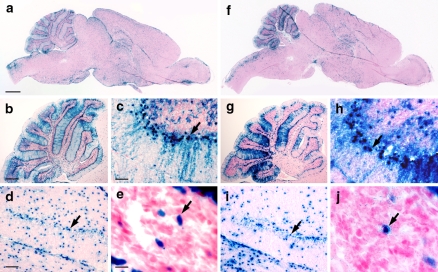
Transgene expression was characterized by histochemistry for β-gal activity in adult mouse brains. In wild-type mice, occasional histochemical staining was noted in cells of the pial membranes, representing endogenous β-gal activity (data not shown) (Chow et al. 2006). In contrast, GFAP-CreER TM brains exhibited specific and reproducible patterns of β-gal activity that were virtually identical between the two mouse lines. Cells expressing β-gal were scattered throughout all regions of the brain (Fig. 2a, f), however, the specific pattern in certain areas was notable. In the cerebellum, many β-gal positive cell bodies aligned along the Purkinje cell layer with cellular processes extending into the molecular layer (Fig. 2b, c arrows, g, h, arrows), a morphological feature consistent with Bergmann glia. In the dentate gyrus, β-gal positive cells tended to cluster in the inner layer of this structure (Fig. 2d, i arrows) where neural precursor cells are known to reside (Doetsch 2003). Histochemical staining for β-gal activity was also noted in cells of the subventricular zone surrounding the lateral ventricle (Fig. 2e, j arrows) where Gfap-expressing adult neural stem cells have also been identified (Doetsch 2003). No β-gal activity was detected in a panel of tissues from GFAP-CreER TM adult mice including muscle, skin, lung, thymus, heart, liver, spleen, adrenal gland, pancreas, bladder, uterus, ovary and epididymus (data not shown). β-gal activity was detected in the gastrointestinal tract, kidney and testis however this likely represents endogenous activity as similar staining was also noted in non-transgenic control animals (data not shown).
Fig. 2.
Transgene expression analysis of adult GFAP-CreER TM brains by β-gal histochemistry. (a–e) GFAP-CreER TM A brain. (f–j) GFAP-CreER TM B brain. (a, f) Sagittal sections of brain. Scale bar in (a) is 1 mm and applies to (f). (b, c, g, h) Sagittal sections of cerebellum at low (b, g) and high (c, h) power. The arrows in (c) and (h) indicate the location of Bergman glia. Scale bar in (b) is 0.5 mm and applies to (g) and that in (c) is 50 μm and applies to (h). (d, i) Sagittal sections through dentate gyrus. The arrows indicate the inner layer of the dentate gyrus, the location of neural progenitor cells. Scale bar in (d) is 100 μm and applies to (i). (e, j) Sagittal sections through subventricular zone. The arrows indicate a transgene-expressing cell in the subventricular zone. Scale bar in (e) is 10 μm and applies to (j)
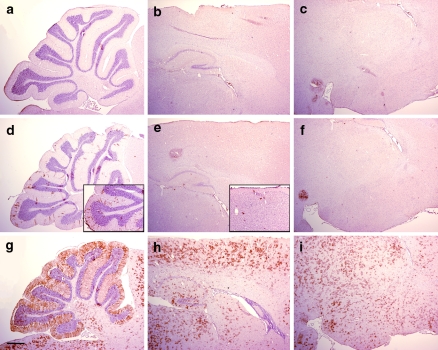
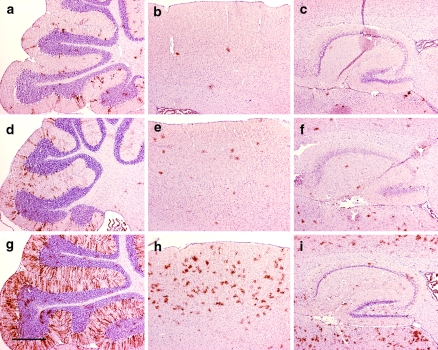
Reporter mice were used to assess the activity of the CreERTM fusion protein following treatment with tamoxifen or vehicle. GFAP-CreER TM mice were crossed with Z/EG reporter mice in which the cDNA encoding enhanced green fluorescent protein (GFP) is expressed only after Cre-mediated excision of a LoxP-flanked stop cassette (Novak et al. 2000). Mice were injected starting at 40–50 days of age intraperitoneally with tamoxifen at a dose of 9 mg/40 g body weight or vehicle alone daily for five injections, then sacrificed at least 5 days after the last injection and tissues analyzed by immunohistochemistry for GFP. In single transgenic mice (either GFAP-CreER TM or Z/EG alone), no GFP immunoreactivity was detected in the brain (Fig. 3a–c and data not shown). In double transgenic mice injected with vehicle, occasional GFP positive cells were present, particularly in cerebellar Bergmann glia (Figs. 3d–f and 5a–c). This tamoxifen-independent Cre-mediated recombination accumulated slowly over the period of 14 months remaining predominantly localized to Bergmann glial cells (Fig. 3d, e insets) and represents the “leakiness” of this inducible Cre strain. In contrast, tamoxifen-injected mice displayed widespread GFP expression mirroring the pattern of β-gal activity in GFAP-CreER TM single transgenic mice (Figs. 3g–i and 5d–f). Under higher magnification, the cortical staining pattern for GFP revealed cells exhibiting a stellate morphology consistent with mature astrocytes (Figs. 4a and 5g). Furthermore, GFP-expressing cells were detected in the inner layer of the dentate gyrus (Figs. 4b and 5h arrows) and in the subventricular zone (Figs. 4c and 5i) consistent with β-gal activity (Fig. 2d, e, i, j). GFP-expressing cells were also noted in the corpus callosum (Figs. 4d and 5j).
Fig. 3.
Cre-mediated recombination in adult brains by immunohistochemistry for GFP reporter. (a–c) Z/EG single transgenic mouse injected with 9 mg/40 g tamoxifen daily for 5 days. (d–f) GFAP-CreER TM A; Z/EG double transgenice mouse injected with vehicle daily for 5 days. Inset represents images from an uninduced 14-month-old double transgenic mouse whereas all other mice presented are ~8 weeks of age as indicated in the text. (g–i) GFAP-CreER TM A; Z/EG double transgenic mouse injected with 9 mg/40 g tamoxifen daily for 5 days. Brain regions presented are sagittal sections through the cerebellum (panels a, d, g), cerebral cortex and hippocampus (panels b, e, h) and thalamus and hypothalamus (panels c, f, i). Scale bar is 0.5 mm and applies to all panels
Fig. 4.
Cre-mediated recombination in adult GFAP-CreER TM A; Z/EG brains by immunohistochemistry for GFP. Mice were treated with tamoxifen as in Fig. 3. (a) Cerebral cortex. The stellate appearance of a cortical GFP-stained cell resembling an astrocyte is presented. (b) Dentate gyrus. The arrow indicates a GFP-stained cell located in the inner layer of the dentate gyrus which may represent a neural precursor cell. (c) Subventricular zone. These GFP-positive cells may represent self-renewing B cells of the subventricular zone. (d) Corpus callosum. Scale bar in (a) is 10 μm and applies to panels (a, c, d). Scale bar in (b) is 50 μm
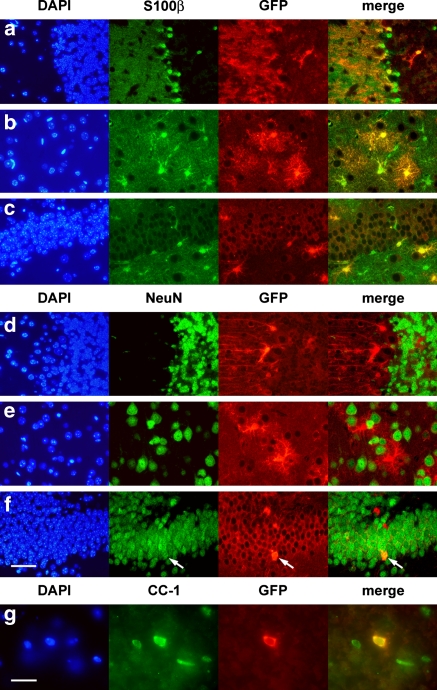
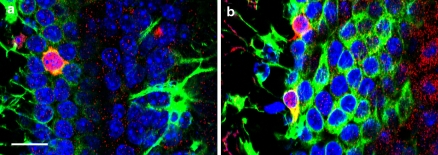
To confirm the cell types targeted for Cre activity, double labeling by immunofluorescence was performed. The majority of GFP-labeled cells in all regions of the brain were found to co-express S100β, a marker for glial cells (Fig. 6a–c). In contrast, almost no GFP-labeled cells co-expressed NeuN, a marker of mature neurons (Fig. 6d–f), although the occasional double-labeled granule neuron in the dentate gyrus, was noted (Fig. 6f arrow). It is possible that such cells were Gfap-expressing neural precursors when tamoxifen was administered, then subsequently differentiated into granule neurons during the 5–10-day-period prior to analysis. Alternatively, this transgenic line may drive CreER expression in the rare mature granule neuron. However, immunophenotypical analyses of multiple GFAP-CreER TM single transgenic mice failed to identify any CreER expression in neurons (data not shown). GFP-expressing oligodendrocytes in the corpus callosum were identified by co-expression of the CC-1 antigen (Fig. 6g). Confocal microscopy was used to confirm Cre activity in occasional Gfap positive neural precursor cells of the subventricular zone (Fig. 7).
Fig. 6.
Identification of cells undergoing Cre-mediated recombination in adult GFAP-CreER TM A; Z/EG brains by double immunofluorescence. Mice were treated with tamoxifen as in Fig. 3. (a–c) Staining with an astrocyte marker and anti-GFP. Co-immunodetection of S100β (green) and GFP (red) is demonstrated in the cerebellum (a), cerebral cortex (b) and dentate gyrus (c). (d–f) Staining with a neuronal marker and anti-GFP. There is no co-immunodetection of NeuN (green) and GFP (red) in cerebellum (d) and cortex (e) while occasional granule neurons of the dentate gyrus (f) express both markers (arrow). (g) Staining with an oligodendrocyte marker and anti-GFP. Cells staining with both CC-1 (green) and GFP (red) were readily identified in the corpus callosum. Scale bar in (f) is 50 μm and applies to panels (a–f). Scale bar in (g) is 20 μm. DAPI: 4′,6-diamino-2-phenylindole (blue). Immunophenotyping results were identical in the GFAP-CreER TM B line (data not shown)
Fig. 7.
Confocal imaging of subventricular zone in adult GFAP-CreER TM A; Z/EG brains. Mice were treated with tamoxifen as in Fig. 3. (a, b) Staining with antibodies against Gfap (green) and GFP (red) with DAPI (blue). Occasional cells co-expressing these two markers in the subventricular zone were identified in these 1 μm optical sections. Scale bar in (a) is 10 μm and applies to both panels
The degree of Cre-mediated recombination was assessed by examining sections serially stained for S100 and GFP proteins in various regions of the brain (Table 1). Overall, astrocyte recombination ranged from 35% to 70% and was more efficient in the cerebellar Bergmann glia than in other parts of the brain. Interestingly, <1% of all cells in the subventricular zone showed cre-mediated recombination under the experimental conditions tested and probably represent Cre activity in the Gfap-expressing B cells (Fig 7) (Doetsch 2003). This population has been reported to comprise 20–25% of cells in the subventricular zone (Doetsch et al. 1997). Increasing the dose of tamoxifen administered did not result in further elevations in the number of GFP positive cells (data not shown), however, recombination could be restricted to fewer astrocytes by titrating the drug dose from 9 to 1 mg/40 g body weight in a single injection (Fig. 8).
Table 1.
Quantitation of Cre-mediated recombination in different brain regions of GFAP-CreER TM A mice
| Region | % Recombination (SEM) |
|---|---|
| Cerebellar astrocytes (Bergmann glia) | 70.8 (2.5) |
| Cortical astrocytes | 52.9 (6.1) |
| Hippocampal astrocytes | 44.3 (3.9) |
| Thalamic/hypothalamic astrocytes | 35.1 (3.4) |
| Corpus callosum oligodendrocytes | <1.0 |
| Subventricular zone cells | <1.0 |
Fig. 8.
Dose–dependent regulation of Cre-mediated recombination by decreasing tamoxifen. Immunohistochemistry for GFP was performed. (a–c) GFAP-CreER TM A; Z/EG double transgenice mouse injected with a single 1 mg/40 g dose of tamoxifen. (d–f) GFAP-CreER TM A; Z/EG double transgenice mouse injected with a single 3 mg/40 g dose of tamoxifen. (g–i) GFAP-CreER TM A; Z/EG double transgenice mouse injected with a single 9 mg/40 g dose of tamoxifen. Brain regions presented are sagittal sections through the cerebellum (panels a, d, g), cerebral cortex (panels b, e, h) and hippocampus (panels c, f, i). Scale bar is 0.5 mm and applies to all panels
Discussion
The GFAP-CreER TM mouse lines described in this report represent useful tools for investigators interested in studying the effects of gene manipulation in mature astrocytes. Virtually no Cre activity is present in neurons, specifically in the cortex, allowing for the study of cell autonomous phenomena in this cell population. This may be particularly relevant to the fields of brain injury and repair as well as to gliomagenesis. Furthermore, the presence of detectable Cre activity in a subset of adult neural precursor cells in the dentate gyrus and the subventricular zone may also facilitate the study of regenerative and oncogenic potentials of this increasingly scrutinized cell population.
Recently, similar transgenic mouse lines have been reported in which the CreERT2 fusion protein (Indra et al. 1999) was expressed under the control of the identical GFAP promoter (Casper et al. 2007; Ganat et al. 2006; Hirrlinger et al. 2006). One report described CreER activity in young (five-day-old) mice and found that neural precursor cells were significantly targeted at this age (Ganat et al. 2006). Interestingly, these cells went on to differentiate into mature neurons, oligodendrocytes and astrocytes in vivo taking residence in all regions of the brain. We have yet to examine CreER activity in our juvenile mice. Two other groups have documented CreER activity in adult astrocytes (Casper et al. 2007; Hirrlinger et al. 2006). One line appears to have broad activity in astrocytes, although quantitation was not reported (Casper et al. 2007), while the other is more restricted to certain regions of the brain (Hirrlinger et al. 2006). Neither group commented on CreER activity in adult neural precursor cells. Other astrocyte-specific inducible Cre mouse strains have also been described with activity in adult neural progenitor cells (Mori et al. 2006; Slezak et al. 2007). We describe CreER activity in a broad range of adult astrocytes as well as in a restricted population of adult neural progenitor cells. While the pattern of expression is largely similar between our two lines, the degree of CreER expression and consequently of recombination in astrocytes appears to be slightly greater in the GFAP-CreER TM A line (compare Figs. 2a–f and 3h, i to 5e, f). In addition, by using a less sensitive variant of the CreER fusion protein, we demonstrated an easily achievable dose-response effect to tamoxifen that may be advantageous for certain studies, such as cell-fate mapping and tumorigenesis. We have successfully generated spontaneous models for diffuse astrocytomas using our GFAP-CreER TM A line (L.M.L.C. and S.J.B., unpublished data). Together, these various CreER mouse lines provide novel and powerful tools for investigators to probe normal and pathological astrocyte functions.
Acknowledgements
We thank D. Gutmann and A. McMahon for the gift of reagents and L. Li for technical expertise in generating the transgenic mice. J. Mitchell’s role in genotyping mice was indispensable. We acknowledge J.M. Lahti and V.A. Valentine from the Cancer Center Core Cytogenetics Laboratory for FISH analyses. L.M.L.C. is a recipient of the Jean-François St.-Denis Fellowship in Cancer Research from the Canadian Institutes of Health Research. S.J.B is supported by grants from the National Institutes of Health and the American Lebanese and Syrian Associated Charities of St. Jude Children’s Research Hospital.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22(14):5100–5113. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Messing A. GFAP transgenic mice. Methods. 1996;10(3):351–364. doi: 10.1006/meth.1996.0113. [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14(3 Pt 1):1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31(4):676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45(5):292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- Chow LM, Tian Y, Weber T, Corbett M, Zuo J, Baker SJ. Inducible Cre recombinase activity in mouse cerebellar granule cell precursors and inner ear hair cells. Dev Dyn. 2006;235(11):2991–2998. doi: 10.1002/dvdy.20948. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93(20):10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64(21):7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26(33):8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: insights into brain function and diseases. Pharmacol Ther. 2007;113(3):619–634. doi: 10.1016/j.pharmthera.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- He F, Sun YE. Glial cells more than support cells? Int J Biochem Cell Biol. 2007;39(4):661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54(1):11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27(22):4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13(3):174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte–Duclos disease. Nat Genet. 2001;29(4):404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14(8):994–1004. [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132(1):165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia. 2006;54(1):21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28(3–4):147–155. doi: 10.1002/1526-968X(200011/12)28:3/4<147::AID-GENE90>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Slezak M, Goritz C, Niemiec A, Frisen J, Chambon P, Metzger D, Pfrieger FW. Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55(15):1565–1576. doi: 10.1002/glia.20570. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31(2):85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]