The incorporation of carbon-11 into small molecules has been paramount to the success of positron emission tomography (PET) for in vivo molecular imaging and drug research and development.[1] However, many of the properties that make [11C] an ideal radionuclide for PET have impeded its chemical development. For instance, the short half-life (t1/2 = 20.4 min), which allows for repeated studies within a short time span, necessitates rapid chemical syntheses and purifications. Moreover, high specific activity, which provides an ability to image low concentration receptors and molecular targets, places the working concentration range of [11C]-labeling reagents in the low nanomolar range. But perhaps the biggest challenge in the synthesis of [11C]-labeled compounds is the lack of available labeling reagents. Bear in mind, nearly all carbon-11 syntheses begin with a nuclear reaction [14N(p,α)11C] using a cyclotron that produces 11CO2 or 11CH4 from which labeling reagents must be prepared.
By far the most common, almost canonical method, to label a molecule with [11C] is through methylation, typically with 11CH3I.[2] While pendant methyl groups appear quite frequently in relevant compounds and [11C]-methylation has led to many successful radiotracers, reliance on methylation limits the range of potential probes. Consequently, there exists a need for new reaction development to focus on methods to incorporate [11C] in skeletal positions of target molecules and several research groups have developed or adapted synthetic methods for [11C]-incorporation into benzene rings, carbocycles, and heterocycles as well as non-pendant locations.[3] By using carefully designed organic reactions, each of these has expanded the types of radiotracers that can be accessed.
[11C]Formaldehyde has shown great promise as a labeling reagent for the preparation of PET compounds. Due to its versatile oxidation state, [11C]formaldehyde has used in synthesis of compounds otherwise unlabelable, through reductive methylations,[4] ring-closure reactions,[5] and electrophilic aromatic substitutions,[6] among others.[7] However, the widespread development and use of synthetic methods for [11C]formaldehyde in the preparation of PET-compounds has been hindered by access to it. Several methods have been developed for the synthesis of [11C]formaldehyde from [11C]methanol beginning in 1972 here at Brookhaven National Laboratory,[8] improved over time with new catalysts,[9] and quite elegantly synthesized enzymatically.[10] While each of these methods has found utility, they each have disadvantages preventing more prevalent use.
Recognizing the power of [11C]formaldehyde as a labeling reagent, demonstrated in these previous reports, we sought to develop a very simple and rapid method that would provide access to [11C]formaldehyde without the need for any new equipment. In order to make [11C]formaldehyde instantly available to all carbon-11 chemists, we also constrained our method to use only commercially available starting materials, mild conditions, and short reaction times. Herein, we report a high yielding method for the production of [11C]formaldehyde that meets all of these criteria.
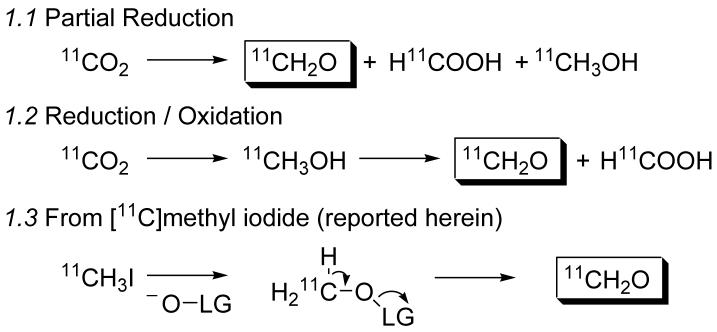
Previous methods for the preparation of [11C]formaldehyde have relied on the partial reduction of 11CO2, or the complete reduction of 11CO2 to 11CH3OH followed by oxidation, Scheme 1. We surmised that using [11C]methyl iodide to access [11C]formaldehyde would be advantageous to these methods for several reasons. First, 11CH3I is routinely produced at virtually every location where carbon-11 compounds are synthesized. Second, we anticipated we could capitalize on the incredible amount of effort that has gone into the development of methods and equipment (now commercially available) for the gas phase synthesis for high specific-activity 11CH3I. Each of the existing methods for [11C]formaldehyde relies on a reduction step that occurs in solution, typically with lithium aluminum hydride, which often causes a reduction in specific activity avoided by gas-phase production of 11CH3I.[2] Finally, since methyl iodide is decidedly electrophilic, we suspected that we could use more mild reagents and conditions than are used for methanol oxidation. Thus, our efforts began with the simple mechanistic oxidation premise outlined in Scheme 1.3.
Scheme 1.
Methods for the production of [11C]formaldehyde
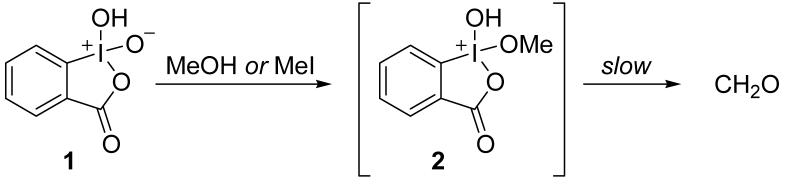
Using 1H-NMR spectrometry, we screened the reaction of sub-stoichiometric methyl iodide with a number of oxygen nucleophiles containing a pendant leaving group (LG). Many inorganic compounds were effective, but led to over oxidation. Our first lead resulting exclusively in formaldehyde was found by comparing the reaction of o-iodoxybenzoic acid (IBX, 1) with methanol and methyl iodide, Scheme 2. The common intermediate 2 was observed, which converted to formaldehyde over the course of several days.[11] It was evident from this study that oxidation rather than methylation was rate limiting. To improve the reaction rate, we turned to trialkylamine-N-oxides, which we predicted would provide a better leaving group.
Scheme 2.
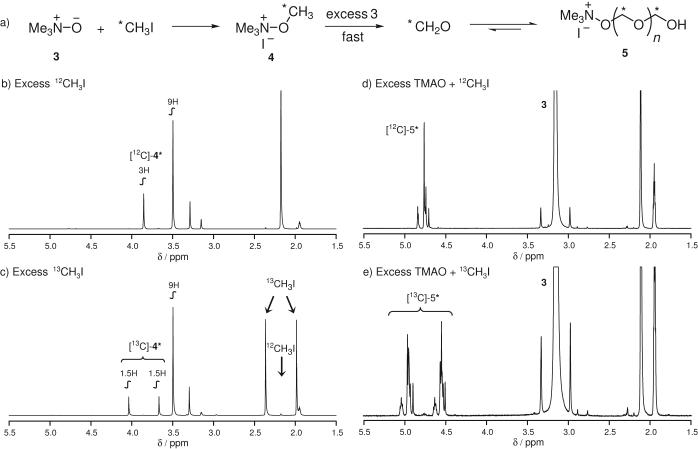
By reacting trimethylamine-N-oxide (TMAO, 3) with methyl iodide, we observed methylation (4) and subsequent formaldehyde formation immediately (as determined by the presence of a 1H resonance at ca. 9.5 ppm).[12] In fact, the decomposition of solid 4 to formaldehyde at high temperatures was reported nearly a century ago when the structure of TMAO was under debate.[13] Given excess TMAO, 4 underwent elimination to form formaldehyde at RT. This process occurred quantitatively at slightly elevated temperatures. Triethylamine-N-oxide and N-methylmorpholine-N-oxide were also effective, but necessitated longer reaction times whereas pyridine-N-oxide was ineffective under all condition screened. Upon formation, formaldehyde oligomerized in the presence of TMAO giving rise to multiple methylene resonances at 4.3 - 4.8 ppm (as is the case in the presence of water).[14] We used 13CH3I to determine if the use of TMAO introduced any isotopic dilution during course of the reaction and to assign all resonances resulting from methyliodide, Figure 1. We found that the ratio of 12C/13C remained 1/100 throughout the reaction, suggesting that methyl groups of TMAO would not impact the specific activity of reactions performed with 11CH3I.
Figure 1.
1H-NMR spectra (400 MHz, CD3CN) of (a) the reaction between TMAO and MeI. (b-c) Using excess MeI, intermediate 4 was observed. (d-e) In the presence of excess TMAO, an equilibrium mixture of formaldehyde and its oligomers (5) was observed. Axes are ppm relative to the solvent (1.95 ppm). Full 1H-spectra as well as 13C-spectra are available in supporting information.
To test the viability of this method for the preparation of [11C]formaldehyde, we assayed the effect of solvent, TMAO concentration, and reaction time and temperature with the dimedone precipitation method used in previous [11C]formaldehyde reports, (a partial list of conditioned screened is given in Table 1). Briefly, an aliquot of the C-11 reaction mixture was added to carrier formaldehyde, which was subsequently precipitated as a dimedone adduct. The precipitate was separated by filtration or centrifugation and the % radioactivity in the precipitate was determined. As a control, we omitted TMAO from the reaction and performed the formaldehyde analysis, e.g. Table entry 5, and found <5% of the radioactivity was associated with the precipitate, which could be removed with washing. We found that DMF was an excellent solvent for the transformation, providing high yields of [11C]formaldehyde in under 2 min at only 70 °C.
Table 1.
[11C]Formaldehyde yield optimization
| Entry | Solvent[[a]] | Time [s] | TMAO [mg] | T [°C] | Yield [%][[b]] |
|---|---|---|---|---|---|
| 1 | MeCN | 120 | 4.0 | 70 | 27 ± 7 |
| 2 | MeCN | 120 | 25.0 | 70 | 58 ± 8 |
| 3 | DMSO | 120 | 4.0 | 70 | 23 ± 7 |
| 4 | THF | 120 | 4.0 | 70 | 26 ± 4 |
| 5 | DMF | 120 | 0.0 | 70 | 3 ± 2[[c]] |
| 6 | DMF | 120 | 1.0 | 70 | 80 ± 2 |
| 7 | DMF | 30 | 4.0 | 70 | 72 ± 5 |
| 8 | DMF | 60 | 4.0 | 70 | 86 ± 4 |
| 9 | DMF | 120 | 4.0 | 70 | 89 ± 4[[d]] |
| 10 | DMF | 120 | 4.0 | 20 | 38 ± 6 |
Reactions (300 μL) were performed with ∼1 mCi 11CH3I.
Average ± stdev (n = 3) based on dimedone precipitation method.
Radioactivity lost from precipitate with exhaustive washing.
Recommended conditions (n = 12).
We found that the reaction was reproducible and robust. The reaction was not sensitive to small amounts of water (up to 10 μL were added) and the dihydrate form of TMAO, which is less expensive, was an equally effective reagent. Added base was tolerated so long as it did not competitively methylate. However, the addition of acid lowered reaction yields or prevented [11C]formaldehyde formation altogether. Higher temperatures were equally effective with no over-oxidation observed.
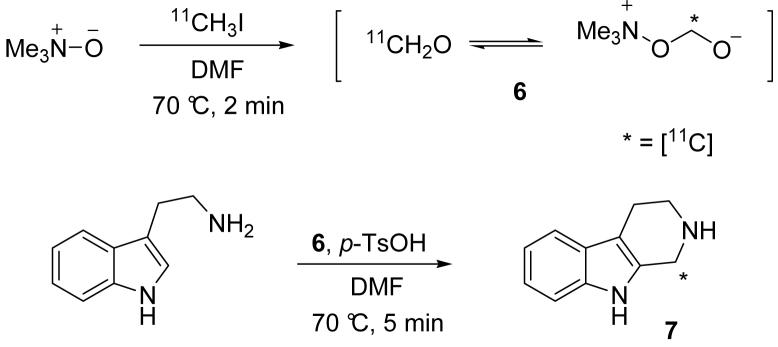
In cold (i.e. C-12) test experiments, we successfully used the formaldehyde/oligomer mixture (5) in a variety of reaction scenarios including reductive amination of anilines, oxime and hydrazone formation, and cyclization reactions. To highlight the efficacy of this mixture as no carrier added [11C]formaldehyde and to determine specific activity, we performed a Pictet-Spengler condensation with tryptamine, Scheme 3.
Scheme 3.
The reaction of tryptamine with 6 occurred readily under acidic conditions affording [11C]-2,3,4,9-tetrahydro-1H-beta-carboline 7 in excellent radiochemical yield. The product was isolated by semi-preparative HPLC using a C18 column and the mass was determined by absorbance at 254 nm. Using a portion of [11C]MeI from a clinical production run, we determined 7 had a specific activity of 3.0 Ci/μmol (as compared to 4.5 Ci/ μmol for the clinical product).[15]
In addition, to further verify TMAO itself does not contribute formaldehyde to the reaction solution, we ran two critical negative control reactions under the same preparative-scale conditions. In the first, we omitted MeI and found no product formation occurred. In the second, TMAO was omitted, and no [11C]-7 was observed.
In summary, we have developed a simple and accessible method for production of isotopically labelled formaldehyde. We demonstrated that commercially available, inexpensive trimethylamine-N-oxide is highly effective at converting [11C]methyl iodide to [11C]formaldehyde under mild conditions with no erosion of specific activity. We anticipate easy access to [11C]formaldehyde will result in the increased use and scope of reactions such as electrophilic aromatic substitution, Mannich-type condensations, and cyclization reactions for the preparation of [11C]-labelled compounds. Current studies are underway to expand the scope and utility of [11C]formaldehyde as a labelling reagent for the synthesis of PET radiotracers from molecules often considered unlabellable.
Experimental Section
General procedure for the conversion of 11CH3I to 11CH2O: A mixture of trimethylamine-N-oxide or its hydrate (4 mg) and DMF (300 μL) cooled to -40 °C was used to capture 11CH3I produced using PETtrace MeI Microlab (GE Medical Systems, Milwaukee, WI, USA). The sealed vessel was heated to 70 °C for 2 min and then cooled in an ice bath.
Supplementary Material
Footnotes
This work was carried out at Brookhaven National Laboratory (contract DE-AC02-98CH10886 with the U.S. Department of Energy and supported by its Office of Biological and Environmental Research). J.M.H. was supported by the NIH (1F32EB008320-01) and a Goldhaber Fellowship at BNL. M.S. and H.S. were supported by Deutscher Akademischer Austauschdienst (DAAD) and BNL.
Contributor Information
Jacob M. Hooker, Medical Department, Brookhaven National Laboratory, Upton, NY 11973-5000.
Matthias Schönberger, Johannes Gutenberg-Universitaet Mainz, Mainz, Germany.
Hanno Schieferstein, Johannes Gutenberg-Universitaet Mainz, Mainz, Germany.
Joanna S. Fowler, Medical Department, Brookhaven National Laboratory, Upton, NY 11973-5000.
References
- [1].Phelps ME. Proc. Nat. Acad. Sci. USA. 2000;97:9226. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]a).Långström B, Lundqvist H. Int. J. Appl. Radiat. Isot. 1976;27:357. doi: 10.1016/0020-708x(76)90088-0. [DOI] [PubMed] [Google Scholar]; b) Larsen P, Ulin J, Dahlstrøm K, Jensen M. Appl. Radiat. Isot. 1997;48:153. [Google Scholar]
- [3]a).Reviewed in:Wuest FR. Trends in Organic Chemistry. 2003;10:61.Itsenko O, Norberg D, Rasmussen T, Långström B, Chatgilialoglu C. J. Am. Chem. Soc. 2007;129:9020. doi: 10.1021/ja0707714.and references therein.
- [4]a).Straatmann MG, Welch MJ. J. Nucl. Med. 1975;16:425. [PubMed] [Google Scholar]; b) Marazano C, Mazière M, Berger G, Comar D. Int. J. Appl. Radiat. Isot. 1977;28:49. doi: 10.1016/0020-708x(77)90159-4. [DOI] [PubMed] [Google Scholar]; c) Mazière M, Berger G, Comar D. J. Label. Comp. Radiopharm. 1977;13:196. [Google Scholar]; d) Berger G, Mazière M, Marazano C, Comar D. Eur. J. Nucl. Med. 1978;3:101. doi: 10.1007/BF00251632. [DOI] [PubMed] [Google Scholar]; e) Berger G, Mazière M, Knipper R, Prenant C, Comar D. Int. J. Appl. Radiat. Isot. 1979;30:393. doi: 10.1016/0020-708x(79)90049-8. [DOI] [PubMed] [Google Scholar]
- [5]a).Nader MW, Zeisler SK, Theobald A, Oberdorfer F. Appl. Radiat. Isot. 1998;49:1599. [Google Scholar]; b) Roeda D, Sipila HT, Bramoulle Y, Enas JD, Vaufrey F, Dolle F, Crouzel C. J. Label. Comp. Radiopharm. 2002;45:37. [Google Scholar]; c) Van der Mey M, Windhorst AD, Klok RP, Herscheid JDM, Kennis LE, Bischoff F, Bakker M, Langlois X, Heylen L, Jurzak M, Leysen JE. Bioorg. Med. Chem. 2006;14:4526. doi: 10.1016/j.bmc.2006.02.029. [DOI] [PubMed] [Google Scholar]
- [6].Langer O, Krcal A, Schmid A, Abrahim A, Minetti P, Celona D, Roeda D, Dollé F, Kletter K, Mueller M. J. Label. Comp. Radiopharm. 2005;48:577. [Google Scholar]
- [7].Pike VW, Palmer AJ, Horlock PL, Perun TJ, Freiberg LA, Dunnigan DA, Liss RH. Int. J. Appl. Radiat. Isot. 1984;35:103. doi: 10.1016/0020-708x(84)90192-3. [DOI] [PubMed] [Google Scholar]
- [8].Christman D, Crawford EJ, Friedkin M, Wolf AP. Proc. Nat. Acad. Sci. USA. 1972;69:988. doi: 10.1073/pnas.69.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roeda D, Dollé Fi. J. Label. Comp. Radiopharm. 2003;46:449. doi: 10.1002/jlcr.2992. [DOI] [PubMed] [Google Scholar]
- [10]a).Slegers G, Lambrecht RHD, Vandewalle T, Meulewaeter L, Vandecasteele C. J. Label. Comp. Radiopharm. 1984;25:338. [PubMed] [Google Scholar]; b) Svärd H, Antoni G, Jigerius SB, Zdansky G, Langström B. J. Label. Comp. Radiopharm. 1984;21:1175. [Google Scholar]; c) Hughes JA, Jay M. Nucl. Med. Biol. 1995;22:105. doi: 10.1016/0969-8051(94)00073-s. [DOI] [PubMed] [Google Scholar]
- [11].For reaction kinetics of IBX with MeOH by 1H NMR see:De Munari S, Frigerio M, Santagostino M. J. Org. Chem. 1996;61:9272.
- [12].Dahn H, Pechy P. Magn. Res. Chem. 1996;34:723. [Google Scholar]
- [13].Meisenheimer J. Justus Liebig’s Annalen der Chemie. 1913;397:273. [Google Scholar]
- [14].Le Botlan DJ, Mechin BG, Martin GJ. Anal. Chem. 1983;55:587. [Google Scholar]
- [15].“Environmental” formaldehyde is found in many chemicals and solvents and should be rigorously excluded for very high specific activity syntheses.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.